?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The chemical composition and nutritional quality in the meat of soldier crab (Mictyris brevidactylus) were analysed and evaluated. Soldier crab was rich in phosphorus, calcium and magnesium, and also was a good mineral source of copper, iron and zinc. The major fatty acids were docosahexaenoic acid (22:6n-3), eicosapentaenoic acid (20:5n-3) and palmitic acid (16:0). Crab meat was an n-3 polyunsaturated fatty acids (PUFAs)-rich food, as the ratio of n-3/n-6 PUFAs was 3.10. Glutamic acid (204 mg/g of protein) was the dominant amino acid in soldier crab protein, followed by glycine (162 mg/g), aspartic acid (100 mg/g), lysine (87 mg/g), leucine (84 mg/g) and alanine (82 mg/g), and the contents of methionine, histidine, serine and cysteine were low. Tryptophan and methionine were the first and second limiting amino acid, respectively, in soldier crab. The ratio of essential to non-essential amino acids was 0.58. The results indicated that soldier crab could be an ideal resource of nutritional food material with a desirable chemical composition.
1. Introduction
Soldier crab (Mictyris brevidactylus) mainly lives in the intertidal and supratidal zone of the Indo-Pacific Ocean (Unno Citation2008; Davie et al. Citation2013). The name ‘soldier crab’ comes from its high density (up to several hundred crabs/m2) and speed during the falling tide, just like marching armies (Shih Citation1995). Soldier crab meat has a delicious umami taste (Liu et al. Citation2018). In Beihai, one of the coastal cities in South China, soldier crab is used to make crab sauce in its maturation period. When making crab sauce, the viscera was removed, and crab meat with carapace was mashed and mixed with 30% salt, and then fermented for 35 days. Crab sauce is a favourite umami seasoning in local (Liu, Xia et al. Citation2019).
Nutritionally speaking, crab is an ideal nutritional food, as it contains high-quality proteins, polyunsaturated fatty acids and various minerals (Celik et al. Citation2004; Naczk et al. Citation2004; Chen et al. Citation2007; Yan-Ru et al. Citation2014; Wang et al. Citation2018). Proteins are vital biomacromolecules for human beings. To some extent, the quality of proteins depends on the contents and constitutes of essential amino acids, which must be taken in from foods and cannot be synthesized independently. In the previous report, the amino acid composition of swimming crab, mud crab and Chinese mitten crab were quite balanced, and crabs were a good source of protein (Chen et al. Citation2007; Jiang et al. Citation2014).
Fatty acid is a very important nutrient, especially those essential fatty acids (Simopoulos Citation1991; FAO Citation1994; Elahe and Ali Citation2014). Numerous research has shown that crab, especially the salt-water crabs, contains large quantity of essential fatty acids, such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) (Celik et al. Citation2004; Naczk et al. Citation2004; Cherif et al. Citation2008; Sato et al. Citation2015). DHA and EPA play important roles in preventing cardiovascular disease and improving immunity function (Simopoulos Citation1991).
What is more, minerals also contribute to the nutritional value of crab meat (Skonberg and Perkins Citation2002). Crab meat was rich in calcium, magnesium, phosphorous, copper, iron, zinc etc., and it was a good resource of minerals (Akpan Citation1997; Chen et al. Citation2007; Yan-Ru et al. Citation2014).
Although there are some reports about the metal-accumulating ability of the soldier crab (Yeh et al. Citation2009), soldier crab food resources and its influence on the fatty acid composition of the crab tissue (Takagi et al. Citation2010), to the best of our knowledge, there is no research concerning the nutritional quality of soldier crab. The objectives of the present study were to analyse the chemical characteristics and evaluate the nutritional quality of soldier crab.
2. Materials and methods
2.1. Sample preparation
Wild soldier crabs (individually weighed 3–5 g, diameter 30–40 mm) were collected at the Daguansha intertidal zone, Beihai city, China (21°24′51.3″N 109°10′25.2″E) during their maturation period in October 2016. Soldier crab is used for making crab sauce in local in autumn, and in other seasons, the local people seldom catch the crab, so the ‘maturation period’ was selected in this study. Five kilograms of fresh soldier crabs were transported to the laboratory under ice fresh condition (∼4 h transport) and cleaned immediately. Because of its small size, the males and females were not separated in the analysis. The crabs were divided into three groups by random, and each of the three groups was considered as a replicate, and then the crab meat (from abdomen, legs and claws) was manually separated (the viscera was completely separated from meat as the viscera is separated distribution from the meat; but some thin and soft carapace is interval distributed in the abdomen meat, so some tiny carapace was in the meat sample) and homogenized at ice water bath. Finally, the processed crab meat was vacuum packed and stored (−20°C) prior to further use. All analyses were done in two weeks.
2.2. Proximate composition analyses
All analyses were carried out in conformity to the methods of AOAC (Citation2005). Moisture content, crude fat, crude protein and ash content were measured using AOAC Official Method 934.01 (drying the sample in an oven at 105°C to constant weight), 948.15 (Soxhlet extraction method), 976.05 (automated Kjeldahl method) and 938.08 (combusting the sample in an furnace at 550°C for 10–12 h until a constant weight was acquired), respectively. Nitrogen-to-protein conversion factor of 6.25 was used for the determination of total crude protein of soldier crab. Analyses were performed in triplicate.
2.3. Mineral analysis
The elements of potassium (K), sodium (Na), calcium (Ca), iron (Fe), magnesium (Mg), manganese (Mn), zinc (Zn) and copper (Cu) were measured by inductively coupled plasma optical emission spectrometry (ICP-OES) after microwave-assisted digestion with HNO3/H2O2, using inductively coupled plasma emission spectrometer (7700e, Agilent, Japan) (Skonberg and Perkins Citation2002). And samples were digested in HNO3/HCIO4 (volume ratio 4:1) for phosphorus (P) determination by spectrophotometric methods (AOAC Citation2005). Standard curves were applied to quantitate the elements. Analyses were done in triplicate.
2.4. Fatty acid analysis
Fatty acid extraction and fatty acid methyl esters (FAMEs) preparation were carried out on the basis of the method by Chen et al. (Citation2007). Firstly, the Soxhlet extraction method was used to obtain the crab oil from 50 g crab sample. Subsequently, the crab oil was saponified and followed by esterification. Then 2 ml saturated sodium chloride and 2 ml hexane were added and mixed. Finally, anhydrous sodium sulphate was used to dehydrate the supernatant. And then the dehydrated supernatant containing FAMEs were used for fatty acid analysis by GC/MS (7890B-5975C, Agilent, USA).
One microlitre of supernatant was injected into the GC/MS. And the GC conditions were shown below: capillary column, HP-INNOWAX (30 m × 0.25 mm I.D., 0.15 μm film thickness, Agilent); carrier gas, pure nitrogen with a flow rate of 0.65 ml/min. The injection temperature was 250 °C and the temperature-programmed condition was from an initial temperature of 50°C (2 min hold), rising to 220°C at 4 °C/min, and then held isothermal for 15 min. MS conditions were as follows: using full scan mode method and scan range 40–450 m/z; an ionization energy of 70 eV; an ion source temperature of 230°C; a detector interface temperature of 250°C; by matching the mass spectra with those in the NISTDEMO library to identify the peak components and by contrast with GC retention times of the standard compounds to identify fully; using the peak area normalization method to quantitatively analyse (% of total fatty acids) the important FAMEs. Analyses were repeated in triplicate.
2.5. Amino acid analysis
Amino acid analysis was carried out on the basis of the method reported by Chen et al. (Citation2007). Approximately 0.2 g crab sample (wet basis) and 8 ml of (6.0 N) HCl were put in a 25 ml ampoule. The ampoules were vacuum-sealed, and samples were hydrolysed in a thermoelectric thermostat drying box (DHG8090, Jinghong, Shanghai) at 110 ± 1°C for 24 h. After acid hydrolysis, 1 ml of hydrolysate was vacuum dried at 45°C to drive HCl and then 5 ml deionized water was used to re-hydrated it, and dried three times. Subsequently, dried hydrolysate was dissolved in 5 ml acetate buffer (pH 2.0) and filtered through a 0.22 μm filtration membrane to filter impurities. Then 10 μl of the sample was injected into the automatic amino acid analyzer (Hitachi, L-8900, Tokyo, Japan) for amino acid analysis. Seventeen of mixed standard amino acids (Sigma) were used for qualitative and quantitative analysis of the amino acids. Amino acid analysis was done in triplicate.
Because the acid-hydrolysed method can destroy tryptophan, in order to obtain the complete amino acid composition, the tryptophan was released by alkaline (Hugli and Moore Citation1972). In a thermoelectric thermostat drying box, ∼0.2 g samples were hydrolysed in 5 ml of NaOH (5.0 N, containing 5% SnCl2 (w/v)) for 20 h at 110 ± 1°C. After alkaline hydrolysis, the hydrolysate was neutralized with HCl (6.0 N) and then centrifuged. One microlitre of supernatant was used for amino acid analysis, using pre-column AccQ-Flour special derivative reagent derivatization. Tryptophan was separated and determined by Waters 2996 HPLC (Waters, USA) using a 3.9 × 150 mm Waters AccQ-Tag column. The column temperature was controlled at 37°C. The eluents used were (A) Waters AccQ-Tag stock solution, (B) acetonitrile and (C) ultrapure water. The flow rate was set to 1.0 ml/min and the elution programme was as described by Hugli and Moore (Citation1972). The wavelength of the detector was set at UV 248 nm. The identity and quantity of the amino acids were determined by comparison with the retention times and peak areas of the standard tryptophan (Sigma). Tryptophan was tested in triplicate.
2.6. Amino acid score
Amino acid score (AAS) is calculated with respect to FAO/WHO/UNU reference amino acid requirements for adults (WHO Citation1985), and it is determined by the following equation,
If the AAS of each essential amino acid in protein is >100, the amino acid composition of this protein is of great balance with high quality. Conversely, if the AAS of an essential amino acid is less than 100, this amino acid is regarded as the limiting amino acid.
2.7. Statistical analysis
All experiments were done in triplicate (n = 3). Results were presented as mean ± standard deviation (SD).
3. Results and discussion
The proximate composition of soldier crab is shown in . In the present study, protein was the main constituent of dry matter; ash content was relatively abundant but lipid content was low.
Table 1. Proximate composition of soldier crab (wet weight; mean ± SD; n = 3).
The moisture content of soldier crab (75%) was similar to that observed in other species, such as spider crab (79%) (Marques et al. Citation2010) and Chinese mitten crab (78%) (Chen et al. Citation2007). The protein content of soldier crab (11.4%) was lower compared with other crab, such as spider crab (14.5%) (Marques et al. Citation2010), green crab (17%) (Naczk et al. Citation2004), blue crab (16%) (Gokoglu and Yerlikaya Citation2003) and Chinese mitten crab (19%) (Chen et al. Citation2007). The lower protein content might be due to the higher ash content of soldier crab as well as the feeding habits and growth environment. Because of the small size of the soldier crab, some tiny carapace containing large quantities of mineral (Benjakul and Sutthipan Citation2009; Jun et al. Citation2019) was remained in the abdomen meat in the sample preparing, so the ash content of soldier crab meat (9.4%) was ∼6–8 times than that of other crabs. Nowadays, various marine-based protein plays an important role as nutritional ingredient for the food industry, so soldier crab seemed to be a nutritious protein material, too. The crude fat content of soldier crab meat was 1.1%, and it was in the range of the general crustacean products (0.5–1.5%) (Celik et al. Citation2004; Naczk et al. Citation2004; Marques et al. Citation2010).
The mineral content of soldier crab is presented in . Phosphorus, sodium, calcium, magnesium and potassium are macro elements, and their contents were high in crab meat, while the trace elements, such as iron, zinc, copper and manganese, their contents in crab meat were relatively low.
Table 2. Mineral content of soldier crab (wet weight; mean ± SD; n = 3).
As some tiny carapace which is rich in calcium, phosphorus and magnesium (Benjakul and Sutthipan Citation2009; Jun et al. Citation2019) was in the meat, the contents of calcium, phosphorus and magnesium in soldier crab were significantly higher than those of other crabs (Skonberg and Perkins Citation2002; Marques et al. Citation2010), which was consistent with the higher ash content. Minerals play a significant role in the health of the human body. According to the dietary reference intake values (DRI) of macro and trace elements presented for adults (Skonberg and Perkins Citation2002), soldier crab was a good mineral source of copper, iron and magnesium, and also the contents of phosphorus, zinc and calcium were relatively rich.
As presented in , a total of 28 fatty acids in crab meat were identified by GC/MS, including 13 saturated fatty acids (SFA), 6 monounsaturated fatty acids (MUFA) and 9 polyunsaturated fatty acids (PUFA). Compared with SFA (33.60%) and MUFA (18.97%), the crab oil was dominated by PUFA which accounted for 35.88% of the total fatty acids. The major fatty acids detected here were DHA (22:6n-3), EPA (20:5n-3), palmitic acid (16:0), stearic acid (18:0) and oleic acid (18:1). The fatty acids composition was consistent with the previous reports, and DHA and EPA were the dominant fatty acids in salt-water crab meat, such as blue crab (Celik et al. Citation2004) and green crab (Cherif et al. Citation2008). The fatty acids profile in the crab meat was somewhat different from that in the hepatopancreas, as arachidonic acid (20:4n-6) was also one of the major PUFA in the hepatopancreas besides DHA and EPA (Takagi et al. Citation2010).
Table 3. Fatty acid composition of soldier crab (wet weight; mean ± SD; n = 3).
Because n-3 and n-6 PUFAs have different biological roles, the balance between the n-3 and the n-6 PUFAs in the diet is of great importance. FAO experts have recommended that the ratio of n-3/n-6 PUFAs in the diet should be between 1:10 and 1:5, and individuals with a ratio below 1:10 should be encouraged to consume more n-3 PUFAs-rich foods such as seafood (FAO Citation1994). In our normal diet, the amount of n-3 PUFAs was significantly lower than that of the n-6 PUFAs, so the ratio of n-3/n-6 was considered to be an important nutritional evaluation standard of fatty acids. A higher ratio of n-3/n-6 PUFAs has often been regarded as an index of high nutritional value, and the United Nations Department of Health recommended the ideal n-3/n-6 ratio of fish oil was 4 (Simopoulos Citation1991). In the present study, the ratio of n-3/n-6 PUFAs in solder crab was 3.10, which was a little lower than the recommended ideal ration of fish oil and was approximately equal to blue crab (3.18) (Celik et al. Citation2004), and higher than those of green crab (1.47) (Cherif et al. Citation2008) and Chinese mitten crab (0.45) (Chen et al. Citation2007). Therefore, taking into consideration of PUFAs, solder crab was an n-3 PUFAs-rich food and it would be beneficial for human health.
The amino acid composition of soldier crab is summarized in . Glutamic acid (204 mg/g of protein) was the most abundant amino acid in soldier crab protein, followed by glycine (162 mg/g), aspartic acid (100 mg/g), lysine (87 mg/g), leucine (84 mg/g), alanine (82 mg/g), and the contents of methionine, histidine, serine, cysteine and tryptophan were low.
Table 4. Amino acid composition of soldier crab (wet weight; mean ± SD; n = 3).
The amino acid composition of soldier crab was corresponded with the investigation of other sea crabs (Marques et al. Citation2010; Jiang et al. Citation2014). Soldier crab had lower ratio of essential to non-essential amino acids (0.58) when compared with Atlantic spider crab meat (0.86) (Marques et al. Citation2010), but it was higher than that in green crab meat (0.49) (Naczk et al. Citation2004). The amino acid composition, especially the essential amino acid composition is very important in nutrition. Usually, the ratio of essential to non-essential amino acids in seafood more than 0.5 indicates a useful source of dietary proteins (Marques et al. Citation2010), and the ratio in soldier crab protein was 0.58, which meant that soldier crab was a valuable source of protein.
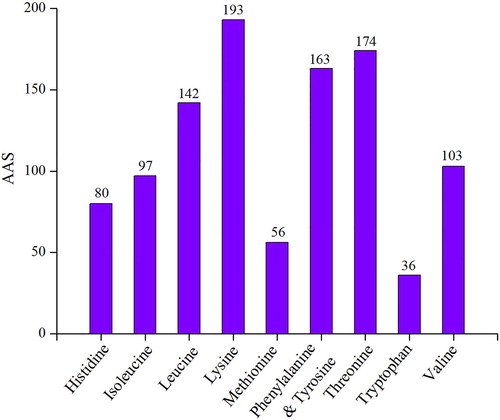
The AASs are given in . When compared with the reference protein and amino acid requirements in human nutrition (WHO Citation1985), except isoleucine, histidine, methionine and tryptophan, the AASs of the other amino acids were higher than 100 in soldier crab. Taking into consideration the abovementioned AASs, tryptophan and methionine were the first and second limiting amino acid in soldier crab, respectively, and other essential amino acid composition was reasonable. Methionine is a sulphur-containing amino acid which was partially lost during the acid hydrolysis (Hugli and Moore Citation1972); therefore, its actual AAS should be higher than the detected value. Tryptophan is typically available in low content in seafoods (Venugopal and Gopakumar Citation2017). The limiting amino acids in soldier crab was coincidence with other crabs, as tryptophan and sulphur-containing amino acids were regarded as the limiting amino acids in the meat of green crab (Naczk et al. Citation2004) and Chinese mitten crab (Chen et al. Citation2007), respectively.
4. Conclusion
In short, soldier crab is an ideal resource of nutritional food material with desirable mineral source, higher n-3/n-6 PUFAs ratio and higher essential to non-essential amino acids ratio.
Acknowledgements
This work was funded by ‘National Natural Science Foundation of China (No. 31660448)’ and ‘Guangxi Natural Science Foundation (No. 2016GXNSFAA380290)’.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Akpan EJ. 1997. Proximate composition of edible blue crab Callinectus sapidus. J Food Sci Techn-Mys. 34(1):59–60.
- AOAC. 2005. Official methods of analysis. Washington (DC): Association of Official Analytical Chemists.
- Benjakul S, Sutthipan N. 2009. Comparative study on chemical composition, thermal properties and microstructure between the muscle of hard shell and soft shell mud crabs. Food Chem. 112(3):627–633.
- Celik M, Tureli C, Celik M, Yanar Y, Erdem U, Kucukgulmez A. 2004. Fatty acid composition of the blue crab (Callinectes sapidus Rathbun, 1896) in the north eastern Mediterranean. Food Chem. 88(2):271–273.
- Chen D, Zhang M, Shrestha S. 2007. Compositional characteristics and nutritional quality of Chinese mitten crab (Eriocheir sinensis). Food Chem. 103(4):1343–1349.
- Cherif S, Frikha F, Gargouri Y, Miled N. 2008. Fatty acid composition of green crab (Carcinus mediterraneus) from the Tunisian Mediterranean coasts. Food Chem. 111(4):930–933.
- Davie PJ, Wisespongpand P, Shih H. 2013. A new species of Mictyris Latreille, 1806 (Crustacea: Decapoda: Brachyura: Mictyridae) from the Andaman coast of Thailand, with notes on its ecology and behaviour. Zootaxa. 3686(1):65–76.
- Elahe A, Ali SM. 2014. Long-chain polyunsaturated fatty acid sources and evaluation of their nutritional and functional properties. Food Sci Nutr. 2(5):443–463.
- FAO. 1994. Joint, F. A. O. Fats and oils in human nutrition: report of a Joint Expert Consultation. No. 57. FAO.
- Gokoglu N, Yerlikaya P. 2003. Determination of proximate composition and mineral contents of blue crab (Callinectes sapidus) and swim crab (Portunus pelagicus) caught off the Gulf of Antalya. Food Chem. 80(4):495–498.
- Hugli TE, Moore S. 1972. Determination of the tryptophan content of proteins by ion exchange chromatography of alkaline hydrolysates. J Biol Chem. 249(9):2828–2834.
- Jiang K, Zhang F, Pi Y, Jiang L, Yu Z, Zhang D, Sun M, Gao L, Qiao Z, Ma L. 2014. Amino acid, fatty acid, and metal compositions in edible parts of three cultured economic crabs: scylla paramamosain, portunus trituberculatus, and eriocheir sinensis. J Aquat Food Prod Technol. 23(1):73–86.
- Jun J, Jung M, Jeong I, Kim G, Sim J, Nam S, Kim B. 2019. Effects of crab shell extract as a coagulant on the textural and sensorial properties of tofu (soybean curd). Food Sci Nutr. 7(2):547–553.
- Liu T, Liang Z, Fan S, Xia N, Chen D. 2018. Analysis of characteristic taste components of soldier crab (Mictyris brevidactylus). Food Sci. 39(14):236–241.
- Liu T, Xia N, Wang Q, Chen D. 2019. Identification of the non-volatile taste-active components in crab sauce. Foods. 8(8):324.
- Marques A, Teixeira B, Barrento S, Anacleto P, Carvalho ML, Nunes ML. 2010. Chemical composition of Atlantic spider crab Maja brachydactyla: human health implications. J Food Compos Anal. 23(3):230–237.
- Naczk M, Williams J, Brennan K, Liyanapathirana C, Shahidi F. 2004. Compositional characteristics of green crab (Carcinus maeas). Food Chem. 88(3):429–434.
- Sato T, Ohgami S, Kaneniwa M. 2015. Seasonal variations in free amino acids, nucleotide-related compounds, and fatty acids and meat yield of the coconut crab Birgus latro. Fish Sci. 81(5):959–970.
- Shih JT. 1995. Population densities and annual activities of Mictyris brevidactylus (Stimpson, 1858) in the Tanshui Mangrove Swamp of Northern Taiwan. Zool Stud. 34(2):96–105.
- Simopoulos AP. 1991. Omega-3 fatty acids in health and disease and in growth and development. Am J Clin Nutr. 54(3):438–463.
- Skonberg DI, Perkins BL. 2002. Nutrient composition of green crab (Carcinus maenus) leg meat and claw meat. Food Chem. 77(4):401–404.
- Takagi KK, Cherdsukjai P, Mimura I, Yano Y, Adulyanukosol K, Tsuchiya M. 2010. Soldier crab (Dotilla myctiroides) distribution, food resources and subsequent role in organic matter fate in Ao Tang Khen, Phuket, Thailand. Estuar Coast Shelf Sci. 87(4):611–617.
- Unno J. 2008. A new species of soldier crab, Mictyris occidentalis (Crustacea: Decapoda: Brachyura: Mictyridae) from Western Australia, with congener comparisons. J R Soc West Aust. 91(1):31–35.
- Venugopal V, Gopakumar K. 2017. Shellfish: nutritive value, health benefits, and consumer safety. Compr Rev Food Sci Food Saf. 16(6):1219–1242.
- Wang Q, Wu X, Long X, Zhu W, Ma T, Cheng Y. 2018. Nutritional quality of different grades of adult male Chinese mitten crab. Eriocheir Sinensis. J Food Sci Tech-Mys. 55(3):944–955.
- WHO. 1985. World Health Organization. Energy and protein requirements: report of a joint FAO/WHO/UNU expert consultation.
- Yan-Ru G, Sai-Qi G, Xi-Chang W, Lin-Min Z, Jin-Yuan Z. 2014. Comparison of fatty acid and amino acid profiles of steamed Chinese mitten crab. Fish Sci. 80(3):621–633.
- Yeh H, Chen I, Chen P, Wang W. 2009. Heavy metal concentrations of the soldier crab (Mictyris brevidactylus) along the inshore area of Changhua, Taiwan. Environ Monit Assess. 153(1–4):103–109.