?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This study aimed at assessing productive growth performance, blood physiology, intestinal histology, body composition and bone mineralization parameters of silver catfish juveniles fed diets containing different inclusion levels of sorghum, considering three combinations (0%, 50% or 100%), supplemented or not with phytase enzyme (0 and 1500 FTU kg−1). The apparent feed conversion and specific growth rate were benefited by supplemented diets (P < 0.05). The highest protein efficiency rate was obtained for the supplemented diet containing corn (P < 0.05). The viscerosomatic index was lower when corn/sorghum diet (P < 0.05) was offered, whilst the hepatosomatic index was lower in corn and corn/sorghum diets regardless of supplementation (P < 0.05). Regarding blood parameters, variations were observed (P < 0.05), however they were kept within the limits for the species. Histomorphometry variables also displayed variations, which were observed in fish fed with sorghum and sorghum/corn diets, supplemented or not (P < 0.05). Proximate composition of carcass varied for both aether extract and mineral matter for supplemented diets (P < 0.05). A variation of Ca content (P < 0.05) regarding bone mineralization was observed when corn and sorghum were used in the same proportion. Sorghum replacement of corn in diets for R. quelen may be a feasible alternative, and phytase supplementation with improves fish performance.
Introduction
The recent advances in animal production require new technologies directed for the elaboration of nutritionally balanced diets, aiming to improve the performance of several species, in order to maximize the use of feeds in formulations, which may directly reduce production costs.
The elaboration of fish diets has included increasing quantities of plant-based ingredients, which are in some cases its exclusive components, justified by its low cost, wide availability and easiness of acquisition (Guimarães et al. Citation2008). As a consequence, the food industry has been testing processes to promote higher availability of nutrients in plant-based foods, which due to the presence of phytic acid, tannin, gossypol and others, inhibit the action of several enzymes, making several minerals unavailable and compromising protein absorption of diets (Bergamin et al. Citation2013).
Among the five most used cereals worldwide, sorghum (Sorghum bicolor) is intended to meet human nutritional needs, as well as agricultural and economic needs (FAO Citation2016). However, its production is mainly directed for animal feeds, as an alternative ingredient to replace corn in diets for swine, birds and bovines, aiming to reduce production costs while maintaining the nutritional quality of the feed and the animals’ performance (Goes et al. Citation2013; FAO Citation2016).
Even though sorghum is a potentially adequate ingredient for diets, it contains anti-nutritional factors like phytic acid, forming stable in vitro complexes with many minerals, thus jeopardizing its absorption (Duarte et al. Citation2011). Although there are a variety of energy ingredients available to be used in animal diets, low-tannin sorghum has nutritional characteristics that are similar to corn, with higher levels of crude protein and starch, as well as a higher fraction of amylose and high antioxidant effect (Ratnavathi and Komala Citation2016; Rostagno Citation2017).
The presence of phosphorus in sorghum mainly in its phytate form, reduces its availability due to the phytic acid molecule, complexes with essential minerals such as Ca, Fe, Zn, Cu, Na and Mg, thus producing insoluble complexes of amino acids, energy and starch (Alkarawi and Zotz Citation2013; Joshi and Satyanarayana Citation2015). The use of phytase has the purpose of making the dietary energy available, thus increasing the capacity of nutrient absorption (Azeke et al. Citation2011).
The addition of phytase in plant-based diets for fish assists in the digestion and absorption of nutrients, consequently improving the animals’ productive performance and health status (Dersjant-Li et al. Citation2015; Zhu et al. Citation2015; Da Silva-Cerozi and Fitzsimmons Citation2017).
The silver catfish Rhamdia sp. presents several adaptations to climate oscillations and rapid growth even in the coldest periods of the year (Fracalossi et al. Citation2004), as well as high productive potential facing optimal temperatures (18.0°C to 28.0°C) (Carneiro et al. Citation2002). In addition, due to its insertion in the consumers market, it has been thoroughly investigated with the purpose of providing and intensifying its breeding in intensive systems (Silveira et al., Citation2013; Garcia et al., Citation2017).
Therefore, the present study aimed at assessing the influence different levels of corn replacement by low-tannin sorghum and phytase supplementation in diets offered for silver catfish juveniles, regarding fish productive performance, blood physiology, intestinal histology, body composition and bone mineralization.
Material and methods
Animals and experimental design
The experimental procedures adopted in this study were approved by the Committee for Ethics in Use of Animals (CEUA) under protocol N° 63/14 the a Universidade Estadual do Oeste do Paraná.
A total of 360 silver catfish juveniles with average initial weight of 16.02 ± 0.58 g were randomly distributed in 24 net-tanks with usable volume of 1 m3 at a density of 15 fish per tank, distributed in a 200 m2 masonry tank. A factorial scheme with six treatments and four repetitions was designed, considering the replacement of corn by sorghum at three different combinations (0, 50 and 100%) supplemented or not with phytase enzyme (0 and 1500 FTU kg−1).
Experimental diets
Fish were fed four times a day, at 8h 00, 12h 00, 02h 00 pm and 05h 00 pm until apparent satiety throughout a 90-day period, with isoproteic (34% crude protein) and isoenergetic feeds (3565 kcal of Digestible Energy kg−1) ().
Table 1. Percentage composition of diets with substitution of corn for sorghum, supplemented with phytase enzyme for silver catfish juveniles raised in net-tanks.
Regarding feeds formulation, the ingredients were ground in hammer-type grinder, weighed, manually homogenized and extruded (Ex-Micro®). The phytase used was in granular and it was blended with the diets according to Rocha et al. (Citation2007). The pellets were dried in an air-circulating stove for 24 h at 55°C and stored at −20°C for later use.
Water quality
Throughout the experimental period, the mean values of physical-chemical parameters of water quality were temperature (25.50 ± 0.72°C), pH (8.27 ± 0.31), dissolved oxygen (7.99 ± 1.15 mg L−1), and electric conductivity (41.95 ± 3.28µS cm−1). These parameters remained within the tolerable limits for the species in captivity (Souza et al. Citation2005), which were weekly monitored with the aid of a portable digital potentiometers.
Productive performance
At the end of the experiment, fish were fasted for 24 h, then anesthetized with a benzocaine solution (100 mg L−1) (Gomes et al. Citation2001), weighed and measured, in order to obtain the following parameters: weight gain (WG (g) = final body weight (g) – initial body weight (g)); feed conversion rate (FCR = feed supplied (g)/weight gain (g)); specific growth rate (SGR (%.day−1) = ([exp (ln final weight (g) – ln initial weight (g) – 1] x experimental period−1) x 100); protein efficiency rate (PER (%) = 100 x (weight gain (g) / crude protein consumed (g))); and survival (SU (%)= final fish number/initial number of fish)x 100).
Plasmatic biochemical and hematological parameters
Three animals from each experimental unit were submitted to blood collection by puncture of the caudal vein, using disposable syringes containing EDTA (ethylenediamine tetracetic acid) at 10%. Plasmatic biochemical analysis was performed with samples of the obtained plasma by centrifugation at 2500 rpm during five minutes for quantitative determination of glucose (mg.dL−1), triglycerides (mg.dL−1) and cholesterol HDL (mg.dL−1) with the aid of commercial analysis kits (Gold analisa Diagnostica, Brazil®) and posterior reading in a spectrophotometer.
The hemogram consisted of the determination of the percentage of microhematocrit by the method described by Goldenfarb et al. (Citation1971); hemoglobin by the method of cyanometahemoglobin proposed by Collier (Citation1944); and erythrocyte count, performed in a Neubauer's chamber. Data were obtained using the Heterocyclic Indexes of Wintrobe (Citation1934), comprising the mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH) and mean corpuscular hemoglobin concentration (MCHC). The blood extensions were stained by the method proposed by Rosenfeld (Citation1947). Total and differential leukocytes counts and total thrombocytes counts were performed with light microscopy and blood extension proposed by Ranzani-Paiva et al. (Citation2013). The leukocytes were classified as total thrombocytes, lymphocytes, neutrophils, monocytes, special granulocytic cells and immature lymphocytes.
Histological analyses and somatic indexes
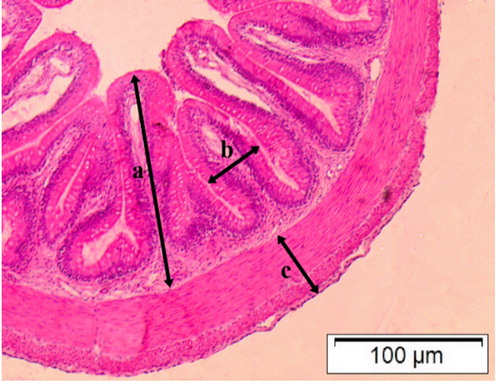
Twelve animals were euthanized in a benzocaine solution (200 mg L−1) (Gomes et al. Citation2001) and the material was collected from the coelomic cavity for the calculation of both viscerosomatic fat index (VFI (%) = 100 x [visceral fat weight (g)/ body weight (g)]) and hepatosomatic index (HSI (%) = 100 x [liver weight / final body weight (g)]). Regarding histomorphometric analyses of the intestinal villi, the transversal segment of the medial portion of the fish intestines was removed, which was then preserved in alcohol 70° to remove the fixer until they are processed. Tissues were dehydrated in different concentrations of alcohols, clarified in xylene and included in paraffin. The histological cuts were obtained by serial sections of 7 µm and coloured in Hematoxylin–Eosin (HE) and periodic acid-Schiff (PAS) and alcian blue (AB) pH 2.5. Subsequently, the blades were analysed in light microscopy (), and the following measurements were performed: villus height (distance from villus top to the beginning of the muscular layer), villus width (distance from the top of an enterocyte to the top of the enterocyte at the opposite side), tunic thickness (total distance from the circular and longitudinal muscular layer), number of goblet cells and total number of villi. The analyses were performed using 40X objective lens, with the aid of the software cellSens Standard 1.15®.
Carcasses proximate composition
Regarding the proximate determination, the eviscerated carcass of the slaughtered fish was kept in a freezer at −20°C for further analyses (AOAC Citation2016). Three different fish from each experimental unit were slaughtered for determination of bone mineral composition by Furuya et al. (Citation2001) in Flame Atomic Absorption Spectrophotometry (FAAS).
Statistical analysis
Productive performance, physiological and mineral composition data were verified for normality and homoscedasticity assumptions, and were submitted to a factorial variance analysis, in order to check the interaction of sorghum inclusion levels and supplementation with phytase; when significant, means were compared by the Duncan test at a 5% probability level. All analyses were performed using the software Statistica 7.0®.
Results
A significant interaction between sorghum inclusion levels and phytase supplementation was observed (P < 0.05) regarding silver catfish’ productive performance. FCR and PER parameters displayed higher results when fish were submitted to a corn-based diet supplemented with phytase. An effect of phytase inclusion was observed on SGR and the obtained results were higher in fish fed with supplemented diets (P < 0.05) ().
Table 2. Productive performance and somatic indexes of silver catfish fed with diets that replace corn for sorghum, supplemented or not with phytase enzyme.
An interaction effect was observed (P < 0.05) between feeds and supplementation on VFI index, with the greatest results being observed in fish fed a corn/sorghum diet without phytase supplementation (). HSI displayed effects among the different tested feeds (P < 0.05), and the lowest values occurred with the use of corn/sorghum diet, regardless of phytase supplementation ().
The biochemical components found in plasma showed that diets, when supplemented with phytase provided variations in glucose contents, while triglycerides and HDL cholesterol suffered influence of phytase and sorghum interaction when these were included in the diets ().
Table 3. Plasma biochemical composition of silver catfish fed with diets where corn was substituted for sorghum, supplemented or not with phytase enzyme.
Regarding hematimetric indices’ data, significant differences were observed for both hemoglobin and MCHC. Hemoglobin rate ranged between 4.49 and 7.60 g dL−1, and MCHC varied from 14.66 and 23.18%, where fish fed with corn with phytase supplementation were higher in comparison to other treatments, however being within the expected range ().
Table 4. Mean of absolute hematimetric indices of silver catfish fed with diets that substituted corn for sorghum, supplemented with phytase enzyme.
Mean values for total leukocytes presented significant differences (p < 0.05) among treatments, ranging from 66,552.90–174,051.00 × 103µl ().
Table 5. Distribution of total leukocytes and differential of silver catfish fed with diets that substituted corn for sorghum, supplemented with phytase enzyme.
In concern of histomorphometric analyses (), an interaction effect between feeds and phytase supplementation was observed for villi height, tunic thickness and number of goblet cells (P < 0.05) (). On the other hand, width and number of villi were influenced by the used levels of the used feed (P < 0.05).
Table 6. Histomorphometry of intestine of silver catfish fed with diets that substituted corn for sorghum, supplemented or not with phytase enzyme.
Proximate composition parameters of the evaluated carcasses displayed significant differences for aether extract and mineral matter contents of fish fed supplemented diets (P < 0.05) (). Regarding bone mineralization, an effect for mineral Ca was observed in fish fed the different tested diets (P < 0.05) ().
Table 7. Proximate composition of carcass of silver catfish fed with diets that substituted corn for sorghum, supplemented or not with phytase enzyme.
Table 8. Bone mineral composition of silver catfish fed with diets that substituted corn for sorghum, supplemented or not with phytase enzyme.
Discussion
Most of the existing protein in sorghum is kafirin, which is characterized by displaying a more rigid structural configuration in the protein matrix involving starch, and having low solubility, as well as to presenting an imbalance of amino acid, factors that are responsible for the low digestibility of food (Wong et al. Citation2010; Chen et al. Citation2017). The increase in FCR of silver catfish fed with corn/sorghum and sorghum diets is probably associated to its negative influence in nutrient absorption by the fish.
The presence of phytase has promoted the break of the phytase molecule, making available a higher amount of phosphorus, thus enabling higher absorption of nutrients present in foods of plant origin (Scottá et al. Citation2014; Da Silva-Cerozi and Fitzsimmons Citation2017).
According to Rocha et al. (Citation2007), phytase supplementation in diets for silver catfish promotes a more efficient protein digestibility and availability of minerals, which consequently leads to a better intestinal absorption of nutrients, providing a linear increase in weight gain and specific growth. Diet supplementation with phytase did not influence these variables, contradicting these authors’ findings.
Sorghum-based diets supplemented with phytase provided higher contents of visceral fat. This fact may have occurred due to the higher energetic availability of sorghum that the silver catfish absorbed, which consequently resulted in higher accumulation of fat, as suggested by Signor et al. (Citation2016).
The synthesis of lipid – particularly phospholipids in the liver, is responsible for the production of energy to be used in different body functions or stored. In case it is not directed to some metabolic function, it is converted into fat in liver cells (Hall Citation2011).
Factors such as food supply, nutrient balance and their quality influence the proper metabolism of fish; one of which is the phosphorus concentration in feeds (Pontes et al. Citation2015). Under adequate nutritional conditions, phosphorus promotes nutrients availability, which are directed to protein synthesis and as a consequence, a higher fat accumulation occurs in the liver, due to increases in the availability of minerals that are provided by both sorghum and corn when these two ingredients are used in the same proportion (Hill et al. Citation2012; Silva et al. Citation2012).
Glucose levels are associated with phosphorus mobilization, which acts in the stimulation for the use of phosphate ions, participating in the phosphorylation of intermediary glycolytic pathways as moderator in the control and stimulation of glycolytic enzymes (Nelson and Cox Citation2018). Phytase supplementation make phosphorus available, as well as other nutrients present in the diets, thus it may have positively influenced the fish metabolism by reducing the concentration of cholesterol and plasma triglycerides.
When the existing phosphorus in diets is harnessed by the fish due to the action of the phytase enzyme, an improvement on the use of protein is verified (Bomfim Citation2013). Sorghum use by the fish is influenced by the kaffirin protein, which belongs to the prolamines group, and act hampering its digestibility, thus consequently delaying the digestion process (Belton et al. Citation2006; Chen et al. Citation2017). With reduced glucose levels promoted by phytase supplementation and reduced protein availability when corn and sorghum are used in the diet, it is suggested that there an induction to glycogen synthesis occurred, as observed in the reduction of the hepatic tissue in fish fed with corn/sorghum diet supplemented with phytase, with these results being similar to those of diets containing only corn in their composition.
Even though significant differences were observed regarding biochemical variables, they were kept within the limits expected for juveniles of silver catfish (Rhamdia quelen) according to Borges et al. (Citation2004).
The effect of phytic acid in corn and sorghum as a limiting factor for phosphorus availability promotes variations in the extracellular balance, with emphasis on phosphate electrolytes, which are involved in blood chemical processes and in the energetic metabolism, thus affecting the functionality of tissues (Motta Citation2003).
The hematological values referring to lymphocytes, neutrophils and SGC displayed variations between normal limits, whilst monocytes and immature lymphocytes remained within the acceptable limits for the species. Erythrocytes, hematocrit, hemoglobin and MCV were similar to those in the control treatment, within the expected range for the species, according to (Tavares-Dias et al. Citation2002).
Fish presented greater and thicker villi when fed the corn-based diet without phytase, in comparison to other treatments, which may explain the highest number of goblet cells in these animals. This possibly occurred due to the presence of higher concentrations of phytic acid, i.e. phosphorus accumulation in the grain, considering that corn presents more of this nutrient in comparison to sorghum (Pinho et al. Citation2009).
Figure 2. Intestinal villi of silver catfish Rhamdia quelen fed with: (a, b and c) diets 0, 50 and 100% without phytase; (d, e and f) diets 0, 50 and 100% with phytase. Presence of goblet cells (letter ‘a' with indicative arrow).
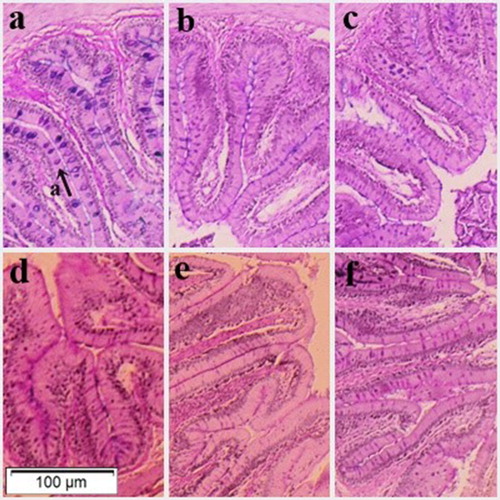
The intestinal muscles may act as an indicative of the animal's nutritional performance, seen that in case of an imbalance of the peristaltic movements and of the absorption activity throughout digestion, an increase of the tissue's activity may occur, as well as changes of its structural characteristic (Junqueira and Carneiro Citation2005).
The presence of phytase probably promoted a reduction of the activity in the muscular tunic, acting in peristaltic movements and motility of the intestinal mucosa (Mello et al. Citation2013).
Moreover, it can influence the absorption and digestion of nutrients, as observed by the FCR results found for the animals fed diets containing only corn. When digestion is hampered, regardless of the affecting factor, cellular activity and the secretion of mucins increases, directing its production through the intestinal tract, in order to better protect the epithelium (Rodwell et al. Citation2017). The high incidence of goblet cells can also result in tissue changes, triggered by distinct feed conditions, because when an aggression process occurs, consequently a greater production of mucus is observed (Schwarz et al. Citation2010; Honorato et al. Citation2011).
When the diet containing corn was supplemented with phytase, a better use of phosphorus was verified, which composes part of fish homeostasis process, and provides a balanced cellular development in the tissue (Schamber Citation2008), as well as differentiations in villi height, tunic thickness and goblet cells of the animals. However, fish development was not compromised, seen that animals fed diets containing sorghum with or without phytase supplementation presented the best zootechnical performance results.
Phytase supplementation promoted higher lipid deposition in fish carcasses, which remained high in fish that were fed corn/sorghum and sorghum diets containing the enzyme. It is noteworthy that the presence of the enzyme also reduced lipid reposition in the fed the diet containing only corn.
Sorghum has a higher energy content in comparison to corn, which meets the demands of the silver catfish, as reported by Signor et al. (Citation2016). Corn-based diets needed higher inclusion levels of an energy source (e.g. vegetal oil), which exerted influence in the fat deposition, an effect that was also observed in the content of visceral fat in the animals.
The enzyme phytase catalyzes the hydrolysis of phytic acid, inositol and orthophosphoric acid, increasing protein and lipid availability of plant-origin to the animals, reducing the activation energy of catalytic reaction and accelerating its reactive process (Gupta et al. Citation2013).
Phytase supplementation in corn diets provided better mineral deposition in fish bones. Diets containing corn/sorghum and sorghum without phytase supplementation presented higher mineral deposition. Increases in available phosphorus to animals, probably makes the availability of other minerals inadequate, resulting in a decrease in the mineral composition of the carcass (Prabhu et al. Citation2016).
When corn and sorghum were used in diets at the same proportion, an influence (P < 0.05) in Ca deposition was observed against corn or sorghum, when ingested in isolation; however, they were still within the 1:1 ratio for the minerals Ca and P, which are responsible for bone mineralization and growth of the animals (Furuya Citation2010; Rocha et al. Citation2016).
The concentration of minerals existing in fish bones varies according to their genetic potential and metabolism, in addition to water composition and interactions of foods that compose the feed (Prabhu Citation2010; Nelson and Cox Citation2018). This latter directly promotes availability of minerals to the organism. The levels of Ca and P used in the maintenance of tissues are associated to factors like availability in the diet, environment, life stage and gastrointestinal tract absorption (NRC Citation2011).
Conclusion
Low-tannin sorghum can fully replace corn in diets for silver catfish Rhamdia quelen without affecting the animal’s development, and phytase supplementation (1500 FTU kg−1) improves its FCR.
Acknowledgments
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001.
Disclosure statement
No potential conflict of interest was reported by the author(s).
ORCID
Mariana Lins Rodrigues http://orcid.org/0000-0003-4957-9626
Milena Souza dos Santos Sanchez http://orcid.org/0000-0003-1131-7081
Jhonis Ernzen Pessini http://orcid.org/0000-0002-9334-6224
Kattia Aparecida Weiler http://orcid.org/0000-0003-1391-0508
Agnaldo Deparis http://orcid.org/0000-0002-5747-7171
Wilson Rogério Boscolo http://orcid.org/0000-0002-1808-0518
Fábio Bittencourt http://orcid.org/0000-0001-5894-7158
Altevir Signor http://orcid.org/0000-0002-4659-6466
References
- Alkarawi HH, Zotz G. 2013. Phytic acid in green leaves. Plant Biol. 16:697–701. doi: 10.1111/plb.12136
- AOAC. 2016. Official methods of analysis. 20th ed. Gaithersburg (MD): Association Analytical Chemists.
- Azeke MA, Egielewa SJ, Eigbogbo MU, Ihimire IG. 2011. Effect of germination on the phytase activity, phytate and total phosphorus contents of rice (Oryza sativa), maize (Zea mays), millet (Panicum miliaceum), sorghum (Sorghum bicolor) and wheat (Triticum aestivum). J Food Sci Technol. 48:724–729. doi: 10.1007/s13197-010-0186-y
- Belton PS, Delgadillo I, Halford NG, Shewry PR. 2006. Kafirin structure and functionality. J Cereal Sci. 44:272–286. doi: 10.1016/j.jcs.2006.05.004
- Bergamin GT, Veiverberg CA, da Silva LP, Pretto A, Siqueira LV, Radünz-Neto J. 2013. Extração de antinutrientes e aumento da qualidade nutricional dos farelos de girassol, canola e soja para alimentação de peixes. Ciência Rural. 43:1878–1884. doi: 10.1590/S0103-84782013001000024
- Bomfim MAD. 2013. Estratégias nutricionais para redução das excreções de nitrogênio e fósforo nos sistemas de produção de peixes no Nordeste: sustentabilidade ambiental e aumento da produtividade. Revista Científica de Produção Animal. 15:122–1402. doi: 10.15528/2176-4158/rcpa.v15n2p122-140
- Borges A, Scotti LV, Siqueira DR, Jurinitz DF, Wassermann GF. 2004. Hematologic and serum biochemical values for silver catfish (Rhamdia quelen). Fishy Physiol Biochem. 30:21–25. doi: 10.1007/s10695-004-5000-1
- Carneiro PCF, Bendhack F, Mikos JD, Schorer M, Oliveira-Filho PRC. 2002. Resultados preliminares sobre o silver catfish, Rhamdia quelen, como espécie importante para a piscicultura na região Sul do Brasil. Anais. In: Simpósio Brasileiro de Aquicultura, 12, 2002, Goiânia. Goiânia: CAUNESP/ESALQ, 403, p. 11.
- Chen H, Zhang S, Park I, Kim SW. 2017. Impacts of energy feeds and supplemental protease on growth performance, nutrient digestibility, and gut health of pigs from 18 to 45 kg body weight. Anim Nutr. 3:359–365. doi: 10.1016/j.aninu.2017.09.005
- Collier HB. 1944. The standardizations of blood hemoglobin determinations. CMAJ. 50:550–552.
- Da Silva-Cerozi B, Fitzsimmons K. 2017. Effect of dietary phytase on phosphorus use efficiency and dynamics in aquaponics. Aquac Int. 25:1227–1238. doi: 10.1007/s10499-016-0109-7
- Dersjant-Li Y, Awati A, Schulze H, Partridge G. 2015. Phytase in non-ruminant animal nutrition: a critical review on phytase activities in the gastrointestinal tract and influencing factors. J Sci Food Agric. 95:878–896. doi: 10.1002/jsfa.6998
- Duarte JO, Garcia JC, de Miranda RA. 2011. Mercado e comercialização. In: Cruz JC, editor. Cultivo do milho. 7ª Ed. Sete Lagoas: Embrapa Milho e Sorgo (Embrapa Milho e Sorgo. Sistema de produção, 1). Disponível em: http://sistemasdeproducao.cnptia.embrapa.br/FontesHTML/Milho/CultivodoMilho_7ed/mercado.htm.
- FAO. 2016. SOFIA: the state of word fisheries and aquaculture. Roma: Food and Agriculture Organization of the United Nations.
- Fracalossi DM, Meyer G, Santamaria FM, Weingartner M, Zaniboni-Filho E. 2004. Performance of silver catfish, Rhamdia quelen, and dourado, Salminus brasiliensis, in earth ponds of southern Brazil. Acta Sci Anim Sci. 26:345–352. doi: 10.4025/actascianimsci.v26i3.1806
- Freitas JMA, Sary C, Luchesi JD, Feiden A, Boscolo W. 2011. Proteína e energia na dieta de silver catfish criados em tanques-rede. Rev Bras Zootec. 40:2628–2633. doi: 10.1590/S1516-35982011001200002
- Furuya WM. 2010. Tabelas Brasileiras para a Nutrição de Tilápias. Toledo: GFM.
- Furuya WM, Gonçalves GS, Furuya HC. 2001. Fitase na Alimentação da Tilápia do Nilo (Oreochromis niloticus) desempenho e digestibilidade. Rev Bras Zootec. 30:924–929. doi: 10.1590/S1516-35982001000400003
- García S, Yasui GS, Bernardes-Júnior JJ, Corrêa da Silva B, Amaral-Júnior H, Zaniboni-Filho E. 2017. Induction of triploidy in Rhamdia quelen (Siluriformes, Heptapteridae) by double-temperature shock. Latin american journal of aquatic research. 45:209–212. doi: 10.3856/vol45-issue1-fulltext-22
- Goes RHTB, Silva LHX, Souza KA. 2013. Alimentos e alimentação animal, 1º ed. Grande Dourados: editora UFGD.
- Goldenfarb PB, Bowyer FP, Hall E, Brosious E. 1971. Reproducibility in the hematology laboratory: the microhematocrit determinations. Am J Clin Pathol. 56:35–39. doi: 10.1093/ajcp/56.1.35
- Gomes LC, Chippari-Gomes AR, Lopes NP, Roubach R, Araujo-Lima CARM. 2001. Efficacy benzocaine as anesthetic in Juvenile tambaqui Colossoma macropomum. J World Aquacult Soc. 32:426–431. doi: 10.1111/j.1749-7345.2001.tb00470.x
- Guimarães IG, Pezzato LE, Barros MM, Tachibana L. 2008. Nutrient digestibility of cereal grain products and by-products in extruded diets for Nile tilapia. J World Aquacult Soc. 39:781–789. doi: 10.1111/j.1749-7345.2008.00214.x
- Gupta RK, Gangoliya SS, Singh NK. 2013. Reduction of phytic acid and enhancement of bioavailable micronutrients in food grains. J Food Sci Technol. 52:676–684. doi: 10.1007/s13197-013-0978-y
- Hall JE. 2011. Tratado de Fisiologia Médica. 12ª ed. Rio de Janeiro: Elsevier.
- Hill RW, Wyse GA, Anderson M. 2012. Fisiologia animal. 2ª Edição. Porto Alegre: Editora Artmed.
- Honorato CA, Cruz C, Carneiro DJ, Machado MRF. 2011. Histologia e histoquímica do intestino anterior de tilápia do Nilo (Oreochromis niloticus) alimentadas com dietas contendo silagem de peixe. Braz J Veterinary Res Animal Sci. 48:281–288. doi: 10.11606/S1413-95962011000400002
- Joshi S, Satyanarayana T. 2015. Review: heterologous expression of yeast and fungal phytases: developments and future perspectives. Indian J Biotechnol. 14:239–311.
- Junqueira LCU, Carneiro J. 2005. Biologia Celular e Molecular. 8ª. Edição. Rio de Janeiro: Editora Guanabara Koogan.
- Mello H, Julieta REM, Niza IG, Moraes FR, Ozório ROA, Shimada MT, Engracia Filho JR, Claudiano GS. 2013. Efeitos benéficos de probióticos no intestino de juvenis de Tilápia-do-Nilo. Pesquisa Veterinária Brasileira. 33:724–730. doi: 10.1590/S0100-736X2013000600006
- Motta VT. 2003. Bioquímica básica 2. ed. São Paulo: Autolab Ltda.
- Nelson DL, Cox MM. 2018. Princípios de Bioquímica de Lehninger, 7° ed. Porto Alegre: Editora Artmed.
- NRC. 2011. Nutrient Requirements of fish and Shrimp. Washington (DC): National Academic Press.
- Pinho RGVP, Borges ID, Pereira JLAR, Reis MC. 2009. Marcha de absorção de macronutrientes e acúmulo de matéria seca em milho. Revista Brasileira de Milho e Sorgo. 8:157–173. doi: 10.18512/1980-6477/rbms.v8n2p157-173
- Pontes TC, Cagol L, Dutra FM, Portz L. 2015. Disponibilidade do fósforo em alimentos de origem Vegetal: atuação na nutrição de peixes. Arquivos de Ciências Veterinárias e Zoologia da Unipar. 18:199–205.
- Prabhu PAJ, Schrama JW, Kaushik SJ. 2016. Mineral requirements of fish: a systematic review. Rev Aquac. 8:172–219. doi: 10.1111/raq.12090
- Prabhu PAJ, Schrama JW, Kaushik SJ. 2010. Mineral requirements of fish: a systematic review. Rev Aquac. 8:172–219. doi: 10.1111/raq.12090
- Ranzani-Paiva MJT, Benites-De-Pádua S, Tavares-Dias M, Egami MI. 2013. Métodos para análise hematológica em peixes. Maringá: Editora Eduem.
- Ratnavathi CV, Komala VV. 2016. Sorghum Grain Quality. In: Ratnavathi CV, Patil JV, Chavan UD, editors. Sorghum Biochemistry: An Industrial Perspective. 1st Edition. London: Academic Press; p. 1–61.
- Reis ES, Feiden A, Signor A, Zaminhan M, Finkler JK, Boscolo WR. 2011. Suplementação de vitamina C na dieta para larvas de jundiá Rhamdia voulezi. Ciência Animal Brasileira. 12:83–89.
- Rocha PMC, Barros MEG, Neto EJ. 2016. Análise morfométrica da parede intestinal e dinâmica de mucinas secretadas no jejuno de frangos suplementados com probiótico Bacillus subtilis cepa C31021. Pesquisa Veterinária Brasileira. 36:312–316. doi: 10.1590/S0100-736X2016000400010
- Rocha BC, Pouey JLOF, Enke DBS, Xavier EG, Almeida DB. 2007. Suplementação de fitase microbiana na dieta de alevinos de silver catfish: efeito sobre o desempenho produtivo e as características de carcaça. Ciência Rural. 37:1772–1778. doi: 10.1590/S0103-84782007000600042
- Rodwell VW, Bender DA, Botham KM, Kennelly PJ, Weil PA. 2017. Bioquímica Ilustrada de Harper, 30° Ed. Porto Alegre: Editora Artmed.
- Rosenfeld G. 1947. Corante pancrômico para hematologia e citologia clínica. Nova combinação dos componentes do May-Grünwald e do Giemsa num só corante de emprego rápido. Memórias do Instituto Butantan. 20:329–334.
- Rostagno HS, editor. 2017. Tabelas brasileiras para aves e suínos: composição de alimentos e exigências nutricionais. 4° ed. Viçosa: Editora da UFV.
- Schamber CR. 2008. Exigência de fósforo para tilápia do Nilo (Oreochromis niloticus). Dissertação de Mestrado em Zootecnia. Universidade Estadual de Maringá. Maringá.
- Schwarz KS, Furuya WM, Natali MRM, Michelato M, Gualdezi MC. 2010. Mananoligossacarídeo em dietas para juvenis de tilapia do Nilo. Acta Sci Animal Sci. 32:197–203. doi: 10.4025/actascianimsci.v32i2.7724
- Scottá BA, Gomide APC, Campos PF, Formigoni AS, Ferreira SV. 2014. Utilização de fitase na alimentação de aves e suínos. Pubvet, 8, Ed. 251, Art. 1660.
- Signor A, Lewandowski V, Silva RA, Fries EM, Schuller JM. 2016. Effect of phytase on digestibility of corn, sorghum and wheat bran by silver catfish (Rhamdia voulezi). Acta Sci Anim Sci. 38:355–359. doi: 10.4025/actascianimsci.v38i4.32054
- Silva CS, Queiroz VAV, Simeone MLF, Guimarães CC, Schaffert RE, Rodrigues JAS, Miguel RA. 2012. Teores de Minerais em Linhagens de Sorgo Para Uso na Alimentação Humana. In: Anais XXIX Congresso Nacional de Milho e Sorgo – 2012, Águas de Lindóia.
- Silveira J., Silva CP, Cargnin-Ferreira E, Alexandre D, Elias MA, Fracalossi DM. 2013. Freshwater catfish jundiá (Rhamdia quelen) larvae are prepared to digest inert feed at the exogenous feeding onset: physiological and histological assessments. Fish Physiol Biochem. 39:1581–1590. doi: 10.1007/s10695-013-9810-x
- Souza LS, Pouey JLOF, Camargo SO, dos Vaz BS. 2005. Crescimento e sobrevivência do catfish de canal (Ictalurus punctatus) e silver catfish (Rhamdia sp) no outono-inverno do Rio Grande do Sul. Ciência Rural. 35:891–896. doi: 10.1590/S0103-84782005000400022
- Tavares-Dias M, Melo JFB, Moraes G, Moraes FR. 2002. Características hematológicas de teleósteos brasileiros. Iv. Variáveis do jundiá Rhamdia quelen (Pimelodidae). Ciência Rural. 32:693–698. doi: 10.1590/S0103-84782002000400024
- Wintrobe M. 1934. Variation in the science and hemoglobin contet of erythrocytes in the blood of various vertebrates. Folia Haematol. 51:32–49.
- Wong JH, Marx DB, Wilson JD, Buchanan BB, Lemaux PG, Perdesen JF. 2010. Principal component analysis and biochemical characterization of protein and starch reveal primary targets for improving sorghum grain. Plant Sci. 179:598–611. doi: 10.1016/j.plantsci.2010.08.020
- Zhu WX, Zhong GF, Hua XM, Ju M, Chen XM, Du ZY. 2015. The optimum active site of acidic phytase in the gastrointestinal tract of channel catfish (Ictalurus punctatus): in vitro and in vivo studies. Aquac Nutr. 22:881–889. doi: 10.1111/anu.12304