?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The aim of this study was to evaluate the effect of intraruminal boluses with sulfamethazine (SM) and selenium (Se) on goat kids naturally infected with Eimeria. Twenty goat kids were selected with an average body weight (BW) of 13.7 kg. They were grouped as follows (n = 5 animals per group). BC: dosed with boluses without drugs; BSM: dosed with boluses containing 4 g of SM; BSe: dosed with boluses containing 90 mg of Se; BSMSe: dosed with boluses with 4 g of SM + 90 mg of Se. All animals showed the presence of the boluses at 10 d after dosing by radiography. DMI/BW at the beginning of the experiment was low: 0.76% in BC and 0.86% in the dosed groups. At the end of the experiment there was an increase: 1.36% in BC and 1.54% in the dosed groups (P > 0.05). BSMSe had the lowest percentage of Eimeria spp (P < 0.05). There were differences between the Eimeria species found before and after the treatments. It was observed that BSM and BSMSe decreased the number of oocysts from 7 to 28 d (P < 0.05). BSMSe had the highest content of blood SM and Se (P < 0.05). The histological alterations were loss of the epithelium and villous atrophy.
1. Introduction
Goat production has been shown to play a significant role in the human diet, providing more than 280,000 tons of meat and 7 million tons of milk. Specifically, underdeveloped countries host 94% of the goat population in the world (FAO Citation2018). In America, Mexico is the country with the highest goat production and a benchmark for goat meat and milk in Latin America. Most goat production systems in México are self-sufficient with the use of natural food resources as their main food intake (Escareño-Sánchez et al. Citation2011). However, infectious diseases and mineral deficiencies have damaged the health and productivity of animals. Statistics show that mortality of sick animals in a system with diurnal grazing and confinement at night can reach up to 58% (Ershaduzzaman et al. Citation2007) because animals are exposed to climate changes, low immunity and malnutrition (França et al. Citation2009). It has been shown that coccidiosis can cause diarrhea and low feed intake, while selenium (Se) deficiency can cause weakness and poor muscle development, these two conditions cause weight loss and growth retardation in the animals (Sharma et al. Citation2017). Eimeria causes productive losses in young animals, induces damage to the intestinal epithelium, atrophy and fusion of villi, resulting in a decrease in nutrient absorption (Chávez Rivera et al. Citation2005). In goat kids, the most pathogenic species are E. ninakohlyakimovae and E. arloingi (Dai et al. Citation2006). In Mexico, the common Eimerias are E. arloingi, E. jolchijevi, E. ninakohlyakimovae, E. hirci, E. christenseni, and E. alijevi (Cepeda-Palacios et al. Citation2015).
On the other hand, Se is incorporated into selenoenzymes and has an important role in the oxidation–reduction, antioxidant defense and redox regulation (Raymond et al. Citation2014). Newborn goat kids require a minimum level of 0.1 μg/g of Se in blood, lower levels can cause muscular dystrophy (Ramírez-Bribiesca et al. Citation2001). Se deficiency is also known to affect late pregnancy by altering fetal growth and consequently affects the birth of these animals. Offspring are born weak and mortality can be up to 60% (Ramírez-Bribiesca et al. Citation2005). Additionally, a lack of Se has been proven to decrease the immune system and alter thyroid function (Hefnawy and Tórtora-Pérez Citation2010).
In Mexico, the lack of Se in soil, together with the high level of antagonistic minerals such as sulfur results in Se deficiency becoming an endemic problem. Previous studies from our research group have indicated that intraruminal boluses containing Se and SM (Gutiérrez-Blanco et al. Citation2006) are suitable to prevent Se deficiency and coccidiosis. This dosage method has not been investigated for the control of coccidiosis and Se deficiency in small ruminants. The aim of this study was to evaluate the effect of dosages of slow release intraruminal boluses (formulated with sodium sulfamethazine and sodium selenite) in goat kids naturally infected with Eimeria. It is expected that dosed boluses can decrease the clinical signs and pathological disorders of Se deficiency and coccidiosis in goat kids.
2. Material and methods
The design of the study was approved by the Internal Committee for the Care and Use of Experimental Animals with registration number C11_02.
2.1. Selection of animals, accommodation, and training of the experimental groups
A herd was identified with 100 goat kids of Alpine breed, weaned, with presence of diarrhea and suspicious signs of coccidiosis. The animals had a history of not having received any preventive or curative treatment against any disease. Twenty goat kids with diarrhea were selected with a body condition of 3 (on a scale of 1–5) (Ghosh et al. Citation2019) without clinical signs of illness. Goat kids had an average body weight (BW) and age of 13.7 ± 2.98 kg and 62 days, respectively. All animals tested positive for Eimeria spp. using the McMaster technique (oocyst per gram of faeces sample). After the selection, all goat kids were housed in individual cages 1.5 m long and 0.5 m wide, with a metal grid floor 1.2 m above the ground. The goat kids were fed with a balanced diet composed of corn grain, soybean paste, molasses, corn fodder, and mineral salts without Se. The diet contained 93% dry matter, 14% protein, and 0.9 Mcal ENg. Water was freely available and provided in individual buckets.
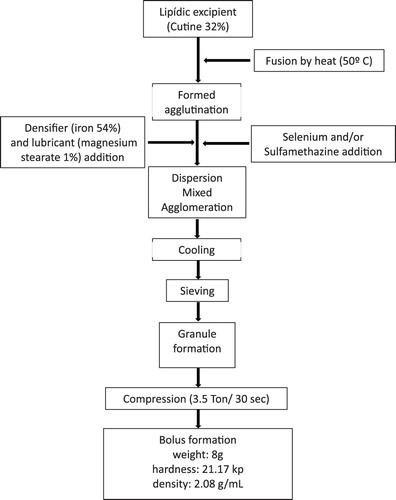
The boluses were manufactured using the granulation by fusion method (Patiño Rodríguez Citation2013) using a densifier (reduced iron) to prevent regurgitation, a binder (cutin) for the binding of excipients and a lubricant (magnesium stearate) to facilitate the sliding of the boluses trough the manufacturing process. The design process is schematized in . The designed boluses were dosed to all goat kids at 9 am, on day zero. One hour before dosage, blood and fecal samples were collected. The treatments were ordered as follows:
BC: dosed with intraruminal boluses without drugs (Placebo group).
BSM: dosed with intraruminal boluses containing 4 g of Sulfamethazine Sodium (SM) (Astroquim México, S.A. de C.V., lot No. 000171.319).
BSe: dosed with intraruminal boluses containing 90 mg of sodium selenite (Na2SeO3). (Valno México, S.A. de C.V., lot No. 0347C7) as source of Se.
BSMSe: dosed with intraruminal boluses with 4 g of SM + 90 mg of Se.
2.2. Radiological diagnostics, variables of performance, and samples collection
2.2.1. X-ray capture
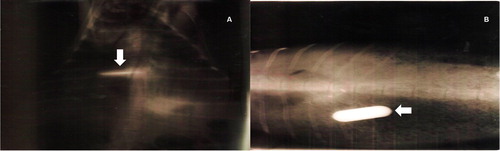
All goat kids were analysed with radiographic plates 10 d after the boluses were administered. A LAICO® model i325v with a high frequency generator was used, the animals were placed in a dorsoventral and ventrodorsal position at 90 degrees, to locate the boluses.
2.2.2. Variables of performance
The animals were fed daily at 8:00 and 16:00 h. The amount of total mixed ration fed to the goat kids was calculated according to the daily feed intake, adjustments were made when needed so that refused feed did not exceed 15% of daily intake. Feed intake was recorded on the basis of dry matter intake (DMI). The goat kids were weighed at the beginning of the experiment and then every 14d using a digital scale with a maximum capacity of 100 kg Hyindoor® and an accuracy of ±0.1 g to measure the body weight (BW). The feed conversion (FC) was calculated by dividing the DMI by the average daily weight gain (ADWG).
2.2.3. Fecal and blood samples collection
Fecal samples were obtained directly from the rectum of the animals using previously identified plastic bags, they were cooled at 4°C, until the oocysts were counted on days 0 7, 14, 21 and 28 post-dosing. Eimeria species were identified 7 days before boluses dosing (0d) and 30 days after post-dosing. Blood samples were taken by venipuncture of the jugular vein using needles and Vacutainer tubes (Vacutainer® Mexico needles caliber 20 G and 38 mm tubes with heparin), they were centrifuged at 4000 rpm for 15 min to separate plasma which was frozen at −20°C until the quantification of SM and Se.
2.2.5. Slaughtering the goat kids and taking histological samples
The goat kids were slaughtered 35 d at the slaughter unit of the University, according with the official Mexican norm (NOM-033-ZOO-1995 Humanitarian Sacrifice of domestic and wild animals). Tissue samples of all goat kids were taken (duodenum, jejunum, ileum and colon) and preserved in 10% formaldehyde.
2.3. Laboratory analysis
2.3.1. Fecal oocysts
Two grams of fecal sample was diluted in 30 mL of saturated saline solution at room temperature (≈ 23°C). Subsequently, 0.5 mL of diluted sample was put in the McMaster counting chamber, and the oocysts per gram of feces were quantified according to López Arellano (Citation2003) using a 10× lens of the optical microscopic (AmScope t390 C) (Cringoli et al. Citation2010).
2.3.2. Eimerias identification
Oocysts in fecal samples of each goat kid were induced to sporulate using a potassium dichromate solution (2.5% w/v) with aeration for 7 d at room temperature (≈ 23°C). In each sample, length, width, and presence of the micropile of the oocysts were measured. Subsequently, Eimeria species were identified based on the morphological norms described by Levine et al. (Citation1962). Optical microscope with graduated-slides was calibrated in 40× ocular micrometer (Harper and Penzhorn Citation1999).
2.3.4. Identification of lesions in the intestinal tissue and presence of schizont by histopathology
Intestinal tissue samples were fixed in 10% formaldehyde and later embedded in paraffin. From the samples, 5-μm-thick paraffin sections were obtained using a microtome (Leica ® México RM-2135, RM) and subsequently incubated at 60°C for 12 h for deparaffinization. The samples were rehydrated by alcohol passes, subsequently they were washed in running water for 10 min. The samples were stained with hematoxylin and eosin following the method described by Alturkistani et al. Citation2015, for histological description.
2.3.5. Sodium sulfamethazine in blood plasma
The SM concentration in the blood plasma was measured by High Resolution Liquid Chromatography (HPLC), modifying the technique described by Dmitrienko et al. (Citation2014). Initially, the samples were thawed at room temperature (≈ 23°C), then 1 mL of blood plasma was mixed with 0.5 mL of internal standard of sulfathiazole (sulfathiazole (Sinbiotik®) and sulfamethazine have a similar chemical form) at a concentration of 40 μg/mL, and the volume topped up to 5 mL with deionized water. Then, the solid phase was obtained with the addition of 2 mL of methanol, using a syringe with a Sep-Pack C18 cartridge Waters® México. Simultaneously with another syringe, 2 mL of phosphate buffer, pH 7.4, was added to the Sep-Pack C18 cartridge and both solutions were infused at a rate of 0.2 mL/sec. In the next step, the sample with SM was introduced into the Sep-Pack C18 cartridge and 1 mL of air was circulated at a rate of 0.2 mL/10 sec. In the end, the recovery of SM was performed with the addition of 2 mL of phosphate buffer (pH 7.4) and 2 mL of methanol. The solid phase was filtered, and the liquid substrate was used to quantify the SM using HPLC filters. The chromatographic conditions were as follows: mobile phase of 80:20 acetic acid and 0.50% acetonitrile, 1 mL/min fluid, run time of 8 min, 20 μL injection volume, wavelength of 266 nm, and an endcapped column Purosfer STAR RP-18 (5 μm).
2.3.6. Selenium in blood plasma
Half a gram of blood plasma was weighed and 5 mL of demineralized H2O (Millipore, Billerica, USA), 2.5 mL of HNO3, and 1 mL of H2O2 (JT Baker, Mexico City, Mexico) were added in Teflon tubes. Subsequently, the samples were digested in a microwave oven (MAR 5, CEM, Falcon, USA) for 10 min. The tubes were removed and cooled, and the solution was poured into 25 mL volumetric flasks (each container was washed three times with 7 M HCl) and gated with 7 M HCl. The total amount of Se was analysed using an atomic absorption device with a Varian® hydride generator. The calibration curve was prepared from 5 to 25 mg Se/L (Standard, High-Purity, 1000 ± 3μg/mL in 2% nitric acid, 99.99 purity, Scientific Company Se Powder Sigma-Aldrich® México) (Ghany-Hefnawy et al. Citation2007).
2.3.7. Statistical analysis
Boluses observed by x-rays and histological lesions caused by Eimeria in the intestinal tissues were only used to confirm the presence of the boluses in the reticulorumen and describe the coccidia infection, respectively. These results were not used to perform a statistical analysis. A non-parametric chi-square test was performed to evaluate the frequency of the Eimeria species. The number of oocysts was transformed to logarithm base 10, decreasing the variance of the McMaster counting technique.
The data of weight, DMI and Feed Conversion were analysed as a completely randomized design, using ANOVA procedure with pen as experimental unit. Each animal was used as an experimental unit in the analysis of oocysts, Se and SM. The oocyst count and levels of SM and Se were analysed with a design of repeated measures over time. All treatments means were conducted using a Tuckey test. The analysis of the results was carried out using the statistical software Statgraphics Centurion XV, version 15.2.05. Statistical Significance was determined at P < 0.05 using the following statistical model:where μ = mean, Ti = effect of the ith treatment (i = 1, 2 … , T), Mj = effect of the j-th sampling time (j = 1, 2 … , M)., TMij = effect of the interaction of the ith treatment for the jth time, and εij = random error for treatment i and time j.
3. Results
3.1. Radiographic location of the bolus
All goat kids showed radiographically the presence of the boluses in the reticulorumen at 10 d after dosing (). The boluses were not regurgitated or degraded at the time of observation, and they were recovered from the reticulorumen during the slaughter of the kids.
3.2. Productive variables
Goat kids started with a weight of 13.5 kg and finished 28 d later with a body weight of 14.8 kg. Animals in the BSe and BSM groups had a decreased body weight at 21 d, which was lower than that of the BC group (P < 0.05). The ADWG and the total gain were not significantly different between the groups at 28 d. The DMI/BW at the beginning of the experiment was low: 0.76% BC group and 0.86% dosed groups. At the end of the experiment (28 d) there was an increase: 1.36% BC group and 1.54% dosed groups. The mean food intake was higher for the BSM and BSMSe groups (8.2%, P < 0.05) than the BC and BSe groups ().
Table 1. Productive variables in goat kids infested naturally with Eimeria spp. and dosed with intraruminal boluses of selenium and sulfamethazine.
3.3. Identification and counting of the Eimeria
Before and after dosing the boluses, E. Ninakohlyakimovae was the most predominant coccidia. E. hirci and E. apsheronica were less common coccidia. BSMSe had the lowest percentage of Eimeria spp. (44%, P < 0.05) (). It was observed that BSM and BSMSe groups decreased the number of oocysts from 7 to 28 d (P < 0.05) ().
Table 2. Percentage of Eimeria spp. registered by species, before and 30 days after dosing the intraruminal boluses in weaned kids.
Table 3. Number of oocysts (log−10) in the fecal matter of goat kids naturally infected with Eimeria spp.
3.4. Levels of sodium sulfamethazine and selenium in blood
The levels of blood SM were highest 7 d after administering the boluses in the BSM and BSMSe groups, then decreased until 28 d. Animals in the BSMSe group had a higher content of blood SM (from 4 to 16 times more, P < 0.05) than those in the BSM group ().
Table 4. Levels of sodium sulfamethazine (μg/mL) in blood of goat kids naturally infected with Eimeria spp.
The average blood Se content of the four groups was 0.86 μg/g. At 7 d, the BSe group increased blood Se levels (P < 0.05). The BSMSe group at 14 d had a Se level higher than all the other groups (P < 0.05). On day 21, the BSe and BSMSe groups had high levels of blood Se, with no significant difference between them (P > 0.05). At 28 d, the Se levels in the blood had dropped in all the groups (P > 0.05) ().
Table 5. Levels of selenium (μg/g) in blood of goat kids naturally infected with Eimeria spp.
3.5. Histopathological findings
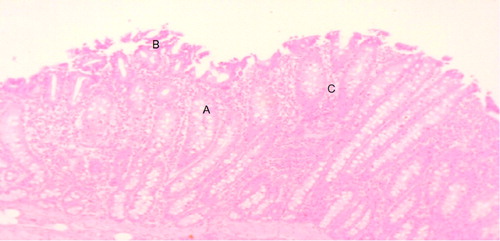
The main histological alterations observed were a moderate loss of the intestinal epithelium and villous atrophy. Some villi were fused together. Eimeria spp. phases were observed, known as schizonts. Intraepithelial lymphocytic infiltrate in the duodenum of BC animals and schizonts in the villi were observed. Some portions of the epithelium had villous destruction ((a)) with significant atrophy of the villi due to parasitic infection ((b)). Structures in the schizonts shows the severe infection suffered by the enterocytes of the small intestine due to the cycle in the infective phases of Eimeria spp ((c)) with intraepithelial lymphocytes surrounding the infected villi cells ((d)).
4. Discussion
4.1. Productive variables
The ADWG and BW in goat kids of this study were low, according to suggested by NRC (2007) and other authors (Academies Citation2007; Quintero Moreno et al. Citation2007; Martínez-Rojer et al. Citation2014). The ADWG would have to be 25 g/day with the offered diet. However, the infection by Eimeria affected the shape of the intestinal villi and consequently the flow of nutrients decreased from the intestine to the portal system (Berriatua et al. Citation1994). Other studies (Dashtizadeh et al. Citation2008; Xu et al. 2017) show that immunosuppression and stress in the kids caused a decrease in feed intake. On the other hand, feed conversion can range from 7 to 9 DMI/kg of weight gain (Ramli et al. Citation2005; Rahman et al. Citation2014). The results of our study were below these values, due to the coccidiosis infection in the goat kids. It was observed that BSe and BSMSe groups did not improve their weight gain trough the experiment; a previous study cited that Se supplementation improved the weight of kids (Awawdeh et al. Citation2015),
however, in the present study the ADWG was low due to Eimeria infection as mentioned by other studies, where the immunological response in infected animals is also evaluated, observing that these animals do not show a good response to the parasite (Khan et al. Citation2011) which could have been a negative characteristic to be able to evaluate the effect of selenium on the feed conversion.
4.2. Identification and counting of Eimerias
The BC group had the highest content of excreted oocysts (88.8%), whereas the BSMSe group had the lowest number of excreted oocysts (44.44%). Previous studies by our research group, using bolus with sulfamethazine, reported quantities of oocysts excreted from 49.9% to 58.4% (Chávez Rivera et al. Citation2005; Gutiérrez-Blanco et al. Citation2006). In this study, the boluses with sulfamethazine were effective against coccidiois in small ruminants. In our study, the combination of SM and Se reduced oocyst excretion in contrast with the other treatments. It has been observed that high levels of oocysts excreted after weaning were due to infection of animals in a dirty pen with the presence of mature oocysts, in addition to the lack of ingestion of colostrum at birth, which affects the ability of immune response of kids to Eimeria infection (Rauprich et al. Citation2000; Chartier and Paraud Citation2012). This is why the importance of coccidiosis prevention treatment in young animals.
Chávez Rivera et al. (Citation2005) described that boluses with sulfamethazine decreased the presence of infectious Eimeria species. In our study, nine species of Eimeria spp. were identified. E. ninakolhyakimovae, was the most predominant and has been reported as one of the most pathogenic in goat kids (Chartier and Paraud Citation2012). Another study conducted with kids in northern Mexico, identified E. arloingi, E. jolchijevi, E. ninakohlyakimovae, and E. hirci species; these species are very virulent causing severe damage to the intestinal epithelium and increase the mortality of goat kids (Cepeda-Palacios et al. Citation2015). Apparently, after all the studies around the world, there is no geographic preference for Eimeria, instead the infection depends on the hygienic conditions and the immune status of the goat kids (Knox and Steel Citation1996).
At 28 d, goat kids in the BSM or BSMSe group no longer excreted oocysts. McDougald and Galloway (Citation1973) observed that sulfonamides eliminated second-generation schizonts from 72 to 96 h post-inculcation. Other studies (Chávez Rivera et al. Citation2005; Gutiérrez-Blanco et al. Citation2006) have also recommend the use of intraruminal boluses with sulfamethazine to eliminate coccidiosis in small ruminants.
It has been observed that Se could act as an immunostimulant against bacterial, parasitic, and fungal infections (Hoffman and Berry Citation2008) in the animals, for example, a study carried out with Se deficient mice infected with Heligmosomoides polygyrus demonstrated that they had less immunity and a higher mortality rate than mice with adequate levels of Se (Smith et al. Citation2005). Therefore, there is a relationship between the Se concentration in the animal and their resistance to infection by pathogens, which could help fight Eimeria infections in goat kids.
4.3. Levels of sodium sulfamethazine and selenium in blood
Before the administration of boluses to goat kids, the Se content of the blood was 0.086 μg/g; it is recommended that the levels of the mineral should be higher than 0.1 μg Se/g in blood. Therefore, the kids in the study had marginal levels of Se (Ramírez-Bribiesca et al. Citation2004). After dosing the intraruminal boluses with Se, there was an increase in the micromineral in the blood. The BSe and BSMSe groups maintained the highest level of Se blood trough the experiment; other studies have reported similar results (Judson et al. Citation1988; Langlands et al. Citation1991; Blanco Ochoa et al. Citation2000) with differences in release rates from 90 to 400 d. In our study, the bolus density and release rate of Se and SM were regulated by the iron matrix, therefore, no animal in the experiment regurgitated the bolus administered.
In our study and a previous study (Chávez Rivera et al. Citation2005) there was an increase in the concentration of SM in the blood with the dosification with boluses with sulfamethazine. Other investigations using baquiloprim/sulfadimidine have been effective in eliminating Eimeria (Svensson Citation1998) in the animals too. However, the BSMSe group had a better SM level in the blood, indicating that there is a synergism between SM and Se in the boluses with this combination.
4.4. Histopathological findings
Goat kids infected with Eimeria had yellowish or dark diarrhea, progressive dehydration, and wasting. During the necropsy, there were macroscopic and microscopic lesions in the jejunum, ileum, and caecum with the presence of progamonts (evolutionary status of Eimeria) and high concentrations of eosinophils and lymphocytes (Khodakaram Tafti and Mansourian Citation2008). The presence of these immune cells indicates an immune response against the parasites. We observed epithelial cell loss, atrophy, and villous fusion, which were associated with the presence of first-generation schizonts (Taylor et al. Citation2003). These lesions, mainly caused by E. ninakohlyakimovae, induce the loss of intestinal absorption surface, affecting the growth and body condition of the kids (Dai et al. Citation2006). The kids had mild and chronic proliferative subacute enteritis and lesions in the mesenteric lymph nodes and the loss of blood in the intestinal epithelium caused anemia, electrolyte loss, a lack of nutrient absorption, and death (Young et al. Citation2011).
5. Conclusion
BSMSe was the treatment that had the lowest excretion of oocysts in contrast to control group, 44% vs. 88%, respectively. Therefore, BSMSe was the most efficient to reduce coccidiosis in goat kids. The concentration of SM and Se in blood tissue remained high for 30d in this group. The intraruminal boluses containing Se did not cause toxicity in the goat kids.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Academies, N.R.C. of T.N. 2007. Nutrients requirementes of small ruminants sheep, goats, Cervids and new world camelids. Washintong, DC: The National Academies Press.
- Alturkistani HA, Tashkandi FM, Mohammedsaleh ZM. 2015. Histological stains: a literature review and case study. Glob J Health Sci. 8:72. doi:10.5539/gjhs.v8n3p72.
- Awawdeh MS, Talafha AQ, Obeidat BS. 2015. Postpartum injection with vitamin E and selenium failed to improve the performance of Awassi ewes and their lambs. Can J Anim Sci. 95:111–115. doi:10.4141/cjas-2014-099.
- Berriatua E, Green LE, Morgan KL. 1994. A descriptive epidemiological study of coccidiosis in early lambing housed flocks. Vet Parasitol. 54:337–351. doi:10.1016/0304-4017(94)90001-9.
- Blanco Ochoa MÁ, Spross Suárez AK, Rosiles Martínez R. 2000. Evaluación de comprimidos intrarruminales de selenio por concentración sanguínea y lanar de corderas semiestabuladas. Vet Mex. 31:121–127.
- Cepeda-Palacios R, González A, López A, Ramírez-Orduña JM, Ramírez-Orduña R, Ascencio F, Dorchies P, Angulo C. 2015. Identification and characterization of eimeria spp. during early natural infection in goat kids in Baja California Sur, Mexico. Trop Subtrop Agroecosystems. 18:279–284.
- Chartier C, Paraud C. 2012. Coccidiosis due to Eimeria in sheep and goats, a review. Small Rumin Res. 103:84–92. doi:10.1016/j.smallrumres.2011.10.022.
- Chávez Rivera Ó, De Lucas Tron J, López Arellano R, Tórtora Pérez J. 2005. Utilización de bolos ruminales de liberación de sulfametazina sódica en el control de la coccidiosis ovina. Arq ciênc vet zool. 8:147–153.
- Cringoli G, Rinaldi L, Maurelli MP, Utzinger J. 2010. FLOTAC: New multivalent techniques for qualitative and quantitative copromicroscopic diagnosis of parasites in animals and humans. Nat Protoc. 5:503–515. doi:10.1038/nprot.2009.235.
- Dai YB, Liu XY, Liu M, Tao JP. 2006. Pathogenic effects of the coccidium Eimeria ninakohlyakimovae in goats. Vet Res Commun. 30:149–160. doi:10.1007/s11259-006-3228-1.
- Dashtizadeh M., Zamiri M. J., Kamalzadeh A., Kamali A. 2008. Effect of feed restriction on compensatory growth response of young male goats. Iranian Journal of Veterinary Research. 9:109–120.
- Dmitrienko SG, Kochuk EV, Apyari VV, Tolmacheva VV, Zolotov YA. 2014. Recent advances in sample preparation techniques and methods of sulfonamides detection – a review. Anal Chim Acta. doi:10.1016/j.aca.2014.08.023.
- Ershaduzzaman M, Rahman MM, Roy BK, Chowdhury SA. 2007. Studies on the diseases and mortality pattern of goats under farm conditions and some factors affecting mortality and survival rates in black Bengal kids. Bangl J Vet Med. 5:71–76. doi:10.1590/s1809-98232013000400007.
- Escareño-Sánchez LM, Wurzinger M, Pastor-López F, Salinas H, Sölkner J, Iñiguez L. 2011. La Cabra Y Los Sistemas De Producción Caprina De Los Pequeños Productores De La Comarca Lagunera, En El Norte De México. Rev Chapingo Ser Ciencias For y del Ambient. XVII:235–246. doi:10.5154/r.rchscfa.2010.10.087.
- FAO. 2018. Statistical Pocketbook 2018. World food and agriculture, Food and Agriculture Organization of the United Nations. doi:978-92-5-108802-9.
- França TGD, Ishikawa LLW, Zorzella-Pezavento SFG, Chiuso-Minicucci F, Da Cunha MLRSM, Sartori A. 2009. Impact of malnutrition on immunity and infection. J Venom Anim Toxins Incl Trop Dis. doi:10.1590/S1678-91992009000300003.
- Ghany-Hefnawy AE, López-Arellano R, Revilla-Vázquez A, Ramírez-Bribiesca E, Tórtora-Pérez J. 2007. The relationship between fetal and maternal selenium concentrations in sheep and goats. Small Rumin Res. 73:174–180. doi:10.1016/j.smallrumres.2007.01.020.
- Ghosh C, Sciences F, Datta S, Sciences F, Mandal D, Sciences F, Das AK, Sciences F. 2019. Body condition scoring in goat: Impact and significance. J Entomol Zool Stud. 7:554–560.
- Gutiérrez-Blanco E, Rodríguez-Vivas RI, Torres-Acosta JFJ, Tórtora-Pérez J, López-Arellano R, Ramírez-Cruz GT, Aguilar-Caballero AJ. 2006. Effect of a sustained-release intra-ruminal sulfamethazine bolus on Eimeria spp. oocyst output and weight gain of naturally infected lambs in the Mexican tropics. Small Rumin Res. 63:242–248. doi:10.1016/j.smallrumres.2005.02.023.
- Harper CK, Penzhorn BL. 1999. Occurrence and diversity of coccidia in indigenous, Saanen and crossbred goats in South Africa. Vet Parasitol. 82:1–9. doi:10.1016/S0304-4017(98)00266-0.
- Hefnawy AEG, Tórtora-Pérez JL. 2010. The importance of selenium and the effects of its deficiency in animal health. Small Rumin Res. 89:185–192. doi:10.1016/j.smallrumres.2009.12.042.
- Hoffman PR, Berry MJ. 2008. The influence of selenium on immune responses. Mol Nutr Food Res. 52:1273–1280. doi:10.1002/mnfr.200700330.The doi: 10.1002/mnfr.200700330
- Judson GJ, Brown TH, Kempe BR, Turnbull RK. 1988. Trace element and vitamin B12 status of sheep given an oral dose of one, two or four soluble glass pellets containing copper, selenium and cobalt. Aust J Exp Agric. 28:299–305. doi:10.1071/EA9880299.
- Khan MN, Rehman T, Iqbal Z, Sajid MS, Ahmad M, Riaz M, Area aS. 2011. Prevalence and associated risk factors of Eimeria in sheep of Punjab, Pakistan. World Acad Sci. 55:443–447.
- Khodakaram Tafti A, Mansourian M. 2008. Pathologic lesions of naturally occurring coccidiosis in sheep and goats. Comp Clin Path. 17:87–91. doi:10.1007/s00580-008-0719-1.
- Knox M, Steel J. 1996. Nutritional enhancement of parasite control in small ruminant production systems in developing countries of South-East Asia and the Pacific. Int J Parasitol. 26:963–970. doi:10.1016/S0020-7519(96)80072-5.
- Langlands JP, Donald GE, Bowles JE, Smith AJ. 1991. Subclinical selenium insufficiency 1. selenium status and the response in liveweight and wool production of grazing ewes supplemented with selenium. Aust J Exp Agric. 31:25–31. doi:10.1071/EA9910025.
- Levine ND, Ivens V, Fritz TE. 1962. Eimeria christenseni sp. n. and other coccidia (Protozoa: Eimeriidae) of the goat. J Parasitol. 48:255–269. doi: 10.2307/3275578
- López Arellano, M.E., 2003. Diagnóstico parasitológico en rumiantes: técnicas tradicionales y avances en biología molecular [WWW Document]. Sitio Argentino Prod. Anim.
- Martínez-Rojer RD, Torres-Hernández G, Martínez-Hernández S. 2014. Caracterización fenotípica, productiva y reproductiva de la cabra blanca Criolla del “Filo Mayor” de la Sierra Madre del Sur en el estado de Guerrero. Nov Sci. 6(1):25–44.
- McDougald LR, Galloway RB. 1973. Eimeria tenella: Anticoccidial drug activity in cell cultures. Exp Parasitol. 34:189–196. doi:10.1016/0014-4894(73)90078-7.
- Patiño Rodríguez G. 2013. Estudio del efecto de la suplementación de selenio sobre biomarcadores de estrés oxidativo de cabra. Universidad Nacional Autónoma de México.
- Quintero Moreno A, Boscán Ocando JC, Rubio Guillén JL, Villasmil Ontiveros YE, Román Bravo RM. 2007. Pesos corporales de cabritos mestizos a diferentes edades. Multiciencias. 7:26–32.
- Rahman MM, Nakagawa T, Abdullah RB, Embong WKW, Akashi R. 2014. Feed intake and growth performance of goats supplemented with soy waste. Pesqui Agropecu Bras. 49:554–558. doi:10.1590/S0100-204X2014000700008.
- Ramírez-Bribiesca E, Hernández-Camacho E, Hernández-Calva LM, Tórtora-Pérez JL. 2004. Efecto de un suplemento parenteral con selenito de sodio en la mortalidad de corderos y los valores hemáticos de selenio. Agrociencia. 38:43–51.
- Ramírez-Bribiesca JE, Tórtora JL, Huerta M, Aguirre A, Hernández LM. 2001. Diagnosis of selenium status in grazing dairy goats on the Mexican plateau. Small Rumin Res. 41:81–85. doi:10.1016/S0921-4488(01)00188-2.
- Ramírez-Bribiesca JE, Tortora JL, Huerta M, Hernández LM, López R, Crosby MM. 2005. Effect of selenium-vitamin E injection in selenium-deficient dairy goats and kids on the Mexican plateau. Arq Bras Med Vet e Zootec. 57:77–84. doi:10.1590/S0102-09352005000100011.
- Ramli MN, Higashi M, Imura Y, Takayama K, Nakanishi Y. 2005. Growth, feed efficiency, behaviour, carcass characteristics and meat quality of goats fed fermented bagasse feed. Asian-Australasian J Anim Sci. 18:1594–1599. doi:10.5713/ajas.2005.1594.
- Rauprich AB, Hammon HM, Blum JW. 2000. Influence of feeding different amounts of first colostrum on metabolic, endocrine, and health status and on growth performance in neonatal calves The onlin. J Anim Sci. 78:896–908. doi: 10.2527/2000.784896x
- Raymond LJ, Deth RC, Ralston NVC. 2014. Potential role of selenoenzymes and antioxidant metabolism in relation to Autism etiology and pathology. Autism Res Treat. 2014:1–15. doi:10.1155/2014/164938.
- Sharma D, Paul S, Rout P, Mandal A, Bhusan S, Sharma N, Kushwah Y. 2017. Caprine coccidiosis in semi-arid India: Dynamics and factors affecting fecal oocysts count. J Adv Vet Anim Res. 4:52–57. doi:10.5455/javar.2017.d190.
- Smith A, Madden KB, Yeung KJA, Zhao A, Elfrey J, Finkelman F, Levander O, Shea-Donohue T, Urban JF. 2005. Deficiencies in selenium and/or vitamin E lower the resistance of mice to Heligmosomoides polygyrus infections. J Nutr. 135:830–836. doi:10.1093/jn/135.4.830.
- Svensson C. 1998. Prevention of Eimeria alabamensis coccidiosis by a long-acting baquiloprim/sulphadimidine bolus. Vet Parasitol. 74:143–152. doi:10.1016/S0304-4017(97)00154-4.
- Taylor MA, Catchpole J, Marshall J, Marshall RN, Hoeben D. 2003. Histopathological observations on the activity of diclazuril (Vecoxan®) against the endogenous stages of Eimeria crandallis in sheep. Vet Parasitol. 116:305–314. doi:10.1016/S0304-4017(03)00256-5.
- Young G, Alley ML, Foster DM, Smith GW. 2011. Efficacy of amprolium for the treatment of pathogenic Eimeria species in Boer goat kids. Vet Parasitol. 178:346–349. doi:10.1016/j.vetpar.2011.01.028.