 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
To investigate the effect of direct fed microbial (DFM) on performance of fattening lamb, 4 treatments (7 animals/treatment) were evaluated; (1) control (without additive; CON); (2) Lactobacillus fermentum and Lactobacillus plantarum (FP); (3) Saccharomyces cerevisiae (SC) plus FP (SCFP) and (4) Megasphaera elsdenii plus SCFP (MSCFP). Feed intake, final body and feed efficiency except average daily gain were not affected by experimental treatment. Digestibility of dry matter and organic matter increased (P < 0.05) in MSCFP lambs versus other treatments. The highest (P < 0.05) amount of N intake, absorbed N and N retention were observed in MSCFP lambs. Blood parameters and enzymatic profiles were not affected (P > 0.05) by the treatments. The lowest and the highest of minimum pH were observed in CON and SCFP lambs. Moreover, concentration of ammonia-N, total and individual volatile fatty acids (except acetate and butyrate concentration) and acetate to propionate ratio were not affected (P > 0.05) by treatment. According to the results, the use of microbial feed additives had a positive effect on growth performance and some digestive and ruminal fermentation parameters.
1. Introduction
Increased concern about the use of antibiotics has increased interest in the use of natural feed additives such as direct-fed microbial (DFM). The DFM are viable organisms that have been shown positive effects on animal health and performance when used as dietary additive (Arowolo and He Citation2018; Rodríguez-Gaxiola et al. Citation2020). Preliminary research into the use of microbial inoculants was conducted by Allison et al. (Citation1964). They used the rumen content of high-grain-adapted animal as microbial inoculants to prevent ruminal acidosis. Several microorganisms known as non-pathogenic are used as DFM such as Lactobacillus spp., Bifidobacterium, Enterococcus, Bacillus spp., lactic acid utilizing bacteria (LUB) and different strains of yeast (Seo et al. Citation2010). The purpose of using DFM is to improve productive performance and prevent disease by maintaining gastrointestinal health and improving gut function (Chaucheyras-Durand et al. Citation2008). In addition, DFM enhance rumen microbial ecosystem (Musa et al. Citation2009; Kewan et al. Citation2019), reduce greenhouse gases emission (Hernandez et al. Citation2017; Vallejo-Hernández et al. Citation2018; Pedraza-Hernández et al. Citation2019) increase digestibility (El-Ghani Citation2004; Kewan et al. Citation2019), nutrient absorption and improve feed conversion ratio (FCR) (Antunović et al. Citation2006; Whitley et al. Citation2009), animal performance and prevent the accumulation of lactic acid (Jouany and Morgavi Citation2007).
Research has shown that the use of lactic acid-producing bacteria (LAB) as bacterial DFM has a positive effect on LUB and prevent ruminal acidosis (Goto et al. Citation2016). Bacterial DFM containing LAB have been reported to stabilize ruminal microbial flora (Chiquette et al. Citation2012), which in turn leads to increased feed intake, weight gain and improved animal health (Timmerman et al. Citation2005). Among the LAB, Lactobacillus plantarum and Enterococcus faecium are mainly used as bacterial DFM (Weinberg et al. Citation2003). The LUB such as Megasphaera elsdenii, Propionibacterium and Selenomonas ruminantium can play a key role in controlling ruminal fermentation (Mackie and Gilchrist Citation1979). M. elsdenii and S. ruminantium are the predominant strains of LUB in the rumen (Henning et al. Citation2010). It has been estimated that M. elsdenii utilize 65% to 95% of the lactic acid in the rumen and leads to prevents a sharp decrease in ruminal pH as a result of the accumulation of lactic acid (Kung and Hession Citation1995). Thus, the LUB can change lactate to volatile fatty acids (VFA), increase the production of propionate compared to lactic acid, increase feed efficiency and increase ruminal pH (Seo et al. Citation2010). Yeast additives lead to an increase the population of rumen bacteria, especially cellulolytic bacteria, through the equilibrium in rumen pH (Beauchemin et al. Citation2006). Hence, live yeast and yeast culture lead to increase the population of rumen bacteria, especially cellulolytic bacteria, through the equilibrium in rumen pH (Beauchemin et al. Citation2006). Therefore, improving ruminal digestion as a result of feeding with yeast additives is associated with increased pH as well as VFA (Dolezal et al. Citation2005).
The impact of using DFM on performance and ruminal fermentation in ruminant has been investigated in several studies (Vallejo-Hernández et al. Citation2018; Kewan et al. Citation2019; Pedraza-Hernández et al. Citation2019), and according to Nocek and Kautz (Citation2006), the use of DFM in dairy cattle has become a commonplace occurrence. The most used commercial products in feeding dairy cows include LAB strains (Jin et al. Citation2000; McAllister et al. Citation2011) alone or in combination with Saccharomyces cerevisiae (SC). Although the use of yeast in feeding small ruminants has been investigated in several articles (Abdelrahman and Hunaiti Citation2008; Titi et al. Citation2008; Soren et al. Citation2013), few studies have been conducted on the use of bacterial DFM in sheep. However, this study was performed to investigate the effect of LAB (Lactobacillus fermentum, Lactobacillus plantarum (FP)) alone or combined with LUB (M. elsdenii (Me)) and SC on growth performance, nitrogen (N) balance, in vivo nutrient digestibility, blood metabolites and rumen fermentation parameters in fattening lambs.
2. Materials and methods
2.1. Animals, housing, diet and treatments
All animal management and sampling procedures were conducted according to The Care and Use of Agricultural Animals in Research and Teaching guidelines (FASS Citation2010). All procedures and guidelines involving animals were approved by the Animal Experiment Committee at Agricultural Sciences and Natural Resources University of Khuzestan, Mollasani, Iran.
Twenty-eight autumn born Arabian lambs (male) with average weight 24 ± 3.7 kg and age 120 days old were used in a completely randomized design with 4 treatments (DFM) and 7 replicates. The lambs were randomly divided into 4 groups and housed in individual pens (1.5 m × 1.3 m). The experiment duration was 77 days (14 days for adaptation and 63 days was the main period of the experiment). During the adaptation period, all lambs were vaccinated against external (Azantole) and internal (Triclabendazole and Levamisole) parasites. Lambs received a diet containing 70% concentrate and 30% forage that formulated according to NRC (Citation2007) – (). The animals were fed with the diet (total mixed ration) ad libitum, twice daily (at 08:00 and 16:00) to ensure remains 5% residual during 24 h. The lambs also had free access to water and salt licks during the experiment.
Table 1. Ingredients (g/kg DM), chemical composition (g/kg DM) and metabolizable energy (ME; Mcal/kg DM) of the experimental diet.
In treatment containing microbial additives, the lamb was inoculated with a 50 mL microbial suspension before morning feeding (daily oral dosed). In the control, 50 mL of water was dosed instead of the microbial suspension. Three microbial additives FP, SC and Me were used in this study and 4 treatments were evaluated according to the type of additive used: (1) control (without additive (only diet); CON), (2) FP (50 mL bacterial suspension containing 4.5 × 108 cfu/day of L. plantarum and L. fermentum (in ratio 50:50)), (3) SC + FP (50 mL microbial suspension containing 4.5 × 108 cfu/day FP and 1.4 × 1010 cfu/day SC; SCFP), and (4) Me + SCFP (50 mL microbial suspension containing 4.5 × 108 cfu/day Me, 4.5 × 108 cfu/day FP and 1.4 × 1010 cfu/day SC; MSCFP). The commercial strain of L. plantarum and L. fermentum (GP10) isolated from the rumen of Najdi goat were used as LAB. M. elsdenii (GU1) isolated from the rumen of Najdi goat was used as LUB. The yeast additive was used from Iran Mollasses company (Mashhad, Iran), that each gram of this yeast contains 7 × 109 cfu/g.
2.2. Feed intake, growth performance, nutrient digestibility, N retention and chemical analysis
During the experiment period for estimating the voluntary feed intake, feed offered and residue were recorded daily before morning feeding, and feed and residue samples were stored at −20°C for subsequent chemical analysis. In order to determine the average daily gain (ADG), and feed efficiency (FE) (gain:feed), the lambs were weighed once every 14 days before morning feeding. The average gain on a daily basis was calculated for each lamb by linear regression analysis of body weight vs. time.
Digestibility coefficients of dry matter (DM), organic matter (OM), crude protein (CP), ash-free neutral detergent fibre (NDF) and ash-free acid detergent fibre (ADF) were estimated using the total faecal collection method (Givens et al. Citation2000). For this purpose, on day 50 of the experiment, 5 lambs from each treatment with the same body weight were transferred to metabolism crate equipped with faeces and urine collectors. The digestibility test lasted 13 days, with 7 days for adaptation and 6 days for sampling. During the collection period, the amount of feed offered, residue, faeces and urine of each lamb was recorded within 24 h. Each day 10% of samples (feed, residue, faeces, and urine) were collected and frozen at −20°C and at the end of the collection period daily samples were pooled of each lamb and thoroughly mixed and then frozen at −20°C for subsequent analysis.
Before the chemical analysis, feed, residue and faeces samples were oven-dried at 55°C for 48 h and then were ground using a mill equipped with a 1-mm sieve (Wiley mill, Swedesboro, USA). After that following the AOAC International (Citation1998) procedure samples were analysed for CP (Number. 988.05), ether extract (EE) (Number. 920.39), ash (Number. 924.05) and ADF (Number. 973.18). Also, NDF was analysed according to Van Soest et al. (Citation1991). Non-fibre carbohydrates (NFC) concentration was calculated by difference as NFC (g/kg DM) = 1000 − (NDF g/kg DM + CP g/kg DM + EE g/kg DM + ash g/kg DM).
Urinary N was also estimated using the Kjeldahl method. Daily N retention was calculated from the difference between the amount of daily N intake and the amount of daily N excreted (total N excreted in urine and faeces).
2.3. Blood sampling and analysis
In order to investigate blood biochemical parameter, blood sample (approximately 10 mL) was collected from jugular veins using tubes (Becton Dickinson, Rutherford, NJ, USA) containing an anticoagulant (heparin) on days 1 (the first day after finishing the adaptation), 14, 28, 42, 56 and 63 of the experimental period just 4 h after morning feeding. The blood samples were centrifuged (3,000 × g for 15 min at 4°C) and the plasma was separated and frozen at −20°C until measuring biochemical parameters. Glucose, triglycerides, total protein, albumin, creatinine, blood urea nitrogen (BUN), cholesterol, low-density lipoprotein (LDL), high-density lipoproteins (HDL), serum glutamic pyruvic transaminase (SGPT) and serum glutamic oxaloacetic transaminase (SGOT) were determined by using enzymatic methods and spectrophotometer (model S Bio-Rad Libra, England) and using kits of the ZiestChem Company (Tehran, Iran).
2.4. Ruminal fermentation parameter
In order to investigate ruminal pH changes during the experimental period on days 1 (the first day after finishing the adaptation), 14, 28, 42, 56 and 63 and 0, 3 and 6 h after morning feeding the rumen fluid was obtained with a stomach tube. The Min and Max of pH were determined by mean of Min and Max pH during sampling days. Ruminal pH was determined immediately by a portable pH meter (Metrohm model, Swiss). To investigate ruminal VFA and ammonia-N, the ruminal fluid that obtained on day 63 (0, 3 and 6 h after morning feeding) was strained through two layers of cheesecloth, and rumen fluid (25 mL) immediately submitted for the evaluation of ammonia-N with 5 mL of HCl 0.2 N and stored at −20°C. The rumen ammonia-N concentration was measured by phenol-hypochlorite assay (Broderick and Kang Citation1980). For analysis of ruminal VFA, 2 mL of strained rumen fluid was preserved, at −20°C, with 0.5 mL of an acid solution containing 200 mL/L orthophosphoric acid and 2-ethyl-butyric acid as internal standard. After thawing, rumen fluid samples were centrifuged (14,000 × rpm for 15 min; 4°C) and the supernatant was used to quantify the VFA. Ruminal VFA were determined by gas chromatography (GC; Chrompack, Model CP-9002, Chrompack, EA Middelburg, Netherlands) that equipped with a 50-m (0.32 mm ID) silica-fused column (CP-Wax Chrompack Capillary Column, Varian, Palo Alto, CA, USA). The helium was used as a carrier and oven initial and final temperatures were 55°C and 195°C, respectively, and detector and injector temperatures were set at 250°C.
2.6. Data analysis
The data obtained from assessing growth performance (7 lambs for each treatment), N balance (5 lambs for each treatment), nutrient digestibility (5 lambs for each treatment) and ruminal fermentation parameter (7 lambs for each treatment) were analysed as a randomized complete design using General Linear Models (GLM) procedure in SAS software (SAS Citation2008) based on the following statistical model:where Yij is observation, µ is the general mean, Ti is the effect of microbial additives and eij is the standard error of term. Also, data obtained from ruminal pH (5 lambs for each treatment) and blood metabolites (5 lambs for each treatment) were analysed as repeated measurements using the MIXED procedures of SAS based on the statistical model:
where Yijk is observation, µ is the general mean, Ti is the effect of microbial additives, Hj is effect of sampling day, (TH)ij is interactions between effect of microbial additives and sampling day and eijk is the standard error of term. Means were compared by the Duncan multiple comparison tests at P < 0.05.
3. Results
3.1. Feed intake and growth performance
Intake of DM, OM, CP, NDF and ADF were not affected (P > 0.05) by dietary supplementation with different microbial additives (). In addition, the final body weight and FE were not affected (P > 0.05) by dosed DFM. However, the ADG increased (P < 0.05) in MSCFP versus CON lambs.
Table 2. Feed intake and growth performance of lambs fed with different sources of microbial additives.
3.2. Nutrient digestibility, N balance and blood metabolites
Digestibility of DM and OM increased (P < 0.05) in MSCFP lambs versus other treatments. The highest (P < 0.05) digestibility of NDF was observed in the MSCFP lambs, but there was no difference with SCFP and CON lambs. However, there were no effect (P > 0.05) among treatments on the digestibility of CP, ADF and ME ().
Table 3. Apparent digestibility (%) and estimated metabolizable energy (Mcal/kg DM) of lambs fed with different sources of microbial additives.
The highest (P < 0.05) DM and OM intake and also digestible organic matter intake (DOMI) were observed in MSCFP lambs during the sampling week (days 57–63). Moreover, for MSCFP lambs, N intake was not higher than SCFP treatment and N retention was not higher than FP and SCFP lambs ().
Table 4. N balance of lambs fed with different sources of microbial additives.
Plasma parameters and enzymatic profiles were not affected (P > 0.05) by the experimental treatments ().
Table 5. Blood chemistry parameters of lambs fed with different sources of microbial additives.
3.3. Ruminal fermentation parameters
The lowest and the highest (P < 0.05) of minimum pH values were observed in CON and SCFP lambs, whereas the maximum pH was not affected (P = 0.443) by experimental treatments. There was no significant difference (P > 0.05) among the experimental treatments in the concentration of ammonia-N, total VFA, propionate, valerate, isobutyrate, isovalerate and acetate to propionate ratio. The highest (P < 0.05) concentration of acetate and butyrate was observed in CON and FP lambs, respectively, while the concentration of butyrate was not different (P > 0.05) between FP and SCFP ( and .).
Figure 1. Effect of different source of microbial additives on ruminal pH of the lambs. □ (CON), without microbial additive (control); ▪ (FP), Lactobacillus fermentum and Lactobacillus plantarum; ▴ (SCFP), Saccharomyces cerevisiae (SC) plus FP; Δ (MSCFP), Megasphaera elsdenii plus SC plus FP. Arrow (↑), morning feeding at 8.
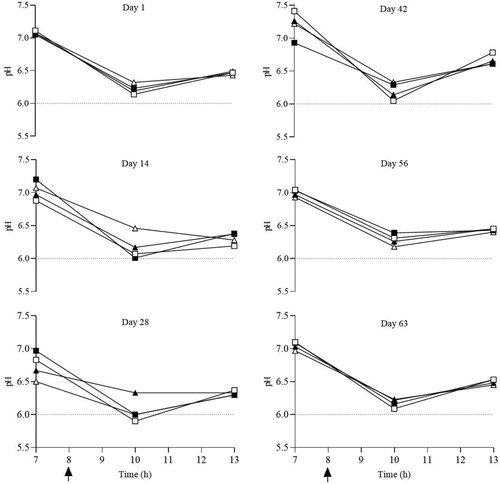
Table 6. Ruminal fermentation parameters of lambs fed with different sources of microbial additives.
4. Discussion
4.1. Feed intake and growth performance
Microbial additives lead to increases feed intake (Chiofalo et al. Citation2004; Antunović et al. Citation2006; Desnoyers et al. Citation2009) and it has been found that increased feed intake affects ruminant performance (Beigh et al. Citation2017). Hassan et al. (Citation2019) reported that the use of microbial supplements (Ruminococcus flavefaciens) increased feed intake. Moreover, final weight, ADG, total gain and FCR were improved in lambs supplemented with Pediococcus acidilactici and Pediococcus pentosaceus after weaning compared to control (Saleem et al. Citation2017). In a meta-analysis have been shown (21 articles were reviewed between 1985 and 2010) by adding DFM containing LAB to milk replacer of calves lead to improved body weight gain and FE compared to controls (Frizzo et al. Citation2011). Overall, DFM by affecting on ruminal pH and nutrient digestibility may affect the amount of intake (Wallace and Newbold Citation1993). Improved animal performance due to the use of probiotics can also result in improved growth of fibre-degrading bacteria and increased digestion of fibre (Russell and Wilson Citation1996). Feed intake and growth performance, of the present study, were not affected by experimental treatments except the ADG. Higher weight gain in lambs dosed with microbial additives could be due to increased microbial protein synthesis and post-ruminal availability of amino acid (Erasmus et al. Citation1992). The lack of effect of microbial additives on performance was attributed to the high level of dietary protein (Titi et al. Citation2008). The addition of yeast to the low-protein diet had a favourable effect on the performance compared to the high-protein diet (Kawas et al. Citation2007). Similarly to our results, in several studies supplementation with LUB (Propionibacterium) and LAB (Enterococcus faecium) (Ghorbani et al. Citation2002) and yeast culture did not have any effect on feed intake (Haddad and Goussous Citation2005; Hernandez et al. Citation2009). Moreover, supplementing the diet with L. acidophilus (Abu-Tarboush et al. Citation1996; Cruywagen et al. Citation1996), a mixture of L. acidophilus and Streptococcus faecium (Higginbotham and Bath Citation1993), a mixture of L. acidophilus and L. plantarum (Abu-Tarboush et al. Citation1996), B. subtilis (Galina et al. Citation2009) or a mixture of L. acidophilus, L. lactis and B. subtilis (Galina et al. Citation2009) had no effect on calf growth performance.
4.2. Nutrient digestibility and N balance
Nutrient digestibility was not different among treatments CON, FP and SCFP, while the combination of all three additives increased the digestibility of DM, OM and NDF. The improved of nutrient digestibility may also be due to the release of the enzyme by DFM and changes in rumen microbial ecology. However, what is known is that the optimal function of DFM on digestibility involves altering the rate of acid production, creating favourable microbial flora, and increasing fibre digestion (McAllister et al. Citation2011). Soren et al. (Citation2013) reported that lambs fed with a diet supplemented by combining S. cerevisiae with L. sporogenes had no effect on the digestibility of DM, OM, and NDF. In other experiments in dairy and beef cattle, it has also been reported that the use of LAB in combination with LUB did not affect nutrient digestibility (Ghorbani et al. Citation2002; Raeth-Knight et al. Citation2007). However, it has been reported that the combination of L. acidophilus NP51 and Propionibacterium freudenreichii NP24 in dairy cows improved the digestibility of CP, NDF and ADF (Boyd et al. Citation2011). In addition, use of Enterococcus faecium and yeast improved DM digestibility of forage in cows during the transition period (Nocek and Kautz Citation2006).
The increase in urinary N excretion in the SCFP and MSCFP lambs was probably due to higher N intake. Increased protein intake and intestinal absorption of more amino acids than needed for tissues and or ammonia-N absorbed from the rumen epithelium (rumen ammonia-N concentration was numerically higher in experimental treatments than CON) lead to increased urinary N excretion (Willms et al. Citation1991). Moreover, the concentration of BUN numerically was higher in lambs of SCFP and MSCFP. Kohn et al. (Citation2005) stated that BUN concentration has a direct relationship with excreted N in the urine. However, the highest N retention was observed in MSCFP lambs that was proportional to the increase in ADG and total gain. That could reflect a greater intake of N and possibly reflect its use by tissue and protein deposition. It has been reported DFM alone or in combination lead to increase N intake (Mohamed et al. Citation2009) and ruminal ammonia-N (Jouany, Mathieu, Senaud, et al. Citation1998). However, several studies have reported that microbial additives had no effect on the amount of N intake, N retention, and N excretion through urine and faeces (Jouany, Mathieu, Bohatier, et al. Citation1998; Hernandez et al. Citation2009).
4.3. Blood metabolites
The concentration of total protein and albumin in this study was not affected by the experimental treatments, which was in agreement with previous studies (Galip Citation2006; Soren et al. Citation2013). The lack of effect of the experimental treatments on the plasma protein indicates an improvement in the nutritional status of the animals and the lack of use of the amino acids and deamination to obtain energy. Bruno et al. (Citation2009) observed that microbial additives lead to better use of N by ruminal bacteria and, thus reduce the concentration of BUN. In present study, the concentration of BUN was not affected by the experimental treatments and numerically was higher in dosed lambs (as results of high protein intake). Since creatinine concentration is constant, an increase in its concentration indicates renal disease (Direkvandi and Kamyab Kalantari Citation2018). The concentration of creatinine was lower the normal range (1.2–1.9 mg/dL) of plasma concentration of creatinine of sheep (Radostitis et al. Citation2007).
As the propionate concentration was not affected by the experimental treatments, the concentration of plasma glucose was not different among treatments. This is may be due to increased propionate production through increased gluconeogenesis lead to increases plasma glucose concentration (Hussein Citation2014). It has been reported that DFM has a positive effect on energy balance as a result of improved metabolic status. Therefore, it results in a decrease in plasma lipids concentration (Baiomy Citation2011). However, in our study concentrations of triglyceride, cholesterol, LDL and HDL were not affected by the DFM. Similarly, to our results, the concentrations of SGPT and SGOT were not affected by DFM, which was agreement with previous studies (Alhidary et al. Citation2016; Mousa et al. Citation2019).
4.4. Ruminal fermentation parameters
Ruminal acidosis occurs when the ruminal pH is reduced to less than 5.6 due to the accumulation of lactic acid and other VFA for 3 h a day (Goto et al. Citation2016). However, in our experiment, no pH was lower than 5.82. The maximum and mean pH were not affected by the experimental treatments, which was in agreement with the results of previous studies (Ghorbani et al. Citation2002; Beauchemin et al. Citation2003; Yang et al. Citation2004). Beauchemin et al. (Citation2003) noted that the lack of effect of bacterial DFM on ruminal pH indicates that providing DFM containing LAB or LUB has little benefit when animal is accustomed to high concentrate rations. On the other hand, the results of some experiments showed that supplementation of Lactobacillus spp. (Huffman et al. Citation1992), M. elsdenii (Kung and Hession Citation1995) and yeast (Desnoyers et al. Citation2009) have a positive effect on pH. In our study, the concentration of ammonia-N was numerically higher than CON. Similarly, Mulaudzi (Citation2018) reported that the use of M. elsdenii, S. cerevisiae and a mixture of both in high concentrate diet had no effect on ammonia-N concentration compared to control treatment.
The decrease in acetate concentration using DFM may be due to the shift in ruminal fermentation to produce propionate. In fact, the decrease in the ratio of acetate to propionate indicates an increase in the fermentation of the non-structural carbohydrates, resulting in an increase in propionate production (Der Bedrosian Citation2009). Consistent with our results Jeyanathan et al. (Citation2016) reported in an in vitro experiment that L. pentosus D31 decreased acetate concentration compared to control. However, contrary to our results, the use of DFM (LAB, LUB and yeast) in the feeding of dairy cows (Philippeau et al. Citation2017; Oh et al. Citation2019), calves (Geiger et al. Citation2014) and sheep had no effect on acetate concentration. Philippeau et al. (Citation2017) attributed the increase in propionate concentration as a result of the use of DFM to the increased amylase activity of the ruminal organisms and M. elsdenii. It has been shown that M. elsdenii converts lactate to propionate and butyrate (Marounek et al. Citation1989; Drouillard et al. Citation2012). This may explain the numerical increase in propionate concentration in MSCFP lambs. Kim et al. (Citation2000) also reported that using different doses of Propionibacterium increased the propionate concentration and numerically reduced the ratio of acetate to propionate. Similarly, to our results, an increase in butyrate concentration has been reported when using DFM (Ghorbani et al. Citation2002; Ramaswami et al. Citation2005; Mamuad et al. Citation2017). Mamuad et al. (Citation2017) reported that higher butyrate concentration than CON indicates increased carbon and energy sources for fatty acid synthesis. However, the lack effect of DFM on the concentration of butyrate has also been reported (Al Ibrahim et al. Citation2010). Consistent with previous research, the concentrations of isobutyrate, valerate and isovalerate were not affected by the experimental treatments (Beauchemin et al. Citation2003; Yang et al. Citation2004; Al Ibrahim et al. Citation2010).
5. Conclusion
The effect of microbial additives on feed intake, performance and blood metabolites was not significant. However, the digestibility of nutrients, especially NDF, improved under the simultaneous use of all three additives (MSCFP). The use of microbial additives had a positive effect on N retention and ruminal fermentation parameters and it can be concluded that between treatments, MSCFP can be a good candidate.
Acknowledgements
The authors gratefully acknowledge the Agricultural Sciences and Natural Resources University of Khuzestan for their financial support.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Abdelrahman MM, Hunaiti DA. 2008. The effect of dietary yeast and protected methionine on performance and trace minerals status of growing Awassi lambs. Livest Sci. 115:235–241. doi: 10.1016/j.livsci.2007.07.015
- Abu-Tarboush HM, Al-Saiady MY, El-Din AHK. 1996. Evaluation of diet containing lactobacilli on performance, fecal coliform, and lactobacilli of young dairy calves. Anim Feed Sci Tech. 57(1):39–49. doi: 10.1016/0377-8401(95)00850-0
- Alhidary IA, Abdelrahman MM, Khan RU. 2016. Comparative effects of direct-fed microbials alone or with a trace minerals supplements on the productive performance, blood metabolites, and antioxidant status in grazing Awassi lambs. Environ Sci Pollut R. 23(24):25218–25223. doi: 10.1007/s11356-016-7684-z
- Al Ibrahim RM, Kelly AK, O’Grady L, Gath VP, McCarney C, Mulligan FJ. 2010. The effect of body condition score at calving and supplementation with Saccharomyces cerevisiae on milk production, metabolic status, and rumen fermentation of dairy cows in early lactation. J Dairy Sci. 93:5318–5328. doi: 10.3168/jds.2010-3201
- Allison MJ, Bucklin JA, Dougherty RW. 1964. Ruminal changes after overfeeding with wheat and the effect of intraluminal inoculation on adaptation to a ration containing wheat. J Anim Sci. 23:1164–1171. doi: 10.2527/jas1964.2341164x
- Antunović Z, Šperanda M, Amidžić D, Šerić V, Stainer Z, Domačinović M, Boli F. 2006. Probiotic application in lambs nutrition. Krmiva. 48:175–180.
- AOAC International. 1998. Official methods of analysis. 16th rev. Arlington (VA): Assoc. Off. Anal. Chem.
- Arowolo MA, He J. 2018. Use of probiotics and botanical extracts to improve ruminant production in the tropics: a review. Anim Nutr. 4(3):241–249. doi: 10.1016/j.aninu.2018.04.010
- Baiomy AA. 2011. Influence of live yeast culture on milk production, composition and some blood metabolites of Ossimi ewes during the milking period. Am J Biochem Mol Biol. 1:158–167. doi: 10.3923/ajbmb.2011.158.167
- Beauchemin KA, Krehbiel CR, Newbold CJ. 2006. Enzymes, bacterial direct-fed microbials and yeast: principles for use in ruminant nutrition. Biol Grow Anim. 4:251–284. doi: 10.1016/S1877-1823(09)70094-3
- Beauchemin KA, Yang WZ, Morgavi DP, Ghorbani GR, Kautz W, Leedle JAZ. 2003. Effects of bacterial direct-fed microbials and yeast on site and extent of digestion, blood chemistry, and subclinical ruminal acidosis in feedlot cattle. J Anim Sci. 81:1628–1640. doi: 10.2527/2003.8161628x
- Beigh YA, Ganai AM, Ahmad HA. 2017. Prospects of complete feed system in ruminant feeding: a review. Vet World. 10(4):424. doi: 10.14202/vetworld.2017.424-437
- Boyd J, West J, Bernard J. 2011. Effects of the addition of direct-fed microbials and glycerol to the diet of lactating dairy cows on milk yield and apparent efficiency of yield. J Dairy Sci. 94(9):4616–4622. doi: 10.3168/jds.2010-3984
- Broderick GA, Kang JH. 1980. Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J Dairy Sci. 63:64–75. doi: 10.3168/jds.S0022-0302(80)82888-8
- Bruno RG, Rutigliano HM, Cerri RL, Robinson PH, Santos JE. 2009. Effect of feeding Saccharomyces cerevisiae on performance of dairy cows during summer heat stress. Anim Feed Sci Tech. 150:175–186. doi: 10.1016/j.anifeedsci.2008.09.001
- Chaucheyras-Durand F, Walker ND, Bach A. 2008. Effects of active dry yeasts on the rumen microbial ecosystem: past, present and future. Anim Feed Sci Tech. 145:5–26. doi: 10.1016/j.anifeedsci.2007.04.019
- Chiofalo V, Liotta L, Chiofalo B. 2004. Effects of the administration of lactobacilli on body growth and on the metabolic profile in growing Maltese goat kids. Reprod Nutr Dev. 44:449–457. doi: 10.1051/rnd:2004051
- Chiquette J, Allison MJ, Rasmussen M. 2012. Use of Prevotella bryantii 25A and a commercial probiotic during subacute acidosis challenge in midlactation dairy cows. J Dairy Sci. 95(10):5985–5995. doi: 10.3168/jds.2012-5511
- Cruywagen C, Jordaan I, Venter L. 1996. Effect of Lactobacillus acidophilus supplementation of milk replacer on preweaning performance of calves. J Dairy Sci. 79(3):483–486. doi: 10.3168/jds.S0022-0302(96)76389-0
- Der Bedrosian M. 2009. The effect of sodium bicarbonate or live yeast culture (Saccharomyces cerevisiae) on the metabolism and production of Lactating dairy cows [Doctoral dissertation]. University of Delaware.
- Desnoyers M, Giger-Reverdin S, Bertin G, Duvaux-Ponter C, Sauvant D. 2009. Meta-analysis of the influence of Saccharomyces cerevisiae supplementation on ruminal parameters and milk production of ruminants. J Dairy Sci. 92:1620–1632. doi: 10.3168/jds.2008-1414
- Direkvandi E, Kamyab Kalantari R. 2018. Fecal volatile fatty acids and blood metabolites in the Turkmen horse associated with type and source of cereal grains. J Appl Anim Res. 46(1):1078–1083. doi: 10.1080/09712119.2018.1464927
- Dolezal P, Dolezal J, Trinacty J. 2005. The effect of Saccharomyces cerevisiae on ruminal fermentation in dairy cows. Czech J Anim Sci. 50:503–510. doi: 10.17221/4255-CJAS
- Drouillard JS, Henning PH, Meissner HH, Leeuw KJ. 2012. Megasphaera elsdenii on the performance of steers adapting to a high-concentrate diet, using three or five transition diets. S Afr J Anim Sci. 42(2):195–199. doi: 10.4314/sajas.v42i2.13
- El-Ghani AA. 2004. Influence of diet supplementation with yeast culture (Saccharomyces cerevisiae) on performance of Zaraibi goats. Small Rumin Res. 52:223–229. doi: 10.1016/j.smallrumres.2003.06.002
- Erasmus LJ, Botha PM, Kistner A. 1992. Effect of yeast culture supplementation on production, rumen fermentation, and duodenal nitrogen flow in dairy cows. J Dairy Sci. 75:3056–3065. doi: 10.3168/jds.S0022-0302(92)78069-2
- FASS. 2010. Guide for the care and use of agricultural animals in agricultural research and teaching. Champaign (IL): Federation of Animal Science Societies.
- Frizzo LS, Zbrun MV, Soto LP, Signorini ML. 2011. Effects of probiotics on growth performance in young calves: a meta-analysis of randomized controlled trials. Anim Feed Sci Tech. 169(3):147–156. doi: 10.1016/j.anifeedsci.2011.06.009
- Galina MA, Ortiz-Rubio MA, Delgado-Pertinez M, Pineda LJ. 2009. Goat kid’s growth improvement with a lactic probiotic fed on a standard base diet. Options Méditerr Sér Sémin Méditerr. 85:315–322.
- Galip N. 2006. Effect of supplemental yeast culture and sodium bicarbonate on ruminal fermentation and blood variables in rams. J Anim Physiol Anim Nutr. 90:446–452. doi: 10.1111/j.1439-0396.2006.00625.x
- Geiger AJ, Ward SH, Williams CC, Rude BJ, Cabrera CJ, Kalestch KN, Voelz BE. 2014. Effects of increasing protein and energy in the milk replacer with or without direct-fed microbial supplementation on growth and performance of preweaned Holstein calves. J Dairy Sci. 97(11):7212–7219. doi: 10.3168/jds.2013-7000
- Ghorbani GR, Morgavi DP, Beauchemin KA, Leedle JA. 2002. Effects of bacterial direct-fed microbials on ruminal fermentation, blood variables, and the microbial populations of feedlot cattle. J Anim Sci. 80:1977–1985. doi: 10.2527/2002.8071977x
- Givens DI, Owen E, Axford RFE, Omed HM. 2000. Forage evaluation in ruminant nutrition. 1st ed. Wallingford: CABI Publishing. 480 pp.
- Goto H, Qadis AQ, Kim YH, Ikuta K, Ichijo T, Sato S. 2016. Effects of a bacterial probiotic on ruminal pH and volatile fatty acids during subacute ruminal acidosis (SARA) in cattle. J Vet Med Sci. 78(10):1595–1600. doi: 10.1292/jvms.16-0211
- Haddad SG, Goussous SN. 2005. Effect of yeast culture supplementation on nutrient intake, digestibility and growth performance of Awassi lambs. Anim Feed Sci Tech. 118:343–348. doi: 10.1016/j.anifeedsci.2004.10.003
- Hassan A, Gado H, Anele UY, Berasain MA, Salem AZ. 2019. Influence of dietary probiotic inclusion on growth performance, nutrient utilization, ruminal fermentation activities and methane production in growing lambs. Anim Biotechnol. 21:1–8. doi: 10.1080/10495398.2019.1604380
- Henning PH, Horn CH, Steyn DG, Meissner HH, Hagg FM. 2010. The potential of Megasphaera elsdenii isolates to control ruminal acidosis. Anim Feed Sci Tech. 157(1–2):13–19. doi: 10.1016/j.anifeedsci.2009.12.011
- Hernandez R, Gonzalez SS, Pinos-Rodriguez JM, Ortega ME, Hernandez A, Bueno G, Cobos M. 2009. Effect of yeast culture on nitrogen balance and digestion in lambs fed early, and mature orchard grass. J Appl Anim Res. 32:53–56. doi: 10.1080/09712119.2009.9706984
- Hernandez A, Kholif AE, Lugo-Coyote R, Elghandour MMY, Cipriano M, Rodríguez GB, Odongo NE, Salem AZM. 2017. The effect of garlic oil, xylanase enzyme and yeast on biomethane and carbon dioxide production from 60-d old Holstein dairy calves fed a high concentrate diet. J Clean Prod. 142(4):2384–2392. doi: 10.1016/j.jclepro.2016.11.036
- Higginbotham G, Bath D. 1993. Evaluation of Lactobacillus fermentation cultures in calf feeding systems. J Dairy Sci. 76(2):615–620. doi: 10.3168/jds.S0022-0302(93)77382-8
- Huffman RP, Karges KK, Klopfenstein TJ, Stock RA, Britton RA, Roth LD. 1992. The effect of Lactobacillus acidophilus on subacute ruminal acidosis. J Anim Sci. 70(Suppl. 1):87 (Abstract).
- Hussein AF. 2014. Effect of biological additives on growth indices and physiological responses of weaned Najdi ram lambs. J Exp Biol Agr Sci. 2:597–607.
- Jeyanathan J, Martin C, Morgavi DP. 2016. Screening of bacterial direct-fed microbials for their antimethanogenic potential in vitro and assessment of their effect on ruminal fermentation and microbial profiles in sheep. J Anim Sci. 94:739–750. doi: 10.2527/jas.2015-9682
- Jin LZ, Marquardt RR, Zhao X. 2000. A strain of Enterococcus faecium (18C23) inhibits adhesion of enterotoxigenic Escherichia coli K88 to porcine small intestine mucus. Appl Environ Microbiol. 66:4200–4204. doi: 10.1128/AEM.66.10.4200-4204.2000
- Jouany JP, Mathieu F, Bohatier J, Bertin G, Mercier M. 1998. The effect of Saccharomyces cerevisiae and Aspergilus oryzae on the digestion of cell wall fraction of a mixed diet in defaunated sheep rumen. Repeod Nutr Dev. 38:401–416. doi: 10.1051/rnd:19980405
- Jouany JP, Mathieu F, Senaud J, Bohaitier J, Bertin G, Mercier M. 1998. The effect of Saccharomyces cerevisiae and Aspergilus oryzae on the digestion of nitrogen in rumen of defaunated and refaunated sheep. Anim Feed Sci Tech. 75:1–13. doi: 10.1016/S0377-8401(98)00194-1
- Jouany JP, Morgavi DP. 2007. Use of ‘natural’ products as alternatives to antibiotic feed additives in ruminant production. Animal. 10:1443e66.
- Kawas JR, García-Castillo R, Garza-Cazares F, Fimbres-Durazo H, Olivares-Sáenz E, Hernández-Vidal G, Lu CD. 2007. Effects of sodium bicarbonate and yeast on productive performance and carcass characteristics of light-weight lambs fed finishing diets. Small Ruminant Res. 67:157–163. doi: 10.1016/j.smallrumres.2005.09.011
- Kewan KZ, Salem FA, Salem AZM, Abdou AR, El-Sayed HM, Eisa SS, Zaki EA, Odongo NE. 2019. Nutritive utilization of Moringa oleifera tree stalks treated with fungi and yeast to replace clover hay in growing lambs. Agroforestry Syst. 93:161–173. doi: 10.1007/s10457-017-0158-6
- Kim SW, Standorf DG, Roman-Rosario H, Yokoyama MT, Rust SR. 2000. Potential use of Propionibacterium acidipropionici, strain DH42, as a direct-fed microbial for cattle. J Anim Sci. 78(Suppl. 1):292 (Abstract).
- Kohn RA, Dinneen MM, Russek-Cohen E. 2005. Using blood urea nitrogen to predict nitrogen excretion and efficiency of nitrogen utilization in cattle, sheep, goats, horses, pigs, and rats. J Anim Sci. 83(4):879–889. doi: 10.2527/2005.834879x
- Kung L, Hession AO. 1995. Preventing in vitro lactate accumulation in ruminal fermentations by inoculation with Megasphaera elsdenii. J Anim Sci. 73:250–256. doi: 10.2527/1995.731250x
- Mackie RI, Gilchrist FM. 1979. Changes in lactate-producing and lactate-utilizing bacteria in relation to pH in the rumen of sheep during stepwise adaptation to a high-concentrate diet. Appl Environ Microbiol. 38(3):422–430. doi: 10.1128/AEM.38.3.422-430.1979
- Mamuad LL, Kim SH, Choi YJ, Soriano AP, Cho KK, Lee K, Bae GS, Lee SS. 2017. Increased propionate concentration in Lactobacillus mucosae–fermented wet brewers grains and during in vitro rumen fermentation. J Appl Microbiol. 123(1):29–40. doi: 10.1111/jam.13475
- Marounek M, Fliegrova K, Bartos S. 1989. Metabolism and some characteristics of ruminal strains of Megasphaera elsdenii. Appl Environ Microbiol. 55(6):1570–1573. doi: 10.1128/AEM.55.6.1570-1573.1989
- McAllister TA, Beauchemin KA, Alazzeh AY, Baah J, Teather RM, Stanford K. 2011. Review: the use of direct fed microbials to mitigate pathogens and enhance production in cattle. Can J Anim Sci. 91:193–211. doi: 10.4141/cjas10047
- Mohamed MI, Maareck YA, Abdel-Magid SS, Awadalla IM. 2009. Feed intake, digestibility, rumen fermentation and growth performance of camel fed diets supplemented with a yeast culture or zinc bacitracin. Anim Feed Sci Tech. 149:341–345. doi: 10.1016/j.anifeedsci.2008.07.002
- Mousa AES, Marghani B, Ateya A. 2019. Effects of supplementation of Bacillus spp. on blood metabolites, antioxidant status, and gene expression pattern of selective cytokines in growing Barki lambs. J Adv Vet Anim Res. 6(3):333. doi: 10.5455/javar.2019.f351
- Mulaudzi T. 2018. In vitro effects of Megasphaera elsdenii ncimb 41125 and Saccharomyces Cerevisiae 1026 on rumen fermentation in early lactating cows [Master of Science thesis]. University of South Africa. p. 30–32.
- Musa HH, Wu SL, Zhu CH, Seri HI, Zhu GQ. 2009. The potential benefits of probiotics in animal production and health. J Anim Vet Adv. 8:313–321.
- Nocek JE, Kautz WP. 2006. Direct-fed microbial supplementation on ruminal digestion, health, and performance of pre-and postpartum dairy cattle. J Dairy Sci. 89:260–266. doi: 10.3168/jds.S0022-0302(06)72090-2
- NRC (National Research Council). 2007. Nutrient requirements of small ruminants. Washington (DC): National Academy Press.
- Oh J, Harper M, Melgar A, Compart DP, Hristov AN. 2019. Effects of Saccharomyces cerevisiae-based direct-fed microbial and exogenous enzyme products on enteric methane emission and productivity in lactating dairy cows. J Dairy Sci. 102(7):6065–6075. doi: 10.3168/jds.2018-15753
- Pedraza-Hernández J, Elghandour MMY, Khusro A, Camacho-Diaz LM, Vallejo-Hernández LH, Barbabosa-Pliego A, Salem AZM. 2019. Mitigation of ruminal biogases production from goats using Moringa oleifera extract and live yeast culture for a cleaner agriculture environment. J Clean Prod. 234:779–786. doi: 10.1016/j.jclepro.2019.06.126
- Philippeau C, Lettat A, Martin C, Silberberg M, Morgavi DP, Ferlay A, Berger C, Noziere P. 2017. Effects of bacterial direct-fed microbials son ruminal characteristics, methane emission, and milk fatty acid composition in cows fed high- or low-starch diets. J Dairy Sci. 100:2637–2650. doi: 10.3168/jds.2016-11663
- Radostitis OM, Gay CC, Blood DC, Hinchliff KW. 2007. Veterinarymedicine. A text book of the diseases of cattle, sheep, goats and horses. 10th ed. London: W.B. Saunders Ltd.
- Raeth-Knight ML, Linn JG, Jung HG. 2007. Effect of directfed microbials on performance, diet digestibility, and rumen characteristics of Holstein dairy cows. J Dairy Sci. 90:1802–1809. doi: 10.3168/jds.2006-643
- Ramaswami N, Chaudhary LC, Agarwal N, Kamra DN. 2005. Effect of lactic acid producing bacteria on the performance of male crossbred calves fed roughage based diet. Asian-Australas J Anim Sci. 18(8):1110–1115. doi: 10.5713/ajas.2005.1110
- Rodríguez-Gaxiola MA, Domínguez-Vara IA, Barajas-Cruz R, Contreras-Andrade I, Morales-Almaráz E, Bórquez-Gastelum JL, Sánchez-Torres JL, Trujillo-Gutiérrez D, Salem AZM, Ramírez-Bribiesca E, Anele UY. 2020. Effect of enriched-chromium yeast on growth performance, carcass characteristics and fatty acid profile in finishing Rambouillet lambs. Small Rum Res. 188:106118. doi: 10.1016/j.smallrumres.2020.106118
- Russell JB, Wilson DB. 1996. Why are ruminal cellulolytic bacteria unable to digest cellulose at low pH? J Dairy Sci. 79(8):1503–1509. doi: 10.3168/jds.S0022-0302(96)76510-4
- Saleem AM, Zanouny AI, Singer AM. 2017. Growth performance, nutrients digestibility, and blood metabolites of lambs fed diets supplemented with probiotics during pre- and post-weaning period. Asian Australas J Anim Sci. 30(4):523–530. doi: 10.5713/ajas.16.0691
- SAS (Statistical Analysis System). 2008. SAS/STAT 9.2 user’s guide. Cary (NC): SAS Institute Inc.
- Seo JK, Kim SW, Kim MH, Upadhaya SD, Kam DK, Ha JK. 2010. Direct-fed microbials for ruminant animals. Asian Australas J Anim Sci. 23:1657–1667. doi: 10.5713/ajas.2010.r.08
- Soren NM, Tripathi MK, Bhatt RS, Karim SA. 2013. Effect of yeast supplementation on the growth performance of Malpura lambs. Trop Anim Health Prod. 45:547–554. doi: 10.1007/s11250-012-0257-3
- Timmerman HM, Mulder L, Everts H, Van Espen DC, Van Der Wal E, Klaassen G, Rouwers SM, Hartemink R, Rombouts FM, Beynen AC. 2005. Health and growth of veal calves fed milk replacers with or without probiotics. J Dairy Sci. 88(6):2154–2165. doi: 10.3168/jds.S0022-0302(05)72891-5
- Titi HH, Dmour RO, Abdullah AY. 2008. Growth performance and carcass characteristics of Awassi lambs and Shami goat kid culture in their finishing diet. J Anim Sci. 142:375–383.
- Vallejo-Hernández LH, Elghandour MMY, Greiner R, Anele UY, Rivas-Cáceres RR, Barros-Rodríguez M, Salem AZM. 2018. Environmental impact of yeast and exogenous xylanase on mitigating carbon dioxide and enteric methane production in ruminants. J Clean Prod. 189:40–46. doi: 10.1016/j.jclepro.2018.03.310
- Van Soest PV, Robertson JB, Lewis BA. 1991. Methods for dietary fibre, neutral detergent fibre, and non-starch polysaccharides in relation to animal nutrition. J Dairy Sci. 74(10):3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2
- Wallace RJ, Newbold CJ. 1993. Rumen fermentation and its manipulation. The development of yeast culture as feed additives. In: Lyons TP, editor. Biotechnology in the feed Industry. Kentucky: Alltech Technical Publications; p. 173–192.
- Weinberg ZG, Muck RE, Weimer PJ. 2003. The survival of silage inoculant lactic acid bacteria in rumen fluid. J Appl Microbiol. 94(6):1066–1071. doi: 10.1046/j.1365-2672.2003.01942.x
- Whitley NC, Cazac D, Rude BJ, Jackson-O’Brien D, Parveen S. 2009. Use of commercial probiotics supplement in meat goats. J Anim Sci. 87:723–728. doi: 10.2527/jas.2008-1031
- Willms CL, Berger LL, Merchen NR, Fahey GC, Fernando RL. 1991. Effects of increasing crude protein level on nitrogen retention and intestinal supply of amino acids in lambs fed diets based on alkaline hydrogen peroxide-treated wheat straw. J Anim Sci. 69:4939–4950. doi: 10.2527/1991.69124939x
- Yang WZ, Beauchemin KA, Vedres DD, Ghorbani GR, Colombatto D, Morgavi DP. 2004. Effects of direct-fed microbial supplementation on ruminal acidosis, digestibility, and bacterial protein synthesis in continuous culture. Anim Feed Sci Tech. 114:179–193. doi: 10.1016/j.anifeedsci.2003.12.010
