ABSTRACT
Although the diseases of the abdomen in camels (Camelus dromedaries) are frequent than those of other systems, various abdominal disorders are passed misdiagnosed or detected incidentally at postmortem examination. This may be because of the large abdominal circumference in camels, and the nature of subacute abdominal pain even in severe conditions. By abdominal ultrasonography, the veterinarian can scan the rumen, reticulum, omasum, abomasum, and small and large intestines and peritoneum in camels. Samples of peritoneal fluid can also be collected under ultrasound guidance. Sonography has been used recently in camels for scanning of the healthy organs as well as evaluation and determining the diagnosis and prognosis of diseased ones. Examples of diseases evaluated by ultrasonography are pneumonia, pleurisy, splenic abscessation, hepatic disorders, nephrolithiasis, hydronephrosis, pyelonephritis, renal abscessation and renal neoplasms. Of the abdominal disorders diagnosed by ultrasonography are intestinal obstruction, volvulus, intussusception, abdominal effusions, peritonitis, abdominal and pelvic abscessations, chronic enteritis due to paratuberculosis, and neoplasia. In such cases, ultrasonography supplements the clinical and laboratory examinations by providing additional information on abdominal disorders for diagnosis antemortem. This review article is written to describe the results of abdominal ultrasonography in healthy camels as well as in camels with some abdominal disorders.
Introduction
In dromedary camels, diseases of the abdomen are frequent than disorders of the cardiopulmonary, musculoskeletal, nervous, hepatic and renal systems. However, because of the large abdominal circumference in camels, and subacute abdominal pain even in advanced conditions, various abdominal disorders are passed misdiagnosed or detected incidentally at postmortem examination. Ultrasonography of the abdomen is applicable in camel medicine and provides valuable diagnostic information on various abdominal affections (Tharwat et al. Citation2012a, Citation2012b; Tharwat Citation2013; Tharwat and Al-Sobayil Citation2016; Tharwat et al. Citation2018; Tharwat Citation2019a, Citation2019b). The procedure supplements the clinical and laboratory examinations by providing additional information on abdominal disorders for diagnosis of such affections antemortem. This review article was written to describe the results of abdominal ultrasonography in healthy camels as well as in camels with some abdominal disorders.
Anatomical background of the stomach and intestines in camels
There are many reports on the subdivisions of the stomach of the camel. From the nineteenth century, descriptions of the stomach in camel have reflected those of cow and sheep. Some investigations regard the camel as a monogastric animal or divide the stomach of the camel into two, three, and four stomachs (Boas Citation1890; Hegazi Citation1950; Hansen and Schmidt-Nielsen Citation1957; Purohit and Rathor Citation1962; Smuts and Bezuidenhout Citation1987; Wang et al. Citation2000). The camel stomach has additional glandular sacs that may have special digestive functions (Wang et al. Citation2000). Although they differ greatly in shape and structure from the typical design encountered in the cow and sheep, in this review we used the same nomenclature as in the cow and sheep as every part of the stomach appeared different by ultrasound examination so was not possible to merge stomach compartments into two or three parts (Tharwat et al. Citation2012c).
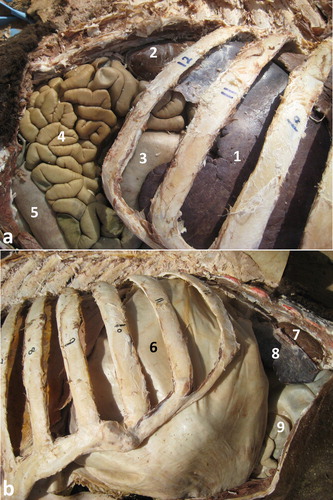
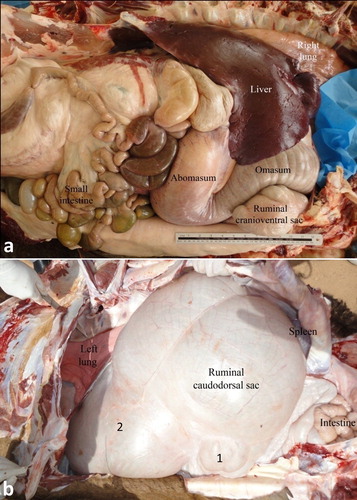
The relative position of the four compartments is such that the rumen lies mainly on the left, the reticulum on the right, the omasum cranially and on the right, and the abomasum on the right side. The rumen occupies the major portion of the abdominal cavity. It extends from the diaphragm to the pelvic inlet, filling the left half of the abdominal cavity. The rumen is divided into a relatively small cranioventral and a large caudodorsal sac; each has its own glandular sac. The caudodorsal sac is large and balloon-like when viewed from the left. The spleen is attached to the caudodorsal surface caudally. The reticulum is situated to the right and cranioventrally to the cardia. Its initial part lies directly cranially to the glandular sac of the caudodorsal compartment. It is bean-shaped, with its greater curvature directed ventrally, while its lesser curvature is attached to the liver by means of the lesser omentum. The omasum is long and sausage-shaped lying cranially below the cardia on the dorsal surface of the cranioventral rumen sac. The abomasum is a relatively short compartment. The transition between omasum and abomasum is not obvious externally (Smuts and Bezuidenhout Citation1987; Tharwat et al. Citation2012c) ().
Figure 1. Anatomical position of the viscera in a camel at postmortem examination. Image (a) shows a right-sided view of the liver, right lung, cranioventral ruminal sac, omasum, abomasum and intestines. Image (b) shows a left-sided view for the left lung, rumen, spleen and intestines. 1 = glandular sac of the caudodorsal ruminal sac; 2 = cranioventral ruminal sac.
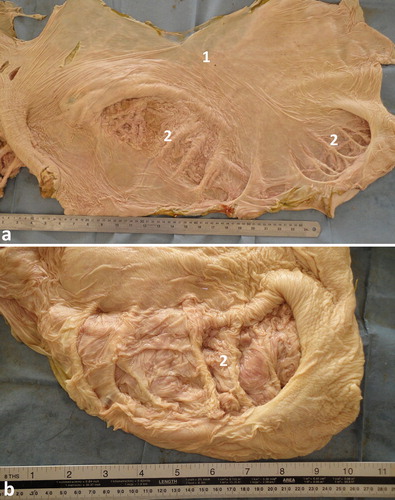
The mucus membrane of the rumen is smooth, unlike that of the other ruminants. Towards the floor of the large caudodorsal sac flat folds are present. A prominent longitudinal muscular pillar is present on the right side. Nine smaller pillars are present at right angles and dorsally to the longitudinal pillar. They in turn are interconnected by muscular folds enclosing square spaces which are 20–30 mm deep. The latter are subdivided by mucus membrane folds to form smaller squares or cells which are lined with glandular mucosa containing tubular glands. This complex of glandular cell units is referred as a glandular sac, formerly named as a water sac (Smuts and Bezuidenhout Citation1987; Tharwat et al. Citation2012c) ().
Figure 2. Anatomy of the interior of the rumen with smooth mucus membrane (a). Image (b) shows a close-up view for the ruminal glandular sacs. 1 = smooth ruminal mucosa; 2 = glandular sacs.
The interior of the reticulum is characterized by a powerful and complex system of radiating pillars which are interconnected by a lattice-work of slender pillars. It resembles the adjoining glandular sac of the caudodorsal rumen sac. An incomplete sphincter marks the transition of reticulum to omasum. The omasum is a long sausage-shaped organ looks very different from the omasum of the other domestic ruminants. Its mucus membrane is arranged in approximately 50 longitudinal folds of up to 20 mm high. Numerous small oblique folds occur in the floor between the longitudinal folds. There is an abrupt change in the appearance of the mucus membrane at the omasoabomasal junction. The mucus membrane of the fundic region of the abomasum is thrown into convoluted folds parallel to the greater curvature, while the pyloric part has a smooth surface. The two parts communicate through a crescent-shaped arch (Smuts and Bezuidenhout Citation1987; Tharwat et al. Citation2012c) ().
Figure 3. Anatomy of the interior of the reticulum, omasum and abomasum (a). Image (b) shows a close-up view for the rumen, reticulum and abomasum. The interior of the reticulum is characterized by a powerful and complex system of radiating pillars which are interconnected by a lattice-work of slender pillars (1). The omasum is a long sausage-shaped organ with a mucus membrane arranged in approximately 50 longitudinal folds of up to 20 mm high (2). The mucus membrane of the fundic region of the abomasum is thrown into convoluted folds parallel to the greater curvature, while the pyloric part has a smooth surface (3).
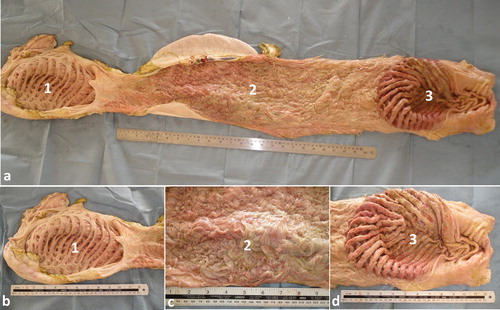
The total length of the small intestine averages 40 m while the large intestine is approximately 19.5 m. The duodenum begins at the pylorus which is situated below the tenth costochondral junction. The cranial part of the duodenum lies against the visceral surface of the liver. It consists of a prominent Ampulla duodeni followed by a slender Ansa sigmoidea which is directed craniodorsally. The convoluted jejunum is placed mainly in the right flank and abdominal region, and on the sternum in the median plane. Its terminal portion is placed on the left of the median plane. A short peritoneal fold, the Plica ileocecalis, attaches it to the lesser curvature of the cecum and marks the beginning of the ileum. The short ileum ends at the ileal orifice which demarcates the cecocolic junction. The latter is on the right of the midline, on the level of the caudal pole of the left kidney. The cecum and the initial part of the colon are much larger in diameter than the small intestine. The cecum is a slightly S-shaped blind tube which is situated chiefly in the right flank medial to the left kidney. The ascending colon is arranged in a flat spiral, the transverse colon is short and the descending colon has a sigmoid flexure in its initial part. The initial part of the ascending colon passes cranially along the ventral surface of the left kidney and to the right of the rumen (Smuts and Bezuidenhout Citation1987; Tharwat et al. Citation2012c) ().
Ultrasonography of the stomach and intestines in camels and normal findings
For abdominal ultrasonography of the camel, it should be firstly pushed to lie down in a sternal recumbency position. The fleece should be clipped and the skin was shaved on both sides of the body. If the camel is irritable, a slight sedation using xylazine (0.2 mg/kg) may be administered intravenously. A 2.5 to 3.5 MHz sector transducer is sufficient for abdominal imaging. The viscera are then scanned transcutaneously from caudal to cranial beginning at the caudal abdomen and extending forward to the level of the sternal pad.
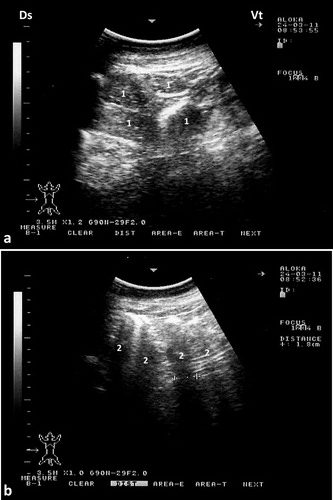
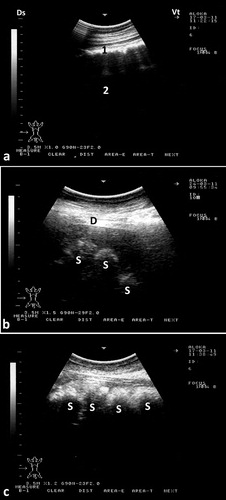
The rumen and its glandular sacs are always imaged occupying most of the abdominal cavity, filling most of the left side of the abdomen. Its wall appears smooth and echogenic and the contents are anechoic because of reflection of the ultrasound waves by gas contents. Reverberation artifacts running parallel to the ruminal wall were seen in the region of the dorsal gas cap. Motility of the rumen is usually seen as shifting, retreating, and eventual replacement of portions of the wall during gastric contraction cycles. In most camels, the cranial glandular sacs are usually imaged deep in the right and left 5th intercostal spaces. The caudal glandular sacs are best seen in the 9th intercostal space with its ventral parts imaged lying against the paramedian region 10 cm to the right of the umbilicus. The glandular sacs are visualized as series of hyperechoic, semicircular protrusions, curving away from the ventral body wall (Tharwat et al. Citation2012c) ().
Figure 5. Ultrasonograms of the rumen and glandular sacs in a healthy camel. The rumen wall is smooth and echogenic and the contents could not be seen because of reflection of the ultrasound waves by gas contents (a). Image was taken from the upper left 10th intercostal space. Image (b) shows the glandular sacs of the cranioventral ruminal sac at the level of the 5th intercostal space on the left side. Image (c) shows the glandular sacs of the caudodorsal ruminal 10 cm cranial to the umbilicus. 1 = Ruminal wall; 2 = Rumen; D = diaphragm; S = glandular sacs; Ds = dorsal; Vt = ventral.
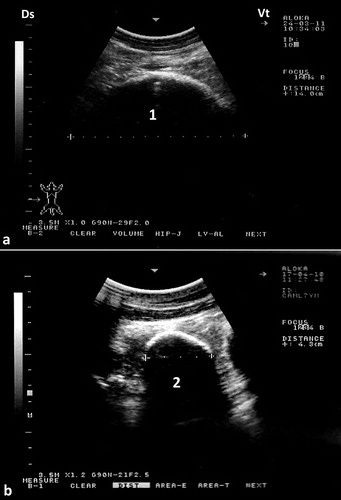
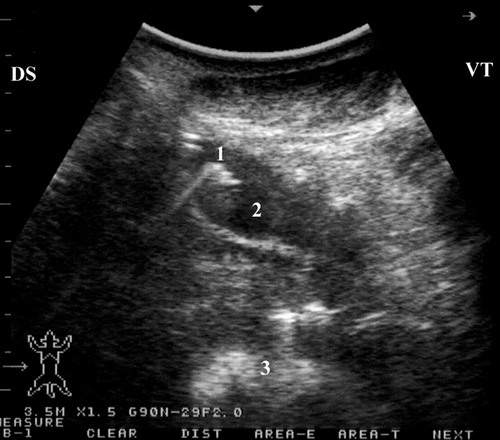
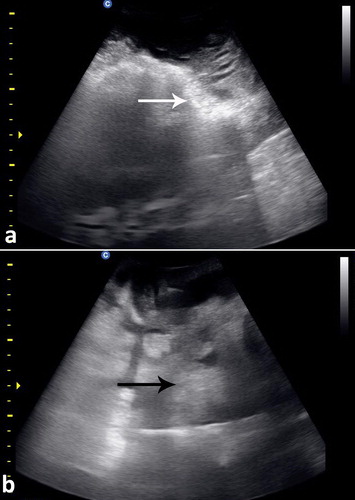
To best image the reticulum, the camel should be placed in a lateral recumbency and the transducer is directed forward in a 30° angle perpendicular to the animal ventrum. The ventral part of the reticulum is best imaged in most camels from the left and right paramedian region just behind the sternal pad. The reticulum has a thick wall with a half-moon-appearance with an even contour. It has a biphasic contraction; the first is incomplete and is followed by an interval of incomplete relaxation; this was followed immediately by a second complete contraction (Tharwat et al. Citation2012c) (). The omasum is imaged in most camels at the level of the right 6th to 8th intercostal spaces. It is visible as a tubular structure extending between these intercostal spaces and coursing along the body wall approximately parallel to the long axis of the camel. Its wall appears as a thick echogenic line and its content are difficult to be seen. The omasal folds appear as fine and smooth moderately echogenic bands. The abomasum is best detected from the right 8th and 9th intercostal spaces. Compared to the omasal folds, the abomasal folds appear as coarse, thick and highly echogenic bands (Tharwat et al. Citation2012c) ().
Figure 6. Ultrasonograms of the reticulum in a healthy camel. It appeared as a half-moon-shaped structure with an even contour. Images were taken at the level of the right paramedian region just behind the sternal pad. Image (a) was taken during reticular relaxation while image (b) was taken during reticular contraction. 1 = diaphragm; 2 = Reticulum; 3 = Ruminal sac; 4 = free peritoneal fluid; Ds = dorsal; Vt = ventral.
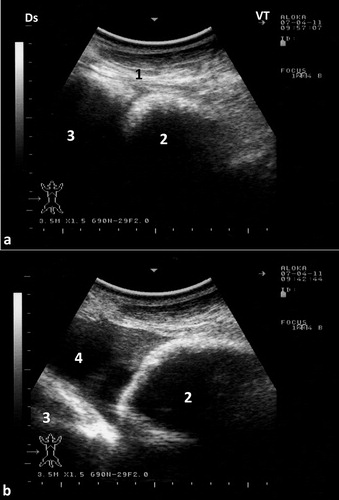
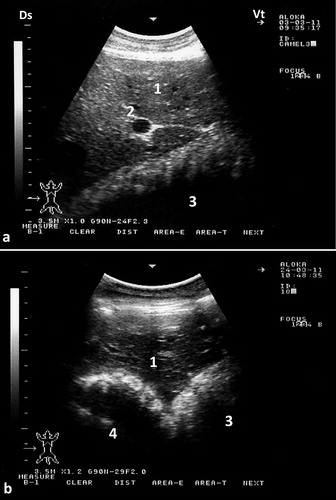
Figure 7. Ultrasonograms of the omasum and abomasum in a healthy camel. Image (a) shows the omasum and image (b) shows the abomasum at the level of the right 6th and 9th intercostal spaces, respectively. The omasal folds in image (a) appeared as fine and smooth moderately echogenic bands, while the abomasal folds in image (b) appeared as coarse and thick highly echogenic bands. 1 = liver; 2 = portal vein; 3 = omasum; 4 = abomasum. Ds = dorsal; Vt = ventral.
The small intestine is best visualized in the lower part of the right flank. Its contents appear heterogeneous and it contracts every few seconds. Boluses of hypoechoic ingesta can be imaged few seconds before the intestine contracts. Individual segments of intestine are difficult to discern in areas of collapsed intestine because of the lack of contrast between wall and lumen. Because of the absence of the gallbladder in camels, it is very difficult to identify and image the duodenum in camels. Therefore, the duodenum, jejunum and ileum cannot be differentiated by ultrasound. The small intestine can be imaged in cross sections and longitudinally (Tharwat et al. Citation2012c) (). The large intestine is easy to differentiate from the small intestine based on its marked gas content and relatively large diameter. Because of the gas, only the wall of the large intestine close to the transducer is imaged where it appeared as a thick echogenic line. The cecum is imaged chiefly in the caudal right flank. Its lateral wall appears as a thick echoic, crescent-shaped line. Owing to the presence of gas, the content of the cecum cannot be imaged. Segments of the colon can be seen in the anterior part of the right flank; its wall appears as thick and echogenic line (Tharwat et al. Citation2012c) ().
Ultrasound-guided collection of peritoneal fluid in camels
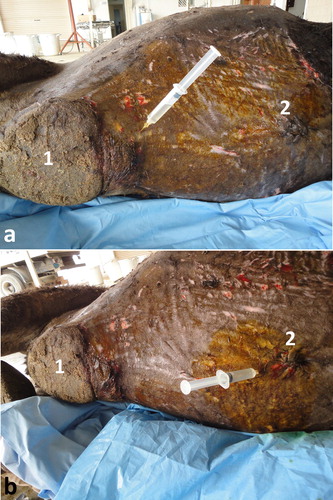
Collection of peritoneal fluid from camels is usually carried out in a lateral recumbency position after sedation using xylazine intravenously (0.1 mg/kg BW). Two sites (10 cm cranial to the umbilicus and 10 cm behind the sternal pad) are the best sites for ultrasound-guided abdominocentesis (). Fluid can be seen in the triangular space between the dorsal ruminal sac and reticulum. It is therefore best visualized during reticular contraction. A 14G × 200 mm spinal needle with a stylet is advanced till visualized within the peritoneal fluid (). The needle is maintained parallel to the plane, and is advanced using an ultrasound-guided, free-hand technique. When the needle is considered to be in the correct position, the internal needle is removed and peritoneal fluid is withdrawn (Tharwat et al. Citation2013).
Application of diagnostic ultrasonography in abdominal disorders
Intestinal obstruction
In camels, intestinal obstruction may be caused by wool balls, massive intestinal parasitic infestations and foreign bodies. Intestinal compression may be occurred externally by an enlarged lymph node, abdominal and pelvic abscessations and abdominal neoplasia (Ramadan et al. Citation2008; Tharwat et al. Citation2012a). Even though, the condition is rare in camels (Köhler-Rollefson et al. Citation2001; Fowler Citation2010). The most common cause of intestinal obstruction in camels is the wool balls that are formed in camels having pica and eating hair, leading to the formation of phytobezoars and trichobezoars that may reach the intestine causing obstruction (Tanwar Citation1985; Tharwat et al. Citation2012a). Other causes of intestinal obstruction include impaction of the spiral colon, dilatation and torsion of the cecum and strangulation of the intestine in inguinal hernia (Fowler Citation2010).
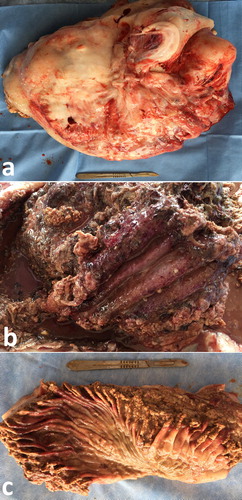
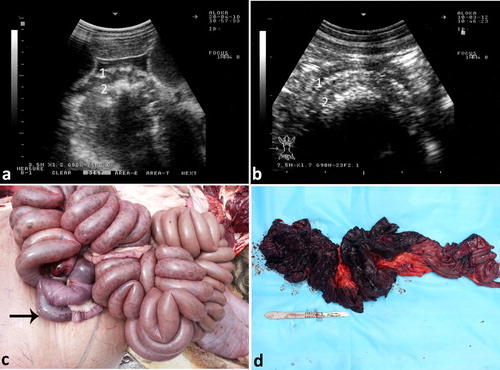
Ultrasonographic findings in camels with intestinal obstruction may include distended intestinal loops and reduced or absent motility. Hyperechoic material consistent with wool balls may be imaged within the intestinal lumen that can be detected at postmortem examination (). Volvulus of the small intestine may occur in young or adult camels, with or without the cecum and spiral colon, or of only the distal third of the jejunum and the proximal portion of the ileum about its mesenteric axis. The volvulus results in intestinal distension, abdominal distension, vascular compromise, intestinal necrosis and eventually death unless surgically corrected (Fowler Citation2010). Ultrasonographic findings include intestinal dilatations, reduced or absent motility, and in advanced cases, hypoechoic fluid with fibrin within the intestinal loops (Tharwat et al. Citation2012a) (). Intussusception may also occur in camels through the invagination of one portion of the intestine into the lumen of an adjacent segment of intestine. As in cattle, jejuno-jejunal intussusception is the most common form in camels (). The contents of rumen can be imaged in camels with depraved appetite due to eating of sand. The sand particles in the latter cases appear hyperechoic that can be detected postmortem ().
Figure 12. Ultrasonograms of intestinal obstruction in 2 camels due to foreign bodies (wool balls; FB) within the intestinal lumen (a & b). Image (c) shows a wool ball within the intestinal lumen detected at postmortem examination (white arrow). Image (d) shows multiple wool balls and other foreign bodies removed from the rumen during rumenotomy. FB = foreign body; J = jejunum.
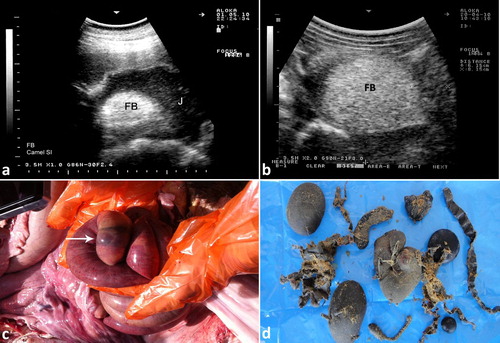
Figure 13. Intestinal volvulus in a female camel showing bilateral abdominal distension (a). Image (b) shows dilated intestinal loops with markedly reduced motility. Image (c) shows mesenteric torsion. 1 = distended intestinal loops; 2 = shows site of volvulus.
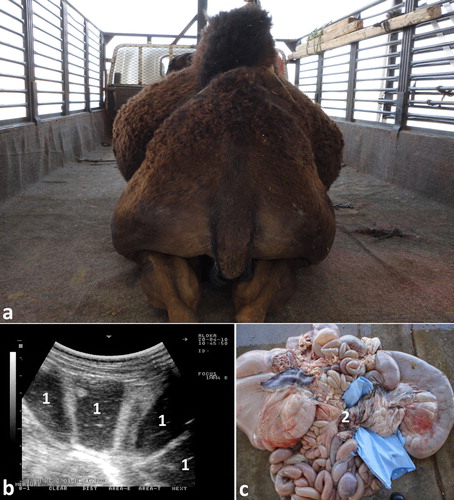
Figure 14. Ultrasonograms of intestinal intussusception in 2 camels (a & b). Image (c) shows a jejuno-jejunal intussusception in a female camel detected at postmortem examination (black arrow). Image (d) shows severe intestinal hemorrhage after jejunal separation of image (c). 1 = intussuscipiens; 2 = intussusceptum.
Abdominal effusions and chronic peritonitis
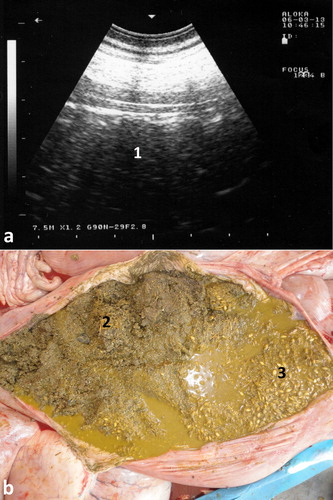
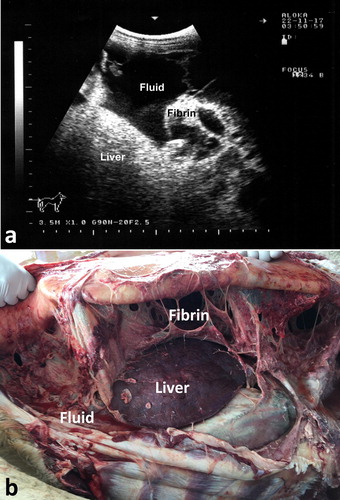
Abdominal effusions are common presentations in camels. The condition may occur due to trypanosomiasis, cardiomyopathy, hypoprotenemia, hepatic and renal diseases, septic peritonitis and diffuse malignant neoplasia (Köhler-Rollefson et al. Citation2001; Fowler Citation2010; Tharwat et al. Citation2012a; Tharwat Citation2013, Citation2019a). In camels with trypanosomiasis, ultrasonography of the abdomen confirms the presence of free abdominal fluid. However, non-inflammatory ascites due to other disorders, such as right-sided cardiac insufficiency, hepatic and renal disorders, and chronic parasitism may cause abdominal effusions. Ultrasonographic findings include accumulation of massive amounts of abdominal fluid, where liver and intestines are floating in a hypoechoic fluid (Tharwat et al. Citation2012a) (). In camels with septic peritonitis, transabdominal ultrasonography reveals bilateral echogenic fibrin threads floating in a hyperechoic peritoneal effusion. Fibrinous peritonitis are usually seen at the sonogram as heterogeneous deposits of echogenic and anechoic materials between the intestines, liver, kidneys rumen, spleen and abdominal wall. Peritoneal effusion appears as echogenic areas of fibrinous tissue deposits interspersed with hypoechogenic areas of fluid pockets (Tharwat Citation2019a) ().
Figure 16. Severe abdominal distension in a camel with trypanosomiasis (a). Images b & c show ultrasonograms with anechoic abdominal effusions where the liver and intestines are floating. Ds = dorsal; Vt = ventral.
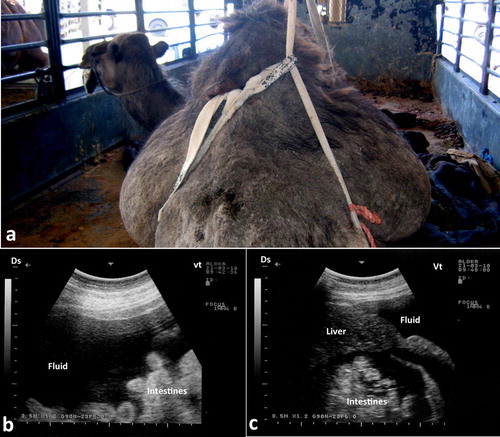
Figure 17. Chronic peritonitis in a female camel. Fibrin and hyperechoic fluid are imaged within the peritoneum exudation (a). Image (b) shows necropsy findings where massive fibrin network and adhesion of the viscera together and to the abdominal wall together with serosanguineous abdominal exudate were detected.
Abdominal and pelvic abscessations
Abdominal and pelvic abscessations may be found in camels as a result of septic infections. The presentation my include symptoms of intestinal obstruction, anuria, difficult defecation and straining. Transabdominal ultrasonography usually verifies the condition. Results of ultrasonographic examination include abdominal or pelvic echogenic mass. Ultrasound guided confirmation should be carried out through aspiration of pus. If the lesion is detected in the pelvic cavity, it may constrict the orifice of the urinary bladder leading to urine accumulation. The bladder in such cases is imaged with echogenic sediments (). The lesion can compress the uterine body or ureter leading to unilateral hydronephrosis (Tharwat and Al-Sobayil Citation2016) ().
Figure 18. Pelvic abscessation in a male camel. Image with hyperechoic pattern was taken transabdominal (a). Two litres of pus was aspirated under ultrasound-guidance. Image (b) shows distended urinary bladder (UB) with hyperechogenic sedimentation.
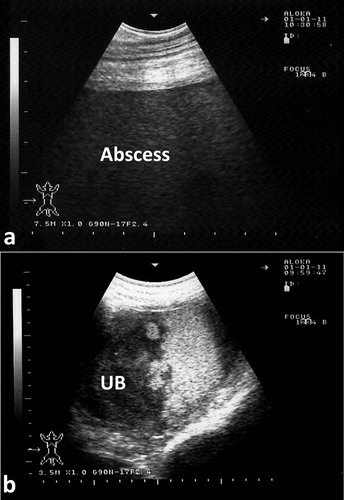
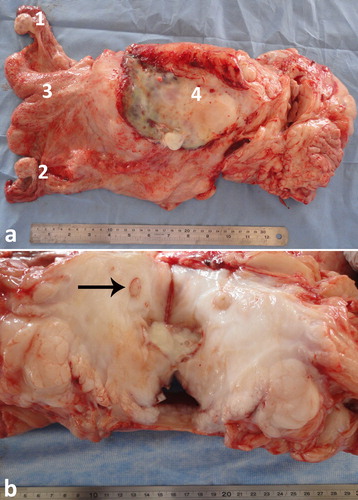
Figure 19. Necropsy findings in a female camel with difficult defecation and straining. Transrectal ultrasonography showed a pelvic mass. Necropsy was performed where abscess was detected near the uterus (a), compressing the left ureter (black arrow; b) and leading to hydronephrosis of the left kidney. Other lesions were fibrinous peritonitis and pelvic adhesions. 1 = left ovary; 2 = right ovary; 3 = uterine body; 4 = abscess.
Chronic enteritis due to paratuberculosis (Johne’s disease)
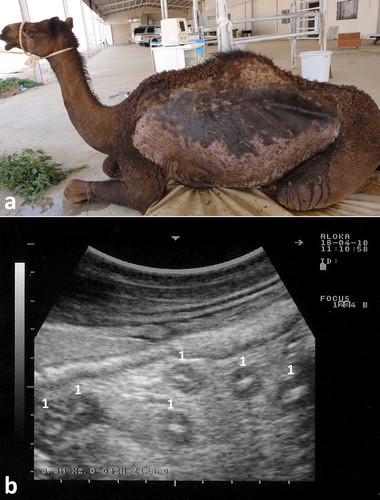
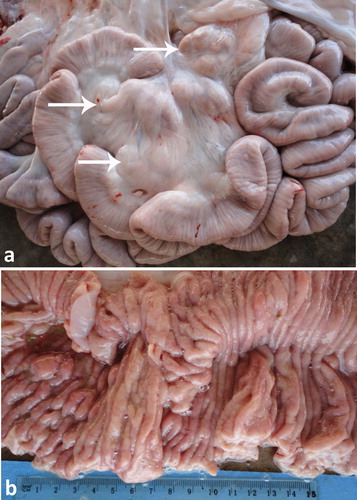
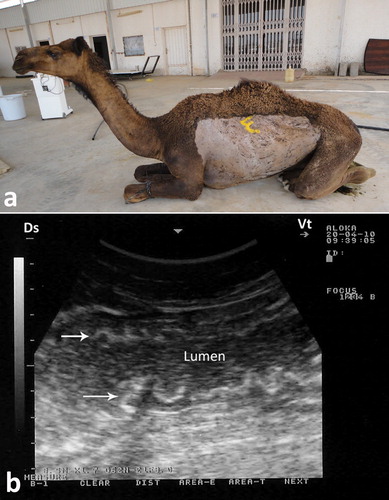
In camels, paratuberculosis or Johne’s disease is a chronic wasting and fatal disease manifested clinically by persistent diarrhea that terminates in death. The causative organism, Mycobacterium avium subsp. paratuberculosis (MAP), may be a cause of Crohn’s disease in human (Stott et al. Citation2005; Chamberlin and Naser Citation2006). Therefore, strict isolation and eradication of infected animals is crucial. Through ultrasonography, the veterinarian can identify suspected camels until the results of PCR appear. Results of ultrasonography in camels with paratuberculosis include thickened and corrugation of the small intestinal walls (). The most outstanding sonographic findings in camels with Johne’s disease are the visible enlargement of mesenteric lymph nodes. Enlarged lymph nodes has either echogenic or anechoic capsule, and with anechoic, echogenic or heterogeneous contents (). Clumps of echogenic tissue interspersed with fluid pockets may also be imaged between the intestinal loops. Other findings include an overall increased brightness of hepatic ultrasonogram, pericardial and pleural effusion. Thickening of intestinal walls and corrugation, and enlargement of the mesenteric lymph nodes are confirmed at postmortem examination (Tharwat et al. Citation2012b) ().
Figure 20. Paratuberculosis in a female camel with progressive weight loss (a). Image (b) shows corrugation of the intestinal mucosa (white arrows).
Gastrointestinal neoplasia
The most common tumours in dromedary camels are squamous cell carcinoma, fibroma, adenocarcinoma, fibromyxosarcoma, leiomyoma, angiosarcoma, schwannoma, lipoma, microcystic adnexal carcinoma, renal cell carcinoma, sertoli-leydig cell tumour and granulosa cell tumour (Tharwat et al. Citation2017; Al-Sobayil et al. Citation2018; Ali et al. Citation2018; Tharwat et al. Citation2018). In a case of gastric adenocarcinoma, transabdominal ultrasonographic examination revealed severely thickened and corrugated omaso-abomasal walls with heterogenous contents (Tharwat et al. Citation2018) (). In the latter case, the abomasum was enlarged at postmortem examination, its wall was thickened and hard and its contents were bloody and malodorous. The abomasal and omasal folds were thickened, congested and ulcerated (Tharwat et al. Citation2018) ().
Acknowledgements
The author would like to express his deep gratitude to Dr N. Peacy, former English professor, Qassim University for language revising.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- Al-Sobayil F, Ali A, Derar D, Tharwat M, Ahmed F, Khodeir M. 2018. Tumors in dromedary camels: prevalence, types and locations. J Camel Pract Res. 25:189–197. doi:10.5958/2277-8934.2018.00026.7.
- Ali A, Derar D, Al-Sobayil F, Tharwat M, Ahmed F, Khodeir M. 2018. Adenocarcinoma in the genital tract of female dromedary camels. J Camel Pract Res. 25:181–187. doi:10.5958/2277-8934.2018.00025.5.
- Boas JEV. 1890. On the morphology of the stomachs of the Camelidae and the Tragulidae; and on the bearing on systematic position. J Morphol. 16:494–524.
- Chamberlin WM, Naser SA. 2006. Integrating theories of the etiology of Crohn’s disease. On the etiology of Crohn’s disease: questioning the hypotheses. Med Sci Monitor. 12:RA27–RA33.
- Fowler ME. 2010. Digestive system. In: Medicine and surgery of camelids. 3rd ed. Ames: Blackwell Publishing; p. 351–402.
- Hansen A, Schmidt-Nielsen K. 1957. On the stomach of the camel with special reference to the structure of its mucous membrane. Acta Anat. 31:353–375. doi: 10.1159/000141291
- Hegazi AH. 1950. The stomach of the camel. Br Vet J. 106:209–213. doi: 10.1016/S0007-1935(17)52827-4
- Köhler-Rollefson I, Mundy P, Mathias E. 2001. A field manual of camel diseases: traditional and modern healthcare for the dromedary. London: ITDG publishing.
- Purohit MS, Rathor SS. 1962. Stomach of the camel in comparison to that of the ox. Indian Vet J. 39:604–608.
- Ramadan RO, Abdin-Bey MR, Mohamed GE. 2008. Intestinal obstruction in camels (Camelus dromedaries). J Camel Pract Res. 15:71–75.
- Smuts MMS, Bezuidenhout AJ. 1987. The viscera. In: Anatomy of the dromedary. Oxford: Clarendon press; p. 105–141.
- Stott AW, Jones GM, Humphry RW, Gunn GJ. 2005. Financial incentive to control paratuberculosis (Johne’s disease) on dairy farms in the United Kingdom. Vet Rec. 156:825–831. doi:10.1136/vr.156.26.825.
- Tanwar RK. 1985. Intestinal obstruction due to phytobenzoars in a camel- a case report. Indian J Vet Med. 5:31–32.
- Tharwat M. 2013. Ultrasonographic findings in camels (Camelus dromedarius) with trypanosomiasis. J Camel Pract Res. 20:283–287.
- Tharwat M. 2019a. Chronic peritonitis in dromedary camels: clinical, hematobiochemical, ultrasonographic and pathologic findings. J Camel Pract Res. 26:169–172. doi:10.5958/2277-8934.2019.00026.2.
- Tharwat M. 2019b. Multiple splenic abscessations in a camel: case report. J Camel Pract Res. 26:273–276. doi:10.5958/2277-8934.2019.00044.4.
- Tharwat M, Al-Sobayil F. 2016. Ultrasonographic findings in camels (Camelus dromedarius) with abdominal disorders. J Camel Pract Res. 23:291–299. doi:10.5958/2277-8934.2016.00049.7.
- Tharwat M, Al-Sobayil F, Ali A, Buczinski S. 2012a. Ultrasonographic evaluation of abdominal distension in 52 camels (Camelus dromedarius). Res Vet Sci. 93:448–456. doi:10.1016/j.rvsc.2011.07.009.
- Tharwat M, Al-Sobayil F, Ali A, Buczinski S. 2012c. Transabdominal ultrasonographic appearance of the gastrointestinal viscera of healthy camels (Camelus dromedaries). Res Vet Sci. 93:1015–1020. doi:10.1016/j.rvsc.2011.12.003.
- Tharwat M, Al-Sobayil F, Ali A, Derar D, Khodeir M. 2017. Renal cell carcinoma in a female Arabian camel: clinical, hematobiochemical, ultrasonographic and pathologic findings. J Camel Pract Res. 24:61–66. doi:10.5958/2277-8934.2017.00009.1.
- Tharwat M, Al-Sobayil F, Ali A, Hashad M, Buczinski S. 2012b. Clinical, ultrasonographic, and pathologic findings in 70 camels (Camelus dromedarius) with Johne’s disease. Can Vet J. 53:543–548.
- Tharwat M, Ali A, Al-Sobayil F, Buczinski S. 2013. Ultrasound-guided collection of peritoneal fluid in healthy camels (Camelus dromedarius) and its biochemical analysis. Small Rum Res. 113:307–311. doi:10.1016/j.smallrumres.2013.04.002.
- Tharwat M, El-Shafaey E, Sadan M, Ali A, Al-Sobayil F, Al-Hawas A. 2018. Omaso-abomasal adenocarcinoma in a female Arabian camel (Camelus dromedarius). J Applied Anim Res. 46:1268–1271. doi:10.1080/09712119.2018.1495644.
- Wang JL, Lan G, Wang GX, Li HY, Xie ZM. 2000. Anatomical subdivisions of the stomach of the Bactrian camel (Camelus bactrianus). J Morphol. 245:161–167. doi:10.1002/1097-4687(200008)245:2<161 doi: 10.1002/1097-4687(200008)245:2<161::AID-JMOR6>3.0.CO;2-B