ABSTRACT
The aim of this study was to determine the semen quality, sexual behaviour and testosterone (T) levels in Saint Croix rams under semi-desert climate conditions. Sixteen rams (70.6 ± 12.5 kg; 24.1 ± 0.3 months old) were randomly divided into a grazing group (GG, n = 8) and a supplemented group (supplemented individually with 1 kg.d−1 of concentrate (156 g CP.kg−1 DM and 2.5 Mcal EM.kg−1 DM). SG, n = 8). Each group grazed on buffel, grass. Every two weeks, semen was collected, and sexual behaviour traits were evaluated. SG rams had greater body weight (P < 0.0001) and greater semen quality than the GG rams. SG rams had greater values for reaction time and lateral approaches than GG rams (P < 0.0001). No interaction was found between treatment and month for T concentration (P < 0.05); the SG rams had greater values during most of the months, but GG rams had greater values during September. Month had a significant effect on T concentration (P < 0.05) with the highest values in autumn. It can be concluded that Saint Croix rams are able to adapt to adverse environmental conditions and that supplementary feed prior to the mating season improves ram sexual behaviour.
1. Introduction
Sheep breeds originating from temperate climates in latitudes greater than 35° show a marked seasonality of breeding activity. Females exhibit oestrous primarily during autumn, whereas the males are able of breeding all year round, but their sexual activity and sperm production is greater during the breeding season (Lincoln and Davidson Citation1977; Ortavant et al. Citation1988; Rosa and Bryant Citation2003). The annual cycle of daily photoperiod has been identified as the determinant factor of this phenomenon, while environmental temperature, nutritional status, social interactions, lambing date and lactation period are considered modulate of the seasonal breeding (Rosa and Bryant Citation2003). On the other hand, hair sheep, breeds originated in the tropics, have been reported to be almost or completely unseasonal (Rosa and Bryant Citation2003; Arroyo et al. Citation2007). Studies on the sexual behaviour of hair sheep rams under tropical conditions are scarce in the literature, but the study of Godfrey et al. (Citation1998) suggest that the sexual behaviour of hair sheep rams is not affected by elevated ambient temperatures.
In hair rams bred at latitudes between 20 and 30° fertility is more associated with feeding level, ambient temperature, and yearly distribution of rainfall, rather than the photoperiod (Kafi et al. Citation2004; Zhang et al. Citation2005; Alexander et al. Citation2012).
The Northeast of Mexico, located at 25° N latitude and 100° W longitude, is known for its hot summers and low humidity, and dry and extreme winters (Sánchez-Dávila et al. Citation2011). Under these extreme conditions, intense thermoregulation activity in the animal is required to keep homeostasis (Martin et al. Citation2010; De et al. Citation2017). Heat stress reduces the reproductive capacity of rams with a negative impact on flock fertility (Oliveira et al. Citation2014), directly affecting spermatogenesis, semen quality and quantity, and sexual behaviour (Cárdenas-Gallegos et al. Citation2015). In extensively reared hair sheep production systems, natural mating is usually performed. The ram reproductive efficiency could be evaluated by semen quality (Hötzel et al. Citation1998; Joshi et al. Citation2003), although an acceptable level of libido and sexual behaviour could be exhibited under variable temperature and environmental conditions throughout the year (Maurya et al. Citation2010). According to Maurya et al. (Citation2016) reproductive characteristics may be affected by genetic and environmental factors. Considering that the metabolic state of each male will depend on the quantity and quality of food consumed, which will influence the reproductive capacity (Zhang et al., Citation2005). In multiple mammal species, the increase in food consumption will improve the production and seminal quality (Maurya et al., Citation2016). However, few studies have been conducted on reproductive behaviour of hair sheep breeds, such as Saint Croix, in semi-desertic areas, where there are variations in forage quality during the months of the year. In addition, there are high temperatures and dry periods, which can affect the reproductive performances (Marai et al. Citation2006). Therefore, the objective of this study was to evaluate the effect of feed supplementation on semen quality, sexual behaviour traits and testosterone (T) blood concentration during the year in adult Saint Croix rams.
2. Materials and methods
2.1. Experimental site description
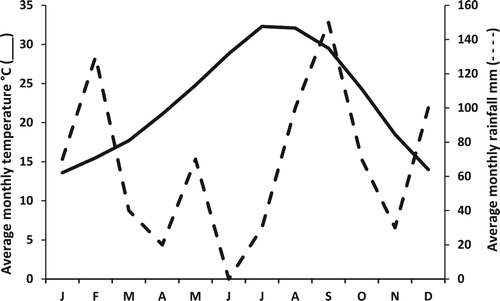
This research was carried out from January to December 2016 at the Laboratorio de Reproducción Animal of the Unidad Académica Marín, Facultad de Agronomía of the Universidad Autónoma de Nuevo León, located in Marin, Nuevo Leon, Mexico, at 25° 50′ 34″ N latitude and 100° 04′ 21″ W longitude and at an altitude of 333 m above sea level. Temperatures range from 18°C to 43°C in summer and −2°C to 10°C in the winter. The average annual temperature is 23.1°C with an average annual rainfall of 70 mm ().
2.2. Animal management
Saint Croix adult’s rams (n = 16; 70.6 ± 12.5 kg; 24.1 ± 0.3 months old) were used with the approval of the Bioethics Committee and Animal Welfare of the Faculty of Veterinary Medicine of Universidad Autonoma de Nuevo León (UANL-Act No. 8). The rams were held in a paddock with an access to a buffel grass (Cenchrus ciliaris) pasture. The field was divided into 6 sub-areas of 200 m2 each. Rotational grazing was carried out with access of 8 h/day in each sub-area, moving the rams according to the availability of forage.
The sixteen rams were divided with similar body weight (BW, 67.2 ± 1.9) and body condition (BC, 2.9 ± 0.08) into two groups of eight animals per group. Grazing group (GG) rams grazed buffel grass pasture only. The supplemented group (GS) grazed the same grass and were additionally supplemented individually with 1 kg.d−1 of concentrate (156 g CP.kg−1 DM and 2.5 Mcal EM.kg−1 DM). Supplemental feed was provided individually in the morning, before the rams went out to grazing. Water and sheep mineral blocks were available ad libitum (8% of phosphorus and 6% of calcium). Ambient temperature (°C) and precipitations (mm) were recorded daily (). There was an adaptation period of 45 days before starting the data collection of each of the rams used in the present study. The breeding season started in June 2014.
2.3. Body weight, scrotal circumference and body condition
The body weight (BW) of each ram was recorded every two weeks using a scale with sensitivity of 0.25 kg (Torrey, Monterrey, Mexico). At the same time, the scrotal circumference (SC) was measured with a metal tape orchidometer (Trueman, Mgf, USA) displacing both testicles to the bottom of the scrotum and then measuring the middle part. The body condition (BC) was evaluated according to criteria described by Kenyon et al. (Citation2014), where 0 was thin and 5 was obese.
2.4. Sexual behaviour traits
Every 2 weeks the rams were evaluated for the degree of libido, using two oestrogenised ewes of the same breed applying 0.5 mg of oestradiol benzoate (Syntex, Virbac, Mexico) for three days prior to the assessment of sexual behaviour traits. A female was presented individually to each ram for a period of 20 min in a 4 m2 pen and the following traits were assessed: Reaction time, number of lateral approaches, anus-genital sniffing’s, flehmen, mount attempts, mounts, mounts with ejaculation, and time to the 1st mount with ejaculate.
To avoid biases due to the effect of dominance between rams. the corral where the sexual behaviour was determined, was isolated and the remaining rams had no visual contact with the ram that was being evaluated. All the assessments were done according to Orihuela Trujillo (Citation2014).
2.5. Semen evaluation
Semen for evaluation was collected from each ram by artificial vagina every 2 weeks, always the day before the assessment of sexual traits. All variables were evaluated based on the technique described by Evans and Maxwell (Citation1990). Each ram was exposed to an oestrogenised ewe and a sample from the semen collected was stored at 37°C to evaluate the following variables: volume, which was measured directly in a graduated conical tube; mass motility, rated from 0 to 5; percentage of progressive motility (PM); semen concentration, which was calculated by multiplying the sperm concentration by the ejaculate volume. To evaluate the seminal quality, was used with a phase-contrast microscope 10X (Primo Star, Zeiss, Minitube America, Wi, EE. UU).
2.6. Blood sampling
Every two weeks, prior to the semen collection, four blood samples were taken, every half an hour to determine the concentration of circulating T. Samples were allowed to clot and centrifuged at 1500 g for 20 min. Serum was separated and stored in 2 ml conical vial Eppendorf at −20°C until analysis. At the time of thawing the serum samples, the 4 samples were mixed to form a pool of them, obtaining a final single sample that was used to determine the concentration of testosterone in serum. All serum samples were analysed by solid-phase radioimmunoassay, using the TKTT-5 kit (Coat-A-Count Testosterone®, Siemens, Los Angeles, California, USA). The intra and inter-assay coefficient of variation for all analysed samples was 6.58 ± 1.3% and 9.3 ± 1.1%, respectively. Testosterone assay sensitivity was 0.44 ± 0.02 ng/ml.
2.7. Statistical analysis
The data were analysed with the SPSS package version 19 (Citation2008). Analyses of variance for body weight, testosterone concentrations, body condition, semen quality and sexual behaviour traits were performed with a linear model that included the effect of treatment, month and the interaction between treatment and month. For the effect of the month, the average of the data collected during the two weeks of evaluation was obtained. Least squares means and standard errors for treatment, month and combinations of treatment and month were obtained and used for multiple mean comparisons using the Tuckey’s test (P < 0.05).
3. Results
3.1. Body weight, scrotal circumference, and body condition
presents the p-values, means and standard errors of the means for the traits considered in this study. Body weight, BC and SC varied with the month of measurement for both groups of rams (P < 0.0001). Treatment affected BW (P < 0.0001), BC (P < 0.0001) and testosterone level (P < 0.05) and there was no interaction between treatment and month. Rams from the SG treatment presented a greater BW through all the months compared to the GG treatment (P < 0.0001). Body condition score showed a similar pattern as BW ().
Table 1. Main effects on semen quality and sexual behaviour traits of Saint Croix hair rams grazed on buffel grass throughout 1 year under semi-desert conditions of North-eastern of Mexico (Mean ± SEM).
Table 2. Effect of feed supplementation and month on the levels of testosterone, scrotal circumference, body weight, and quality semen of Saint Croix hair rams grazed on buffel grass throughout 1 year under semi-desert conditions of North-eastern of Mexico (Means ± SEM).
3.2. Semen quality
Semen quality traits varied through the months (P < 0.0001) (), and only volume was affected by the treatment group (P < 0.05) (). The interaction between treatment and month was significant for mass motility (P < 0.01) and total sperm concentration (P < 0.004).
Total sperm concentration decreased for both groups of rams from January to May, remained low during the hot months (May to August) and increased again until December ((A)). It was observed that the GS rams had greater values of total sperm concentration than the GG rams except for November ((A)).
Figure 2. Effect of feed supplementation on semen concentration (A) and mass motility (B) in Saint Croix hair rams grazed on buffel grass throughout 1 year (Means ± SEM). *P < 0.05.
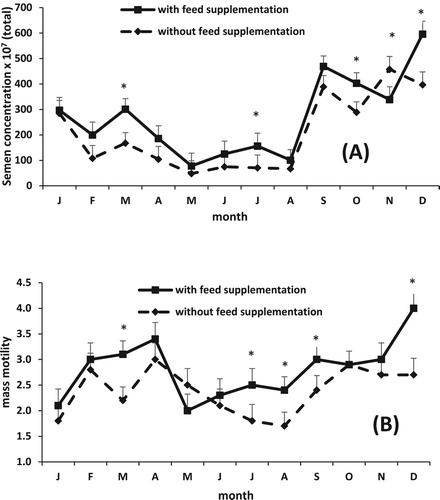
The GS rams had greater values of mass motility than GG rams during March, July, August, September and December ((B)), whereas GG rams had greater values of mass motility than GS rams during May. The mass motility values were greater during the months with less extreme temperatures (February, March, April, and November).
3.3. Sexual behaviour
Month effect () was significant on all the characteristics evaluated for sexual behaviour (P < 0.0001). Treatment effect was significant on reaction time (P = 0.009), anus-genital sniffing (P = 0.001), (). The interaction between treatment and month was significant on anus-genital sniffing (P = 0.03) and flehmen (P = 0.06) (). The GS rams had greater number of sniffs in April, November and December compared to the GG rams ((A)). Both treatment groups presented more flehmens from March to June and later in December. The GS rams had greater number of flehmens than GG rams in April and December, but the opposite was observed in February, during the other months both treatments groups had similar values ((B)).
Figure 3. Effect of feed supplementation on the sexual behaviour: Ano-genital sniffing (A) and Flehmen (B) of Saint Croix hair rams grazing buffel grass throughout 1 year (Means ± SEM).*P = 0.03; ¥ P = 0.06.
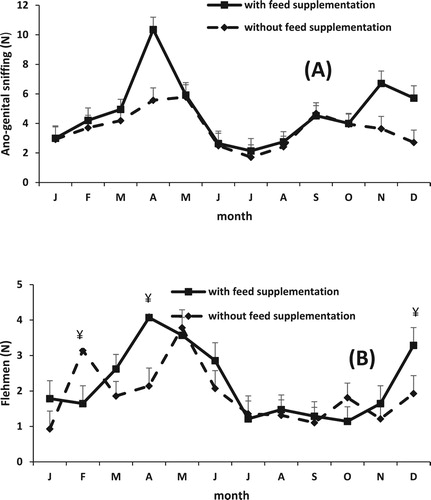
Table 3. Effect of feed supplementation and month on the sexual behaviour traits of Saint Croix hair rams grazed on buffel grass throughout 1 year under semi-desert conditions of North-eastern of Mexico (Means ± SEM).
4. Discussion
Concentrations of T decreased from January to May in both groups of rams, then increased at the beginning of the breeding season (June) and reaching their maximum level (October).
Despite these decreases in T in the first months of the year, this is not an impediment for the rams to have a sufficient sexual activity to stimulate the presence of oestrus in Saint Croix ewes (Aguirre et al. Citation2007) or in wool breed ewes (Clemente et al. Citation2012). Considering that, at these latitudes, there is a seasonal anestrus during the first half of the year and more marked in wool sheep breeds (Aller et al. Citation2012).
From the beginning of the breeding season (June), T concentrations were increased for both treatment groups despite considering that when presenting a decrease from March to May, it is not an impediment to present a sexual response and to be able to stimulate sheep during the seasonal anoestrus (March-May), as reported by Orihuela Trujillo (Citation2014).
The T levels observed in this study are similar to the values reported in Saint Croix rams in Mexico (Aguirre et al. Citation2007) and Morada Nova rams in Brazil (Sousa et al. Citation2014). However, when raised in tropical regions, the effect of season on T is more nutritionally dependent, especially in the case of cross-breeding with wool breeds (Zhang et al. Citation2005; Cárdenas-Gallegos et al. Citation2015). In the present study, T concentrations were greater during January, June, September, and October in both treatment groups; when important rainfall occurred, which increased pasture availability and quality creating an opportunity for the rams to have greater nutrient intakes (Maurya et al. Citation2010). In our study, it was observed that throughout the year the GS rams had greater T concentrations than GG rams, this may have explained by the positive effect of better nutrition on volume of testicular Leydig cells, which increase the production of T as reported by Hötzel et al. (Citation1998). This effect is more evident if exogenous growth hormone (Hamidi et al. Citation2012) or melatonin implants (Egerszegi et al. Citation2014) are used. This may be the result of selection applied in the development of this hair sheep breed originating from Africa (Godfrey et al. Citation2001) where under limited conditions of forage the reproductive performance of rams is not affected (Marai et al. Citation2006; Martin et al. Citation2010).
In the present experiment, BW and BC varied over the months. The rams from the GG treatment group lost more body condition from January to June, then regained BC at the same level as the rams of the GS group during July and August, but again lost BC in November with the lowest score during the year. The weight gain throughout July-August could be due to the start of the rainy season in July, which increased growth and quality of buffel grass, thus crude protein and metabolizable energy requirements of rams grazing buffel grass was meet (Sánchez-Dávila et al. Citation2011). However, this period was very short and was followed by a rpid decrease in BW and BC from October onwards, due to the lower nutritional quality of forage, more marked for the rams grazing only buffel grass without supplementary concentrate, as reported Vasquez-Aguilar (Citation2014) (11.4% CP and 1797 kcal ME kg−1 DM, during a year of study). As autumn approaches and during winter, the nutritional quality of buffel grass is reduced (1291 kcal ME kg−1 DM and 6.6 of CP) in this latitude as reported by Hernández-Calva et al. (Citation2011) and Vasquez-Aguilar (Citation2014).
The reproductive variables, both the seminal quality and sexual behaviour, were affected by month and feed supplementation; having a tendency that in the months of higher temperatures, the reproduction of the rams was reduced. In our study semen quality traits had the lowest values during from May to August in both treatment groups. This may be due to two factors that occur in semi-desert regions: one is the caloric stress that prevails during the summer season (June to September), where there are high ambient temperatures (Sánchez-Dávila et al. Citation2011) and, on the other factor is the scarcity and poor quality of the buffel grass that is available for the animals. In this study both factors were presented during the year. However, supplementary feed improved the quality and preservation of semen in response to these critical periods. Another positive effect of supplementation of rams in these semi-desertic regions is to improve resistance against the increase of gastrointestinal nematodes infestation after the rainy season (Knox et al. Citation2003; Torres-Acosta et al. Citation2012). However, in the particular case of this breed, it has been characterized to be resistant to parasites in the different regions where it is exploited (Torres-Acosta et al. Citation2012). In the GG rams, despite having a decrease in seminal quality during the summer months, they recover quickly when the rainy season occurs and highlights the adaptive capacity of the Saint Croix breed to extreme conditions of temperature, availability of food and resistance against gastro-intestinal parasites (Torres-Acosta et al. Citation2012; Sánchez-Dávila et al. Citation2015). These climatic changes lead to an increase in the quality of the native grass (Hernández-Calva et al. Citation2011) and a decrease of the thermal stress of the animal (Marai et al. Citation2006). Although in contrast to the other sheep breeds that are seasonal, in this hair breed the effect of photoperiod is less determinant on the reproductive efficiency of the rams (Arroyo Citation2011). Considering that, by not supplementing with energy and protein, seminal quality will be affected, even more so when the body condition of the rams is less than 2.5 (Maurya et al. Citation2010). Therefore, it is important nutrient supplementation in the season of low forage availability as the demand for thermoregulation of rams is increased and therefore become gonadally regressed and sexually inactive (Fernandez et al. Citation2004). The Saint Croix has developed evolutionary physiological mechanisms when forage availability and energy supply are low, to redirect any available energy and maintain brain function and cognition thus maintaining reproduction (Martin et al. Citation2008).
Sexual behaviour, measured as the number of sniffing, flehmen, lateral approaches and mounts with ejaculate, decreased from May to September. These results are consistent with those reported by Santos et al. (Citation2015), in the same breed, where they report that during the summer months, sexual behaviour of adult and young rams was also reduced. However, despite this low sexual behaviour, it is interesting to notice the ability of the Saint Croix rams to establish strategies and dosage of their mating. Clemente et al. (Citation2012) report that Saint Croix rams were able of inducing oestrus in and out of the breeding season in Suffolk sheep. Aguirre et al. (Citation2007) evaluated the effect of social hierarchy and reported that even the subordinate rams can keep acceptable sexual behaviour and were able to present a greater proportion of lambing throughout the year, as reported by Godfrey et al. (Citation1998) and Sanchez-Davila et al. (Citation2015). The other sexual behaviour variables, such as reaction time, mount attempts and time to the first mount with ejaculate, presented a different pattern to the other sexual behaviour traits discussed above but were also affected by month. These results agree with the results reported by De et al. (Citation2017) in Malpura rams and Aguirre et al. (Citation2007) in Saint Croix rams. Cardenas-Gallegos et al. (Citation2015) also found that the reaction time increased during dry-hot season in tropical regions in four breeds, with the highest values in Dorper rams.
Likewise, these results highlight the importance and the mechanism of dosage of mounts and sexual activity of the hair breed rams under hot conditions of semi-desertic areas (Maurya et al. Citation2010). Heat stress and the combined effects of heat stress and nutritional restriction reduce the level of T and have a severe impact of reproductive performance of rams (Fernandez et al. Citation2004). An adaptive mechanism of the hair breed rams to re-establish sexual behaviour under elevated temperatures is to release LH, which in turn leads to the production of testosterone in the gonads and therefore sexual behaviour can be maintained (De et al. Citation2017).
It is concluded that Saint Croix rams are able to adapt to adverse environmental conditions of the semi-desertic areas and maintain sexual activity during the year. Supplementary feed can improve sexual behaviour, semen quality and testosterone concentration of rams, which may be a strategy to improve the reproductive efficiency of the flocks from the semi-desert regions of the world.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Aguirre V, Orihuela A, Vásquez R. 2007. Seasonal variations in sexual behavior, testosterone, testicular size and semen characteristics, as affected by social dominance, of tropical hair rams (Ovis aries). Anim Sci J. 78:417–423. doi: 10.1111/j.1740-0929.2007.00456.x
- Alexander BM, Cocket NE, Burton DJ, Hadfield TL, Moss GE. 2012. Reproductive performance of rams in three producer range flocks: Evidence of poor sexual behavior in the field. Small Rum Res. 107:117–120. doi: 10.1016/j.smallrumres.2012.04.003
- Aller JF, Aguilar D, Vera T, Almeida GP, Alberio RH. 2012. Seasonal variation in sexual behavior, plasma testosterone and semen characteristics of Argentine Pampinta and Corriedale rams. Span J Agr Res. 10:345–352. doi: 10.5424/sjar/2012102-389-11
- Arroyo J. 2011. Estacionalidad reproductiva de la oveja en México. Trop Subtrop Agroecosys. 14:829–845.
- Arroyo LJ, Gallegos-Sánchez J, Villa-Godoy A, Berruecos JM, Perera G, Valencia J. 2007. Reproductive activity of Pelibuey and Suffolk ewes at 19° north latitude. Anim Reprod Sci. 102:24–30. doi: 10.1016/j.anireprosci.2006.09.025
- Cárdenas-Gallegos MA, Aké-López JR, Magaña-Monforte JG, Centurión-Castro FG. 2015. Libido and serving capacity of mature hair rams under tropical environmental conditions. Arch Med Vet. 47:39–44. doi: 10.4067/S0301-732X2015000100008
- Clemente N, Orihuela A, Flores-Pérez I, Aguirre V, Ortiz A, Solano J, Valencia J. 2012. Reproductive activity of Suffolk ewes in seasonal anestrus after being exposed to Saint Croix or Suffolk rams. J Appl Anim Res. 40:203–207. doi: 10.1080/09712119.2012.658060
- De K, Balaganur K, Saxena VK, Thirumurugan P, Naqvi SMK. 2017. Effect of thermal exposure on physiological adaptability and seminal attributes of rams under semi-arid environment. J Therm Biol. 65:113–118. doi: 10.1016/j.jtherbio.2017.02.020
- Egerszegi I, Sarlós P, Rátky J, Solti L, Faigl V, Kulcsár M, Cseh S. 2014. Effect of melatonin treatment on semen parameters and endocrine function in Black Racka rams out of the breeding season. Small Rum Res. 116:192–198. doi: 10.1016/j.smallrumres.2013.11.001
- Evans G, Maxwell WMC. 1990. Inseminación artificial de ovejas y cabras. Zaragoza: Editorial Acribia.
- Fernández M, Giráldez FJ, Frutos P, Lavin P, Mantecón AR. 2004. Effect of undegradable protein supply on testicular size, spermiogram parameters and sexual behavior of mature Assaf rams. Theriogenology. 62:299–310. doi: 10.1016/j.theriogenology.2003.10.003
- Godfrey RW, Collins JR, Gray ML. 1998. Evaluation of sexual behavior of hair sheep rams in a tropical environment. J Anim Sci. 76:714–717. doi: 10.2527/1998.763714x
- Godfrey RW, Collins JR, Hensley EL. 2001. Behavioral and endocrine responses of hair sheep ewes exposed to different mating stimuli around estrus. Theriogenology. 55:877–884. doi: 10.1016/S0093-691X(01)00450-2
- Hamidi A, Morteza M, Khalil M, Saleh T, Hedayat-Allah R. 2012. Correlation between blood growth hormone profile and reproduction performance in Arabic rams. Comp Clin Pathol. 21:819–823. doi: 10.1007/s00580-011-1183-x
- Hernández-Calva LM, Ramírez-Bribiesca JE, Salinas-Chavira J, Ducoing-Watty A, Ramírez RG. 2011. Nutritive value of browse plants selected by range goats in the Mexican plateau. J Applied Anim Res. 39:320–323. doi: 10.1080/09712119.2011.607941
- Hötzel MJ, Markey CM, Walkden-Brown SW, Blackberry MA, Martin GB. 1998. Morphometric and endocrine analyses of the effects of nutrition on the testis of mature Merino rams. Reprod Fert Develop. 113:217–230. doi: 10.1530/jrf.0.1130217
- Joshi A, Naqvi SMK, Bag S, Dang AK, Sharma RC, Rawat PS, Mittal JP. 2003. Sperm motion characteristics of Garole rams raised for a prolonged period in a semi-arid tropical environment. Trop Anim Health Prod. 35:249–257. doi: 10.1023/A:1023347514476
- Kafi M, Safdarian M, Hasheim M. 2004. Seasonal variation in semen characteristics, scrotal circumference and libido of Persian Karakul rams. Small Rum Res. 53:133–139. doi: 10.1016/j.smallrumres.2003.07.007
- Kenyon PR, Maloney SK, Blache D. 2014. Review of sheep body condition score in relation to production characteristics. New Zeal J Agr Res. 57:38–64. doi: 10.1080/00288233.2013.857698
- Knox MR, Deng K, Nolan JV. 2003. Nutritional programming of young sheep to improve later-life production and resistance to nematode parasites: a brief review. Aust J Exp Agr. 43:1431–1435. doi: 10.1071/EA03051
- Lincoln GA, Davidson W. 1977. The relationship between sexual and aggressive behaviour, and pituitary and testicular activity during the seasonal sexual cycle of rams, and the influence of photoperiod. J Reprod Fertil. 49:267–276. doi: 10.1530/jrf.0.0490267
- Marai IF, El-Darawany AHA, Ismail ESAF, Abdel-Hafez MA. 2006. Tunica dartos index as a parameter for measurement of adaptability of rams to subtropical conditions of Egypt. Anim Sci J. 77:487–494. doi: 10.1111/j.1740-0929.2006.00376.x
- Martin GB, Blache D, Miller DW, et al. 2010. Interactions between nutrition and reproduction in the management of the mature male ruminant. Animal. 4:1214–1226. doi: 10.1017/S1751731109991674
- Martin B, Golden E, Carlson OD, Egan JM, Mattson MP, Maudsley S. 2008. Caloric restriction: impact upon pituitary function and reproduction. Ageing Res Rev. 7:209–224. doi: 10.1016/j.arr.2008.01.002
- Maurya VP, Sejian V, Kumar D, Naqvi SMK. 2010. Effect of induced body condition score differences on sexual behavior, scrotal measurements, semen attributes and endocrine responses in Malpura rams under hot semi-arid environment. J Anim Physiol and Nutr. 94:e308–e317. doi: 10.1111/j.1439-0396.2010.01012.x
- Maurya VP, Sejian V, Kumar D, Naqvi SMK. 2016. Impact of heat stress, nutritional restriction and combined stresses (heat and nutritional) on growth and reproductive performance of Malpura rams under semi-arid tropical environment. J Anim Physiol Anim Nutr. 100:938–946. doi: 10.1111/jpn.12443
- Oliveira MEF, Teixeira PPM, Silva JCB, Feliciano MAR, Nociti RP, De Oliveira LG, Vicente WRR, Rodrigues LFDS. 2014. Effect of scrotal insulation associated to environmental discomfort on andrologic characteristics in Santa Inês rams. J Anim Sci Adv. 4:1051–1058. doi: 10.5455/jasa.20141016121152
- Orihuela Trujillo A. 2014. La conducta sexual del carnero, Revisión. Rev Mex Cienc Pec. 5:49–89. doi: 10.22319/rmcp.v5i1.3217
- Ortavant R, Bocquier F, Pelletier J, Ravault JP, Thimonier J, Volland-Nail P. 1988. Seasonality of reproduction in sheep and its control by photoperiod. Aust J Biol Sci. 41:69–86. doi: 10.1071/BI9880069
- Rosa HJD, Bryant MJ. 2003. Seasonality of reproduction in sheep. Small Rum Res. 48:155–171. doi: 10.1016/S0921-4488(03)00038-5
- Sánchez-Dávila F, Bernal-Barragán H, Colín-Negrete J, Olivares-Saénz E, del Bosque-González AS, Ledezma-Torres RA, Ungerfeld R. 2011. Environmental factors and interval from the introduction of rams to estrus in postpartum Saint Croix sheep. Trop Anim Health Prod. 43:887–891. doi: 10.1007/s11250-011-9779-3
- Sánchez-Dávila F, Bernal-Barragán H, Padilla Rivas G, del Bosque-González AS, Vasquez-Armijo JF, Ledezma-Torres RA. 2015. Environmental factors and ram influence litter size, birth, and weaning weight in Saint Croix hair sheep under semi-arid conditions in Mexico. Trop Anim Health Prod. 47:825–831. doi: 10.1007/s11250-015-0795-6
- Santos SI, Sánchez-Dávila F, Vázquez-Armijo JF, Ledezma-Torres RA, del Bosque-González AS, Palomera CL, Bernal-Barragán H. 2015. Changes in sexual behaviour and semen quality associated with age and type of enclosure of Saint Croix rams in different seasons of the year. Ital J Anim Sci. 14:678–683. doi: 10.4081/ijas.2015.3890
- Sousa FML, Lobo CH, Menezes ESB, Rego JPA, Oliveira RV, Lima-Souza AC, Fioramonte M, Gozzo FC, Pompeu RCFF, Candido MJD, et al. 2014. Parameters of the reproductive tract, spermatogenesis, daily sperm production and major seminal plasma proteins of tropically adapted Morada Nova rams. Reprod Domest Anim. 49:409–419. doi: 10.1111/rda.12288
- SPSS version 19. 2008. Package for Social Sciences Windows version 12.0. Chicago (IL). 125–155.
- Torres-Acosta JFJ, Sandoval-Castro CA, Hoste H, Aguilar-Caballero AJ, Cámara-Sarmiento R, Alonso-Díaz MA. 2012. Nutritional manipulation of sheep and goats for the control of gastrointestinal nematodes under hot humid and subhumid tropical conditions. Small Rum Res. 103:28–40. doi: 10.1016/j.smallrumres.2011.10.016
- Vásquez-Aguilar NC. 2014. Determinación de fracciones de carbohidratos y proteínas y del valor nutricional de pasto buffel (Cenchrus ciliaris L.) asociado con dos subproductos agroindustriales. Tesis Maestría. UANL. 104 p.
- Zhang S, Dominique B, Margaret AB, Graeme BM. 2005. Body reserves affect the reproductive endocrine responses to an acute change in nutrition in mature male sheep. Anim Reprod Sci. 88:257–269. doi: 10.1016/j.anireprosci.2005.01.001