ABSTRACT
McMaster (McM) method is one of the most widely used techniques for the assessment of faecal parasites shedding in veterinary practices because of its simplicity. However, due to its light sensitivity, recently, the Mini-FLOTAC (MF) has been introduced as a possible alternative for faecal worm egg counts. This study aims to compare the diagnosis performance of MF to McM technique. Faecal samples from 40 animals randomly selected in sheep, goats and rabbits’ farms were collected and examined individually using MF and McM techniques. A statistical difference (p < 0.001) in strongylida egg counts in small ruminants and oocyst of Eimeria spp counts in rabbits using both techniques was observed. However, strongylida eggs per gram of feces in sheep (MF: 202.01 vs McM: 174.75) goat (MF: 147.36 vs McM: 143.75) and oocysts of Eimeria spp per gram of feces in rabbits (MF: 130.75 vs McM: 130.5) revealed no significant difference (p > 0.05). MF showed better diagnostic performance in term of the prevalence (MF: 32.5–100% vs McM: 7.5–70%) and the precision values (MF: 85.52–90.44% vs McM: 49.52–63.07%). This study demonstrated that MF appears to be the more reliable alternative technique for veterinary practices.
Introduction
Parasitic infections of the gastrointestinal (GI) tract represent a serious challenge to the health, welfare and productivity of livestock (Morgan et al. Citation2013) such as large and small ruminants, rabbits, pigs, poultry, etc. Moreover, economic losses are caused by GI parasites in a variety of ways: they cause losses through lowered fertility, reduced work capacity, involuntary culling, a reduction in food intake and lower weight gains, lower milk production, treatment costs, and mortality in heavily parasitized animals (Fikru et al. Citation2006). Internal parasites get out of control and cause damage when their numbers grow beyond what the animal can tolerate (Owusu et al. Citation2016). A combination of treatment and management after a sensitive and accurate diagnosis using coproparasitological diagnosis techniques is necessary to control parasites, so that they will not cause economic losses to the producer (Emiru et al. Citation2013). Due to the emergence of the anthelmintic drugs resistance in GI parasites in livestock across the world, many diagnosis techniques have been recommended by the World Association for the Advancement of Veterinary Parasitology (WAAVP) to monitor drug efficacy against GI nematodes in livestock based on the reduction in faecal egg counts (FEC) (Coles et al. Citation1992). Indeed, the use of FEC in small ruminants and other livestock species has several important purposes according to Bosco (Citation2014). The first is to determine whether animals are infected and to estimate the intensity of infection. The second is to determine whether animals need to be treated to improve their health with the resulting increase of productive performance. The third is to predict pasture contamination by parasitic eggs. The fourth is to determine the efficacy of anthelmintics as well as monitoring control programmes.
The McMaster technique is the most widely used FEC technique in veterinary parasitology and is advocated by the WAAVP in its guidelines for evaluating the efficacy of anthelmintic drugs in ruminants (Wood et al. Citation1995; Cringoli et al. Citation2010) and for detection of anthelmintic resistance (Coles et al. Citation2006). This technique, mostly the modified technique is the most common routine method used in developing countries to diagnose GI parasites in domestic and wild animals because of its simplicity and the low demand for modern laboratory diagnostic materials (Hansen and Perry Citation1995). Unfortunately, this technique exhibits a comparatively limited sensitivity, particularly in samples with light nematode egg counts (Mes Citation2003). Regarding this, in order to obtain lower detection limits, many studies have illustrated substantial differences in precision between other diagnostic techniques developed such as the modified Wisconsin sugar flotation technique, the Stoll egg-counting technique and the FECPAK method (Levecke et al. Citation2012a; Godber et al. Citation2015) with less satisfactory outcomes in term of sensitivity and accuracy.
The FLOTAC technique, a more sensitive, precise and accurate method has been introduced in the past decade as an alternative to the diagnosis techniques previously developed for qualitative and quantitative copromicroscopic diagnosis of parasites in animals and humans (Cringoli et al. Citation2010). The FLOTAC technique is designed as a centrifugation-based method that uses two large flotation chambers each holding 5 mL of floated suspension (Cringoli et al. Citation2010). However, because of the amount of time and specialized equipment that this required to perform this technique, a simplified version, the Mini-FLOTAC technique has been recently developed (Cringoli et al. Citation2017). This technique is based on passive flotation and requires fewer preparation steps than the FLOTAC technique (Cringoli et al. Citation2017). In addition, the Mini- FLOTAC is a low-cost technique, which does not require any expensive equipment or energy source, so it can be comfortably used in developing countries and in the field (Barda et al. Citation2013a) where usually low financial resources are provided for the research.
This study, therefore, was conducted to compare for the first time the performance of the Mini-FLOTAC technique with the common the McMaster technique in several animal species as repetitive manner helping in the choice of precise and accurate diagnostic technique using for the evaluating of the efficacy and anthelmintic drugs resistance emergence in GI parasites in livestock.
Materials and methods
Study area
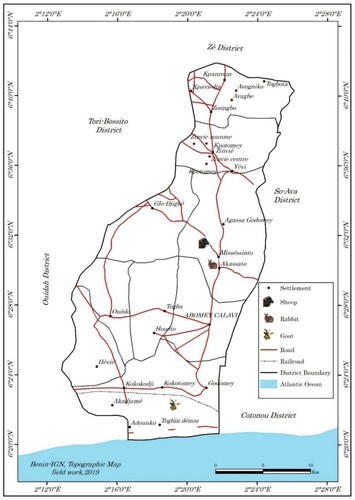
The faecal samples were collected on July 24th to 26th, 2019 in rabbits, sheep and goats’ farms, all located in the municipality of Abomey-Calavi (). This municipality of Abomey-Calavi is located in the Atlantique Department in the south of the Republic of Benin (between 6° 26′ 55″ North, 2° 21′ 20″ East). Limited in the south by the Atlantic Ocean, in East by the municipalities of Sô-Ava and Cotonou, in the West by the municipalities of Tori-Bossito and Ouidah and in the north by the municipality of Zè (). This municipality has a little hilly relief with a sandy strip, spits, a plateau of ‘Terre de barre’ soil and some depressions. Most of the territory of the municipality of Abomey-Calavi is occupied by tropical ferruginous soils and sandy soils and covers an area of 650 km2 with a population of 655,965 people. The climate is of the subequatorial type marked by two rainy seasons and two dry seasons. The rainfall of the locality is 1200 mm. Maximum temperatures are always below 35°C. Minimum temperatures between 20 and 23° C are recorded from July to September. The average monthly temperatures vary between 27 and 31°C and the annual amplitudes vary between 3 and 4°C (Adjahossou et al. Citation2017). The population carry out several activities in different sectors such as agriculture, livestock farming, fishing, trade, crafts, transport, fuelwood exploitation and transformation of products (Biaou Citation2006). Regarding the farming system, small ruminant keeping is more based on low input traditional extensive system where animals are allowed to graze on natural pasture while in rabbits farm, the cut-and-carry system of feeding is more practiced with feed supplementation.
Faecal samples collection
In respectively sheep farm and goats farm, individual faecal samples (at least 20 g) from 40 animals per species aged to five (05) months in average and randomly selected out of 136 (with 86 lambs) and 128 (with 75 kids) animal species were collected directly from the rectum. Out of 187 (with 105 young rabbits) rabbits, 40 animals of four (04) months of age randomly selected were individually placed in separate cages with a clean stall in bottom, and the faecal samples were collected from individual animals from their droppings. Afterwards, all samples were immediately sealed in plastic rectal sleeves and tying a knot halfway up the sleeve. These were stored in a refrigerator (4°C) and analysed the same day.
Parasitological methods
All faecal samples collected were analysed in 24 h elapsed time later using the Mc Master technique and Mini-FLOTAC as described below.
The modified McMaster technique (MAFF,1986) was performed using three grams of feces putting into a container adding 42 ml of saturated sodium chloride solution (NaCl, specific gravity = 1.2) diluted in 1:15 ratio. The faecal suspension was thoroughly homogenized and strained three times through a wire mesh (aperture of 250 m) to remove large debris. The strained suspension was collected in a bowl and thoroughly mixed by pouring it 10 times in one bowl to another. Then, 0.5 ml aliquots were added to each of the two chambers of a McMaster slide. After 10 min, the GI parasites egg counts were performed under the two grids (volume = 0.3 ml) of the McMaster slide (Cringoli et al. Citation2004) under a light microscope using a 100× magnification. FEC values, expressed as EPG or OPG of parasites, were obtained by multiplying the total number of eggs by 50 (McM50).
The Mini-FLOTAC technique was performed using the SOP described in Cringoli et al. (Citation2017). Briefly, two grams of fresh feces were put into the Fill-FLOTAC container ((a)) and 18 ml of NaCl (specific gravity = 1.2) were added (dilution ratio = 1:10). The suspension was then thoroughly homogenized using the homogenizer stick of the Fill-FLOTAC ((a)). The faecal suspension was then filtered through the Fill-FLOTAC ((a)), and used to fill the two chambers of the Mini-FLOTAC ((b)). After 10 min, the top part of flotation chambers was translated and both Mini-FLOTAC chambers were read under a light microscope using a 100× magnification ((b)). The added FEC values from both chambers, expressed as EPG or OPG of parasite species, were obtained by multiplying the total number of eggs by 5.
Figure 2. The Fill -FLOTAC (a) currently associated to the Mini-FLOTAC ongoing to reading under microscope (b); CREMOPAR: http://www.parassitologia.unina.it/flotac/mini-flotac/

The quality of the parasitological investigation of both techniques, McMaster and Mini-FLOTAC was assured as described by Rinaldi et al. (Citation2014) by first, the analysing of the faecal samples collected in each site study within an average of seven h of collection, the verification of specific gravity of the flotation solution using a hydrometer, and the calibration of the scale weighing the faecal samples, finally the reading of the two techniques by at least one expert in the field. Each technique was used to analyse all faecal samples, and each sample was counted in triplicates with both techniques, for a total of six counts on each sample (e.g. three for Mini-FLOTAC and three for McMaster).
In small ruminants, according to Bosco (Citation2014) the number of GIN eggs in a faecal sample varies with factors related to the host and parasite species. This aspect should be taken into account to identify FEC thresholds for treatment. Indeed, not only there are no widely accepted defined FEC thresholds for treatment, but also these thresholds will vary between the different nematode species (Kenyon et al. Citation2009). Some authors suggest that less than 500 EPG is considered a light threshold of GIN infection, between 500 and 1500 EPG as moderate to heavy, and more than 1500 EPG as heavy level of infection (Hansen and Perry Citation1995). According to other authors FEC of ≥200 EPG is regarded to indicate a significant worm burden and is used as basis for the decision for anthelmintic treatment according to the report of Bosco (Citation2014). Other authors suggest a threshold of 300–500 EPG (based on counts of 10 animals) for treatment of sheep flocks (Coles G.C., personal communication), reported by Bosco (Citation2014). Finally, according to Keyyu et al. (Citation2005), an animal was recognized as severely infected when faecal egg counts exceeded 500 eggs per gram of feces (EPG). With regard to these, in the present study, the reference values to use will be: for light infection: FEC< 200 EPG/OPG, moderate infection: 200 OPG/EPG < FEC <500 EPG/OPG and heavy infection: FEC > 500 EPG/OPG.
Statistical analysis
The intensity of parasitism, i.e. the arithmetic means of eggs/oocysts and the one of EPG/OPG were calculated for each parasite and each technique. Differences between the eggs/oocysts and EPG/OPG obtained with the two techniques for each parasite were analysed using two-ways Analysis of Variance (ANOVA). Where significant variations were noted, Tukey–Kramer HSD post hoc test was performed on R software (R core Team Citation2013).
The prevalence (percentage of animal infected) was calculated for each parasite species detected with each technique and the prevalence values between both techniques were compared through the two-proportions z-test in R (R core Team Citation2013) by the function prop.test using.
The techniques precision as described by Noel et al. (Citation2017) was computed as follows: first, a CV (SD divided by the mean count times 100%) was calculated for each set of triplicate counts for each technique for each sample. Mean coefficients of variation with 95% confidence intervals were then calculated for each of the two techniques. A percent precision was calculated for each technique by subtracting the CV from 100. Difference in precision between the two techniques is performed using the khi2 of Pearson test. Afterwards the Pearson correlation coefficient was calculated to examine the strength and direction of the linear relationship between mean of EPG or OPG obtained with bot techniques (McMaster and Mini-FLOTAC) using R software (R core Team Citation2013). Finally, 10 of the counts were timed in order to find an average time investment for each technique, start to finish. Faecal samples of sheep and rabbits were respectively timed from when the faecal sample was weighed to when the last triplicate count was completed on the microscope.
Results
Parasites species identified in the faecal samples’ examination using Mini-FLOTAC and McMaster techniques
Different classes and species of parasites were identified in small ruminants and rabbits’ faecal samples using Mini-FLOTAC (MF) techniques and McMaster (McM). Thus, eggs of gastrointestinal nematodes (GIN) as egg of Strongylida, Strongyloides spp, Nematodirus spp and Marshallagia spp and oocysts of Eimeria spp were found in faecal samples of small ruminants examined with McMaster and Mini-FLOTAC techniques. On the other hand, eggs of Passalurus ambiguus, eggs of Dicrocoelium lanceolatum and oocysts of Eimeria spp were identified in the faecal samples of rabbits.
The intensity of parasitism in small ruminants and rabbits
The intensity of parasitism (mean of faecal egg/oocyst counts and egg/oocyst per gram of feces) in small ruminants and rabbits is shown in . The study revealed a statistical difference (p < 0.001) in the mean of faecal egg counts for all of the parasite species found in small ruminants (sheep and goats) and rabbits using the Mini-FLOTAC compared to the McMaster technique (). However, the mean of egg/oocyst per gram of feces revealed no significant difference (p > 0.05) in small ruminants and rabbits when both techniques, McMaster and Mini-FLOTAC are used (). According to the reference values, a light intensity of parasitism (FEC < 200 EPG) was noted in goats (MF: 147.36 ± 51.95 vs McM: 143.5 ± 41.21) and rabbits (MF: 130.75 ± 47.65 vs McM: 130.5 ± 29.25) using the two techniques. However, moderate intensity of parasitism was observed in sheep using Mini-FLOTAC technique (202.01 ± 99.25 > 200 EPG) whereas it was light with the McMaster technique (174.75 ± 69.15 < 200 EPG) even the difference was not significant.
Table 1. Mean of eggs/oocysts (± ESM) and EPG/OPG (± ESM) of parasites in 40 fecal samples per animal species using two copromicroscopic techniques.
Prevalence of helminth and protozoa parasites in small ruminants and rabbits
The prevalence of helminths and protozoa infections in small ruminants and rabbits using in comparison the Mini-FLOTAC and McMaster techniques is presented in .
Figure 3. Prevalence of gastrointestinal parasites from goats detected by McMaster and Mini-FLOTAC techniques. Each bar of the chart represents the proportion of animals infested. The letters on each bar compare the results obtained by both techniques through the two-proportions z-test in R with the function prop.test using. Different lowercase letters indicate a significant difference between values at p < 0.05.
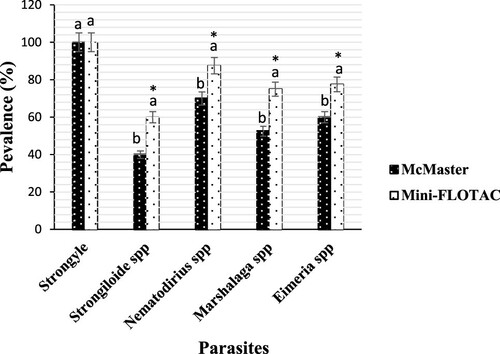
With regard of goats, the number of positive samples according to Mini-FLOTAC and the McMaster techniques reading was 40/40 (100%, IC: 100–100) for strongylida (). However, there was significant difference (p < 0.001) in the prevalence of Nematodirus spp (87.5%, IC: 82.21–92.78 vs 70%, IC: 50.75–89.38), Eimeria spp (77.5%, IC 72.89–92.77 vs 60%, IC 43.50–76.62), Marshallagia spp (75%, IC: 45.68–85.71 vs 52.5%, IC: 38.02–66.92) and Strongyloides spp (60%, IC: 41.60–75.95 vs 40% IC: 28.97–51.01) using Mini-FLOTAC technique in comparison to McMaster technique ().
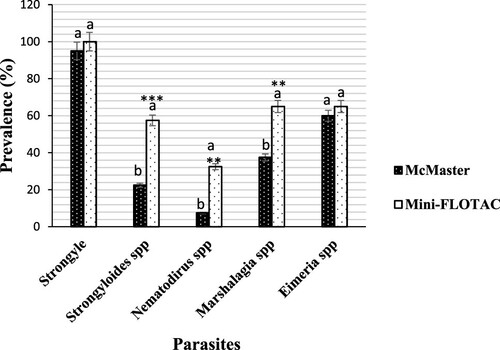
Contrary to strongylida (100%, IC: 100–100) and Eimeria spp (65%, IC: 43.12–88.35 vs 60% IC: 39.50–86.32) detected in all of the faecal samples in sheep using Mini-FLOTAC technique compared to McMaster technique, a statistical difference (p < 0.01) in prevalence of Marshallagia spp (65%, IC 47.12–82.99 vs 37.5%, IC 27.18–47.88), Strongyloides spp (57.5%, IC 41.83–66.92 vs 22.9%, IC 16.58–29.20) and Nematodirus spp (32.5%, IC 23.53–42.97 vs 7.5%, IC 5.43–9.56) were noted ().
Figure 4. Prevalence of gastrointestinal parasites from sheep detected by each of McMaster and Mini-FLOTAC techniques. Each bar of the chart represents the proportion of animals infested. The letters on each bar compare the results obtained by both techniques through the two-proportions z-test in R with the function prop.test using. Different lowercase letters indicate a significant difference between values at p < 0.05.
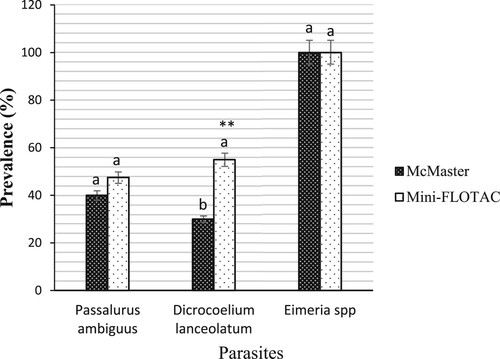
From rabbits, there was no significant difference (p > 0.05) in the prevalence of Eimeria spp (100%, IC: 100–100) and Passalurus ambiguus (47%, IC: 34.19–54.69 vs 40% IC: 31.09–48.55) when the Mini-FLOTAC technique is compared to the McMaster techniques (). On the other hand, significant difference (p < 0.01) in the prevalence of Dicrocoelium lanceolatum was observed between both techniques (55%, IC: 40.01–64.25 vs 30%, IC: 21.82–34.91) ().
Figure 5. Prevalence of gastrointestinal parasites from rabbits detected by McMaster and Mini-FLOTAC techniques. Each bar of the chart represents the proportion of animals infested. The letters on each bar compare the results obtained by both techniques through the two-proportions z-test in R with the function prop.test using. Different lowercase letters indicate a significant difference between values at p < 0.05.
Precision of Mini-FLOTAC and McMaster techniques
The mean of the SD of the two techniques before and after applying the multiplication factors is presented in along with coefficients of variation and precision estimates. The Mini-FLOTAC technique, whatever the type of faecal samples used in the present study was more precise in faecal egg counts of GI parasites than the McMaster technique. Moreover, the results showed difference in coefficients of variation between both techniques (). The precision was 85.52% (95% CI: 80.35–90.68) versus 59.78% (95% CI: 43.30–76.25) in sheep respectively with the Mini-FLOTAC and McMaster using. From goat, the precision was 90.44% (95% IC: 84.97–95.90) versus 63.07% (95% IC: 45.68–80.45), respectively, when the Mini-FLOTAC and the McMaster are used. Concerning, rabbit species by Mini-FLOTAC and McMaster techniques using the precision was, respectively, 89.74% (95% IC: 84.31–95.16) and 49.52% (95% IC: 35.87–63.16) ().
Table 2. Coefficients of variation (CV), standard deviations (SD), and precision for the two egg-counting techniques evaluated in the study.
Relationship between McMaster and Mini-FLOTAC techniques
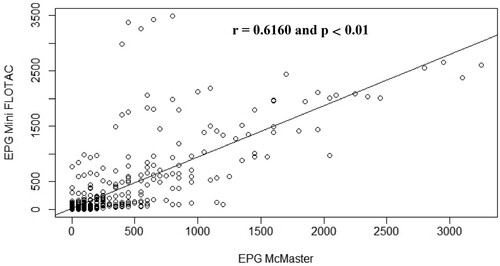
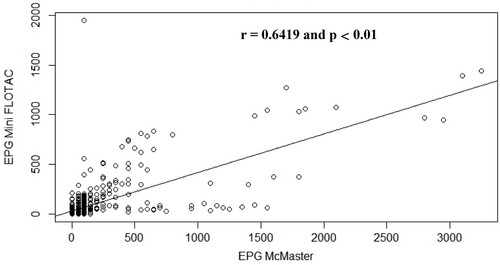
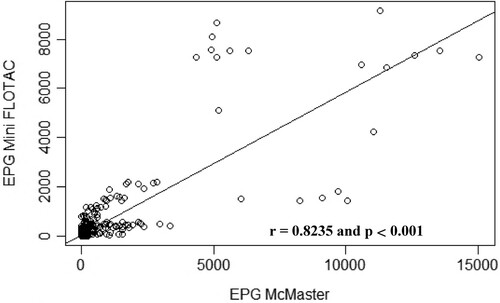
The correlation between the mean of EPG obtained with the Mini-FLOTAC and that obtained with McMaster for each animal species is illustrated by . Concerning the data obtained for sheep faecal samples, the points fall close to the line, which shows that there is a strong linear positive relationship between the techniques (Pearson r = 0.82, p < 0.001) (). Regarding goat () and rabbit () samples, some points are close to the line but other points are far from it, which indicates only a moderate linear positive relationship between the variables (Pearson r = 0.60, p < 0.01) ().
Figure 6. Scatterplot of gastrointestinal parasites egg counts determined with McMaster and Mini-FLOTAC techniques from samples collected from naturally infected sheep.
Timed counts
presents the processing times of 10 of the samples from one species of herbivores (Sheep) and that of lagomorphs (rabbit) for the two evaluated techniques. Time consumption includes preparation and counting of all three replicates. The results show that the Mini-FLOTAC technique took twice as long time than the McMaster technique for the sample analysis from start to finish ().
Table 3. Time taken to generate three replicate counts from 10 different sheep and rabbit fecal samples.
Discussion
Accurate diagnosis of parasitic infections is of pivotal importance for both individual patient management and population-based studies, such as drug efficacy trials and surveillance of parasitic disease control and elimination programmes, in both human and veterinary public health (Cringoli et al. Citation2010). Therein, for any newly introduced diagnostic tool, the knowledge of the specific diagnostic parameters e.g. concerning precision and detection rate compared with established methods is of great interest. Thus, the goal of the present study was to collect data on the performances of Mini-FLOTAC technique in comparison to the established McMaster technique using field derived samples.
In the present study, the Mini-FLOTAC technique exhibited a significant precision than the McMaster technique whatever the type of faecal material examined. The possible explanation could be due to the process of faecal samples examination such as the amount of feces examined and the volume examined according to Torgerson et al. (Citation2012) and Cringoli et al. (Citation2017). Indeed, in previous work, Cringoli et al. (Citation2017) reported that the Mini-FLOTAC technique permits to sample and homogenize a comparatively larger amount of feces (up to 5 g) and afterwards to directly analyse 200 mg of feces contrary to usual techniques such as Kato-Katz (41.7 mg) of stool, employed in humans and McMaster (66.7 mg of feces) in veterinary practices. In addition, Noel et al. (Citation2017) reported that the McMaster has a volume under the counting grid which is 0.15 ml corresponding to a total of 0.30 ml examined while the Mini-FLOTAC possess 1 ml per chamber, for a total of 2 ml examined. Therefore, the larger volume investigated should help enhance the precision by offering a better representation of eggs present in the samples (Noel et al. Citation2017). Similar outcomes have been highlighted in previous studies using the Mini-FOTAC technique in comparison with the McMaster technique (Rinaldi et al. Citation2014; Dias de Castro et al. Citation2017; Noel et al. Citation2017) or with other techniques such as Kato-Katz technique (Barda et al. Citation2013b) the FECPAK technique (Godber et al. Citation2015). However, the precision estimated for the two egg-counting techniques (Mini-FLOTAC, McMaster) in the current study respectively in sheep (85.52%, 59.78%) and in goats (90.44%, 63.07%) except in rabbits (89.74, 49.52) seems to be better than values obtained by Noel et al. (Citation2017) in horse with both techniques (83.24%, 53.70%). The difference in precision values could be likely due to a difference in livestock species (sheep and goats rather than horses) or to slight differences in method, rather than 2 g of feces for Mini- FLOTAC, 5 g was used, and rather than 3 g of feces for McMaster, 4 g was used by Noel et al. (Citation2017). Furthermore, instead of the sodium chloride solution used in this study, a glucose-NaCl solution with a specific gravity of 1.25 was used by Noel et al. (Citation2017).
The accurate detection of the intensity and prevalence of parasitism is the key to understanding the effect of parasites on the biology, behaviour and the conservation of hosts according to Alvarado-Villalobos (Citation2017). Regarding to this, the Mini-FLOTAC technique in the present study demonstrated a better diagnostic performance in terms of the obtained intensity (egg outputs) and prevalence of parasitism in small ruminants and rabbits compared with the McMaster technique. Besides, compared to the reference values, a moderate intensity of parasitism was observed using the Mini-FLOTAC in sheep contrary to the use of McMaster technique where it was light. The good performance of Mini-FLOTAC is also demonstrated by its capacity to detected in a herd the right threshold of parasitic infection. Many studies have reported a significant sensitivity, accuracy and precision demonstrated by the Mini-FLOTAC technique over the commonly used techniques such as the Kato-Katz technique (Barda et al. Citation2013a, Citation2013b) and the McMaster technique (Rinaldi et al. Citation2014; Noel et al. Citation2017; Dias de Castro et al. Citation2017) including its modified version, the FECPAK technique (Godber et al. Citation2015). Outputs from this study are in agreement with data from other studies evaluating the performance of Mini-FLOTAC technique in other animal species. The previous works implemented by Noel et al. (Citation2017) for determining equine strongylida egg counts and Dias de Castro et al. (Citation2017) in GI nematode eggs detecting in horses and cattle, illustrated that Mini-FLOTAC produced significant faecal egg counts than McMaster. Moreover, the faecal egg counting (FEC) was correlated to the variation of the SD and the CV. The three animal species with significant FEC in the present study shown significant variation of CV and significant variation of the SD indicating that the Mini-FLOTAC technique opposite to McMaster technique was more accurate and sensitive. Moreover, Bosco et al. (Citation2014) reported that the larger variation (CV) found with McMaster is due to the large multiplication factors used to provide eggs per gram from the egg counts. For more explanation, Dias de Castro et al. (Citation2017) reported that this may be because the minimum detection limits were 5 EPG for the Mini-FLOTAC and 50 EPG for the McMaster. Consequent, except for the prevalence of strongylida in small ruminants and the prevalence of Eimeria spp in rabbits, globally the data obtained by the Mini-FLOTAC technique resulted in a significant prevalence values of GI parasites than that obtained by the McMaster technique in all of the animal species. The possible reason for the difference in the prevalence rate obtained is related as described above to the precision of the Mini-FLOTAC which overcome that one of the McMaster technique. Similarly, but in human field, Barda et al. (Citation2013a, Citation2013b) reported the significant performance of Mini-FLOTAC in terms of the prevalence of soil-transmitted helminths and intestinal protozoa in human.
This study showed that the Mini-FLOTAC technique produced more precise small ruminants and rabbits’ GI parasites faecal egg counts than the McMaster technique, but also illustrated that this comes with a significant time consumption per sample from start to finish. The time taken to generate three replicate counts from equine faecal samples (38:20 minutes) by Noel et al. (Citation2017) is important than that taken to generate three replicate counts from sheep (30:08 minutes) and from rabbits (30:12 minutes) in the present study. One of the possible explanations could be the difference in animal species faecal samples used on both studies. Likewise, the experience of the technician with the respective technology during the faecal sample analysis could likely be another reason for the difference found in time consumption between both studies. However, in both studies, if several samples were setup simultaneously, this time could likely be reduced because there are 10:00 minutes that is the flotation time which could all be passed. Moreover, reported that the Mini-FLOTAC about the technique feasibility could take more time (13 minutes/sample) than the McMaster (7 minutes/sample) but that this mean time (min/sample) could decrease significantly when processing multiple samples.
Conclusion
Results in the present study showed that the quantitative faecal analysis using the Mini-FLOTAC technique provides more sensitive and precise data than the McMaster technique when counting parasite eggs in sheep, goats and rabbits’ faecal samples. This technique is then a good and valid alternative to the conventional McMaster method for parasitic infections diagnosis in livestock animals. Further studies could compare these techniques when counting other species of parasite eggs from other host species using several flotation solutions including the saturated zinc sulphate solution for trematode eggs and nematode larvae detecting.
Ethics approval and consent to participate
The present study was approved and conducted in accordance with the guidelines of the Ethical Committee of University of Abomey – Calavi (EC approval 2019/1134) and after receiving approval. Data collection was conducted using the guidelines of the World Association for the Advancement of Veterinary Parasitology (WAAVP). In addition, it is important to note that consent was obtained from all participants, especially from the owners of sheep, goats and rabbits’ farms where the faecal samples used in this study were collected.
Acknowledgements
This work was supported mainly by the INTERNATIONAL FOUNDATION FOR SCIENCE (IFS) and the CREMOPAR Regione Campania, Naples, Italy. This study would not have been completed without the small-scale farmers of the municipality of Abomey-Calavi (Southern of Benin) and the staff of the Laboratory of Ethnopharmacology and Animal Health, LESA, FSA, UAC.
Disclosure statement
The Mini-FLOTAC is a device manufactured and patented by Professor Cringoli Giuseppe from the University Federico II of Naples (Italy). Nevertheless, there was no conflict of interest in the present study.
Additional information
Funding
References
- Adjahossou BS, et al. 2017. Importance of Home Gardens in Rural Zone of the Municipality of Abomey-Calavi in south of Republic of Benin. S.A.R. 6(4):150–160. doi:10.5539/sar.v6n4p150
- Alvarado-Villalobos MA. 2017. Flotation techniques (FLOTAC and mini-FLOTAC) for detecting gastrointestinal parasites in howler monkeys. Parasite Vector. 10:1–8. doi:10.1186/s13071-017-2532-7
- Barda BD, et al. 2013a. Mini-FLOTAC. An innovative direct diagnostic technique for intestinal parasitic infections: experience from the field. PLoS Negl Trop Dis. 7(8):1–7.
- Barda BD, et al. 2013b. Mini-FLOTAC and Kato-Katz: helminth eggs watching on the shore of Lake Victoria. Parasite. Vector. 6:1–6.
- Biaou FC. 2006. Monographie de la commune d’Abomey-Calavi, Afrique Conseil, Benin, March, p. 14–21.
- Bosco A. 2014. The coprological diagnosis of gastrointestinal nematode infections in small ruminants. Doctorate thesis, University Federico II of Naples, p. 144.
- Bosco A, et al. 2014. The comparison of FLOTAC, FECPAK and McMaster techniques for nematode egg counts in cattle. Acta Parasitol. 59(4):625–628.
- Coles GC, et al. 2006. The detection of anthelmintic resistance in nematodes of veterinary importance. Vet Parasitol. 136:167–185.
- Coles GC, Bauer C, Borgsteede FH, Geerts S, Klei TR, Taylor MA, Waller PJ. 1992. World Association for the Advancement of Veterinary Parasitology (W.A.A.V.P.) methods for the detection of anthelmintic resistance in nematodes of veterinary importance. Vet Parasitol. 44:35–44.
- Core Team R. 2013. A language and environment for statistical computing: R Foundation for Statistical Computing, Vienna, Austria. [cited 24 Feb 2018]. Available from: http://www.R-project.org/.
- Cringoli G, et al. 2004. The influence of flotation solution. sample dilution and the choice of McMaster area (volume) on the reliability of the McMaster method in estimating the fecal egg counts of gastrointestinal strongyles and Dicrocoelium dendriticum in sheep. Vet Parasitol. 123:121–131.
- Cringoli G, et al. 2010. FLOTAC: new multivalent techniques for qualitative and quantitative copromicroscopic diagnosis of parasites in animals and humans. Nat Protoc. 5:503–515. DOI:10.1038/nprot.2009.235. PMID: 20203667.
- Cringoli G, et al. 2017. The Mini-FLOTAC technique for the diagnosis of helminth and protozoan infections in humans and animals. Nat Protoc. 12(9):1723–1732.
- Dias de Castro LL, et al. 2017. Comparison of McMaster and Mini-FLOTAC fecal egg counting techniques in cattle and horses. Vet Parasitol. 10:132–135.
- Emiru B, et al. 2013. Epidemiology of gastrointestinal parasites of small ruminants in Gechi District, Southwest Ethiopia. Adv Biomed Res. 7:169–174.
- Fikru R, et al. 2006. Epidemiology of gastrointestinal parasites of ruminants in Western Oromia, Ethiopia. J Appl Res Vet Med. 4(1):51–57.
- Godber OF, et al. 2015. . comparison of the FECPAK and Mini-FLOTAC faecal egg counting techniques. Vet Parasitol. 207:342–345.
- Hansen J, Perry B. 1995. Épidémiologie, Diagnostic et Prophylaxie des Helminthiases des Ruminants Domestiques (7nd edn). Rome: FAO.
- Kenyon F, et al. 2009. The role of targeted selective treatments in the development of refugia-based approaches to the control of gastrointestinal nematodes of small ruminants. Vet Parasitol. 164:3–11.
- Keyyu JD, Kyvsgaard NC, Monrad J, Kassukum AA. 2005. Epidemiology of gastrointestinal nematodes in cattle on traditional, small-scale and largescale dairy farms in Iringa District. Tanzania Vet Parasitol. 127:285–294.
- Levecke B, et al. 2012a. The bias. accuracy and precision of faecal egg count reduction test results in cattle using McMaster. Cornell-Wisconsin and FLOTAC egg counting techniques. Vet Parasitol. 188:9–194.
- Mes THM. 2003. Technical variability and required sample size of helminth egg isolation procedures. Vet Parasitol. 115:311–320. doi:10.1016/S0304-4017(03)00219-X.
- Morgan ER, et al. 2013. Global change and helminth infections in grazing ruminants in Europe: impacts. trends and sustainable solutions. Agriculture. 3:484–502.
- Noel ML, et al. 2017. Accuracy and precision of Mini-FLOTAC and McMaster techniques for determining equine Strongyle Egg counts. J Equine Vet Sci. 48:182–187.
- Owusu M, Sekyere JO, Adzitey F. 2016. Prevalence and burden of gastrointestinal parasites of Djallonke sheep in Ayeduase, Kumasi, Ghana. Vet World. 9(4):361–364.
- Rinaldi L, et al. 2014. Comparison of individual and pooled faecal samples in sheep for the assessment of gastrointestinal strongyle infection intensity and anthelmintic drug efficacy using McMaster and Mini-FLOTAC. Vet Parasitol. 205:216–223.
- Torgerson PR, Paul M, Lewis FI. 2012. The contribution of simple random sampling to observed variations in faecal egg counts. Vet.Parasitol. 188:397–440.
- Wood IB, et al. 1995. World Association for the Advancement of Veterinary Parasitology (W.A.A.V.P.) second edition of guidelines for evaluating the efficacy of anthelmintics in ruminants (bovine, ovine, caprine). Vet Parasitol. 58:181–121.