ABSTRACT
This study aimed to evaluate differences in productive performance between primiparous and multiparous Murrah buffaloes and the interrelationships between metabolic traits during the transition period and early lactation. Thirty pregnant buffaloes were monitored during the transition period and at the beginning of the lactation. Animals were randomly assigned within the two experimental groups considering the calving number and the estimated calving date: primiparous (n = 15) and multiparous buffaloes (n = 15). The buffaloes were monitored every week during the last 30 days of pregnancy, and the first 63 days postpartum. Buffaloes were kept in the same environment condition, and management practices. Multiparous buffaloes, at the postpartum period, showed higher milk fat, protein, lactose, total dry extract production, non-fat dry extract contents, and higher milk urea nitrogen and casein contents than primiparous buffaloes. Primiparous buffaloes showed higher urine pH and hematocrit concentration than the multiparous group at the prepartum period and higher leukocyte and lymphocytes concentrations at the postpartum. During the transition period, primiparous buffaloes exhibited negative interrelationships between metabolic traits and productive performance related to variations in their metabolic status. These results may indicate that multiparous buffaloes fewer sensitive to variations of metabolic status during the transition period.
Introduction
Over the years, it was possible to verify the change in the buffalo breeding system, which has shifted from a backyard activity to commercial farms and big companies (Uzun et al. Citation2018). The enormous popularity of products based on buffalo milk and meat has allowed buffalo production to follow the dairy cattle industry (Araújo et al. Citation2012; Uzun et al. Citation2020). Nevertheless, for this species have satisfactory productive performance when submitted to the pressure of intensive production systems, buffalo breeds must be improved, and research should be carried out aiming to evaluate the transition between different physiological stages and alternations in the lipid metabolism (Bauman Citation2000; Campanile et al. Citation2006).
The transition period poses a substantial metabolic and physiological challenge for the animals because, at the same time that it is noticed a reduction of nutrients intake, there is an increase in fetal growth and homeorhetic adaptations (Grummer et al. Citation2004), including mobilization of body reserves. The difference between the lower nutrient intake and increased energy requirements of females for maintenance, own body and fetal growth, and production of colostrum and milk for the newborn are related to the physiological imbalance called negative energy balance (Berry et al. Citation2007; Al Ibrahim et al. Citation2010). During this period occur modifications in blood metabolite and hormone profiles, which, consequently, influence milk yield and fertility.
Buffaloes (Bubalus bubalis) managed in the tropical environment are usually calved for the first time around 34–41 months of age to maximize the economic gain (Khattab et al. Citation1996) even if animals are not yet physically matured at this age. Consequently, buffaloes approaching their first calving are in a differing metabolic state to that experienced by multiparous buffaloes because, during this period, it is necessary nutrients for their continued growth in addition to that of their developing calf. Besides, oxidative stress is related to the body condition score (BCS) and mobilizing body reserves that are different with age (Bernabucci et al. Citation2005).
Few studies are available in the literature comparing periparturient metabolic changes in the first lactation (primiparous) and more mature buffaloes (multiparous)(Hansen et al. Citation2017) and transition period (Delfino et al. Citation2018). Besides, it was highlighted that it was available limited numbers of animals in each age group and information. Available evidence with studies in dairy cows (Coffey et al. Citation2006; Wathes et al. Citation2007) indicates that parity can influence the pattern of changes in metabolic hormones and metabolites following calving but are scarce published data for buffaloes (Fiore et al. Citation2017).
Delfino et al. (Citation2018) assessed the influence of the body condition score at calving on the metabolic status of female Murrah buffaloes in the transition period. They concluded that animals showed variations in the oxidative status during this period due to their metabolic status. Despite this, it is necessary to evaluate the alterations in blood metabolite and hormone profiles, which influence milk yield and composition in primiparous and multiparous Murrah buffaloes in the transition period at early lactation managed in a tropical environment.
Given the context, this study aimed to evaluate differences in productive performance between primiparous and multiparous Murrah buffaloes (Bubalus bubalis) and the interrelationships between metabolic traits during the transition period and early lactation. We hypothesize that the buffaloes with more than one birth show fewer changes in metabolic status, improving the productive performance of these animals.
Materials and methods
Location, animals, experimental design and diets
Data from Murrah buffaloes (Bubalus bubalis) were collected on one commercial dairy farm (Lamarão do Passé) located at São Sebastião do Passé, Bahia – Brazil. The climate in the experimental area according to the Köppen is classified as Af1, with average annual rainfall around 1600 mm and irregular distribution (Latitude: 12°30'50’ ‘South, Longitude: 38°29'43'‘ West) the annual average temperature is approximately 25°C.
The care and handling of the animals were performed in strict conformity with the recommendations of Brazil’s National Council for Animal Experimentation (CONCEA). All animal procedures were undertaken according to the regulations of the Ethics Committee in Animal Use of the School of Veterinary Medicine and Animal Science of the Federal University of Bahia, Bahia State, Brazil (Protocol number 39-2014).
Thirty pregnant buffaloes (Bubalus bubalis) were monitored during the transition period and at the beginning of the lactation. Animals were randomly assigned within the two experimental groups considering the calving number and the estimated calving date: 1) Primiparous buffaloes [body weight (BW), 570 ± 20.6 kg; body condition score (BCS) at calving in the prepartum period, 3.68 ± 0.9 (mean ± SD)]; and the expected calving date (26/10 ± 20.94),(n = 15) and; 2) Multiparous buffaloes [body weight (BW), 670 ± 20.5 kg; body condition score (BCS) in the prepartum period, 4.20 ± 0.8 (mean ± SD)] and the expected calving date (26/10 ± 19.19 d), and buffaloes’ calving number (7.7 ± 2.14)(n = 15). As described later, in the current study, the BCS was evaluated using the body-condition scoring method for Murrah buffaloes as proposed by Anitha et al. (Citation2011).
Animals were weekly monitored during the last 30 days of pregnancy (prepartum = –28, –21, –14 and –7 days), at calving (until 24 h postpartum), and +7, +14, +21, +28, +35, +42, +49, +56 and +63 days (postpartum) and allocated in the environment condition, and management practices. The average milk production of the multiparous buffaloes in the previous lactation was 8.73 kg / buffaloes / day with a total lactation period of 270 days and a total of 2357.1 kg of milk.
In the postpartum period, immediately after milking, buffaloes were fed diet composed of chopped elephant grass (Pennisetum purpureum), with the following nutrient composition, expressed in g/kg dry matter (DM): 250.1 dry matter (DM), 71.0 crude protein (CP), 768.2 neutral detergent fiber (NDF), 115.1 non-fibrous carbohydrates (NFC), ether extract (EE) and concentrate.
Buffaloes were fed the same concentrate (control basal diet) during the pre and postpartum periods, which was comprised of ground corn, soybean meal, cottonseed, urea, limestone, and mineral in the form of total mixed ration (TMR)(). According to Paul and Lal (Citation2010), the nutritional requirement of buffaloes was to meet the requirements of lactating buffaloes producing 6.0 kg/day milk with 7.0% fat and 4.2% crude protein. After the feeding, buffaloes were housed in paddocks with Brachiaria decumbens grass as supplement, with the following nutrient composition, expressed in g/kg dry matter (DM): 216.5 dry matter (DM), 110.1 crude protein (CP), 727.4 neutral detergent fiber (NDF), 142.1 non-fibrous carbohydrates (NFC), 2.21 ether extract (EE) and concentrate.
Table 1. Proportion of ingredients and chemical composition of concentrate.
Sample collection and chemical analysis
During the experimental period, feed samples were collected, dried in a forced air-oven (55°C for 72 h), ground in a Wiley mill with 1-mm screen (AH Thomas, Philadelphia, PA, USA) and stored in air-tight plastic containers until laboratory analyses.
Ground samples were analyzed for dry matter (DM; AOAC 950.15), ash (MM; ID 942.05), ether extract (EE; AOAC 920.39), crude protein (CP = N × 6.25; AOAC 984.13) contents according to the methods described by AOAC (Citation2000).
Samples were analyzed for neutral detergent fiber (NDF) contents according to Mertens (Citation2002), with the aid of a fiber analyser (TE-149 analyser, Tecnal Equipments for Laboratory Inc., Piracicaba, Brazil) using thermostable alpha-amylase and no sodium sulfide sulfit in the detergent. Dietary non-fiber carbohydrates (NFC) content was calculated using the equation proposed by Hall (Citation2000), wherein: NFC = 100 – [(% CP – % CP from urea + % urea) + % EE + % ash + % NDF].
Performance and physiological parameters
Body condition score (BCS) was measured using the body-condition scoring method for Murrah buffaloes following the technique described by Anitha et al. (Citation2011). Buffaloes were scored via visual inspection, and it was used as a graph for the classification of condition on a scale using a five-point system (1–5, with half-point divisions). Measurements were taken every week by two trained evaluators at days –28, –21, –14 and –7 (prepartum), on the calving date, and +7, +14, +21, +28, +35, +42, +49, +56 and +63 (postpartum). The evaluators were the same throughout the process and carried out their evaluations separately without communicating with each other.
Body weight (BW) was obtained on the same days used to determine the changes in body condition score (BCS). The average change body weight change (CBW), which was calculated as follows: Initially, values were obtained at weekly intervals, i.e. at week 14 body weight minus body weight at week 7 postpartum. Putting all these values together at one-week intervals, the mean value of pre-delivery and post-delivery period was determined.
Furthermore, it was measured the physiological parameters, such as rectal temperature and heart rate (beats/minute, which was evaluated using a stethoscope).
Milk sampling, yield and analysis
Buffaloes were milked mechanically once daily (0600 h) and their milk production was recorded using an automatic metre (MM6® DeLaval, Sweden). Energy-corrected milk (ECM; kg/day) was calculated using the following equation: Y = 1 + 0.01155 [(X – 40) + (Z – 31)], where Y is the amount (kg) of FCM equivalent to 1 kg of milk produced and X and Z are the grams of fat and protein present in 1 kg of milk produced, respectively (Di Palo Citation1992).
Milk samples were collected directly from the measurer once weekly at +7, +14, +21, +28, +35, +42, +49, +56 and 63 days, according to the milk production of each animal in each milking. Milk samples were analyzed fresh for fat, protein, lactose, urea nitrogen (MUN), and somatic cell count (SCC) according to the methodology described by Campanile et al. (Citation2006). Besides, milk casein concentration was measured by infrared spectrometry method (method 972.16; AOAC International Citation1999).
Complete blood count and blood metabolites analysis
Blood samples (10 mL) were collected by venipuncture from jugular vein in Vacutainers® tubes before the morning feeding (0800 h) and milking every week at days –28, –21, –14 and –7 (prepartum), on the calving date and, at +7, +14, +21, and +28 days (postpartum). Immediately after blood collection, tubes were cetrifuged (2000 x g for 15 min at room temperature)(Centribio 80-2B centrifuge Centribio, São Paulo, SP, Brazil), to obtain the plasma.
Plasma samples was used for measuring total red blood cells (RBCs), hemoglobin concentration (HGB), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC). Thus, mean corpuscular hemoglobin concentration (MCHC) was determined using the cyanmethemoglobin colorimetric technique, and mean corpuscular volume (MCV), by the micro-hematocrit method.
Besides, blood samples collected were used for analyses of total white blood cells (WBC) and differential leucocytic counts of segmented neutrophils, lymphocytes, and neutrophils using the May-Grunwald Giemsa method. The obtained serum was transferred to plastic tubes and stored at –20°C further analyses. Besides, blood samples were analyzed to measure the serum concentrations of urea (K056, Bioclin®, Brazil), total protein (K031, Bioclin®, Brazil), albumin (K040, Bioclin®, Brazil), total cholesterol (K083, Bioclin®, Brazil), glucose (K048, Bioclin®, Brazil), triglycerides (K117, Bioclin®, Brazil), calcium (K051, Bioclin®, Brazil), and phosphorus (K068, Bioclin®, Brazil) using colorimetric methods with commercial kits. Absorbances were measured on an automatic biochemistry analyzer (BioSystems®, Brazil). The concentration of globulin was calculated by the difference between the total serum protein and the albumin values, according to Robinson et al. (Citation1937).
Sampling and urinary analysis
Urine samples were collected from all animals approximately four hours after the morning feeding, when the buffaloes urinated spontaneously. Urine aliquots (10 mL) at –28, –21, –14 and –7 days (prepartum), at calving (until 24 h postpartum), and +7, +14, +21, +28, +35, +42, +49, +56 and +63 days (postpartum) were immediately diluted into a sulfuric acid solution (40 mL; 0.036 N) to maintain the pH below 3, thus avoiding uric acid precipitation (Chen and Gomes Citation1992) and ammonia volatilization (Plaizier et al. Citation2000). Samples were then stored in plastic containers at −20°C until analysis.
Urinary pH was evaluated in concentrated urine samples (without addition of sulfuric acid solution) and measured using a digital pH metre (MB-10, Marte Científica, Minas Gerais, Brazil). In these samples, it was also used Bioclin® biochemical kits with the aid of an automatic biochemistry analyzer (BioSystems®) according to the following methods: urea (fixed-time kinetic method; K056, Bioclin®, Brazil); uric acid (UA; enzymatic colorimetric method - K139, Bioclin®, Brasil); calcium (Ca; endpoint colorimetric method - Arzenazo III - K051, Bioclin®, Brazil); and sulfur (S) and chlorine (Cl; mercury thiocyanate colorimetric method - K050, Bioclin®, Brazil). Also, it was evaluated potassium (K) in urine samples, but it was determined using an MH 9180 ion-selective device (MH LabISE® 9180, Belo Horizonte, Brazil).
Statistical analyses
Data were analyzed using the PROC MIXED procedure of SAS (Version 9.1, Citation2004, SAS Institute Inc., Cary, NC), according to the model as repeated measures over time, verifying the normality of residuals and homogeneity of variances by PROC UNIVARIATE procedure.
The following model was used to estimate the effect of physiological stage (week), group (Group 1: primiparous; Group 2: multiparous), and their interaction on metabolic status indices (blood and urinary parameters): Yijk = µ + Wi + Gj + (W x G)ij + eijk
Where Yijk = dependent variable; µ = overall mean of the population; Wi = mean effect of the physiological stage (weeks) [i = prepartum (–28, –21, –14 and –7 days); postpartum (+7, +14, +21, +28, +35, +42, +49, +56 and +63 days)] with the physiological stage evaluated as a repeated factor; Gj = mean effect of group (j = 1 and 2); and eijk = unexplained residual element assumed as independent and normally distributed.
Analyzed variables were subjected to three covariance structures: compound symmetry, autoregressive order, and unstructured covariance. Thus, the best fitting covariance structures of the repeated measures were chosen according to the lower Akaike’s information criterion with correction. The data were analyzed on sampling days relative to the calving date, with day 0 representing the calving date. Average means obtained of groups were obtained using the LSMEANS procedure of SAS. Significance was declared at P < 0.05.
Results
Performance and physiological parameters
An interaction effect between group and week was observed for BW during the pre (P<0.001) and postpartum (P<0.001) periods (; A). Also, an interaction effect was noted on BCS (P = 0.039; B) and RT (P = 0.049; D) values during the postpartum period.
Figure 1. Body weight (BW)(A), body condition score (BCS)(B), change body weight (CBW)(C), and urinary pH values (D), in buffaloes. Primiparous, lactation number = 1; Multiparous = lactation number > 1. *P < 0.05, difference between primiparous and Multiparous groups in the week.
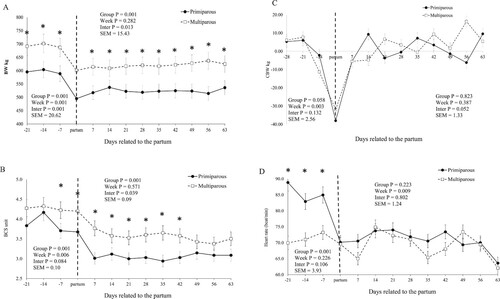
Table 2. Body weight, body condition score and clinical parameters for the different buffaloes groups during the prepartum and postpartum periods (mean ± SEM).
During the prepartum period, higher urinary pH (P<0.001) and heart rate (P<0.001) values were observed in the primiparous group than multiparous buffaloes (). In contrast, multiparous buffaloes had higher BCS during the pre and postpartum (P<0.001 and P<0.001, respectively). Higher RT values (P = 0.028) were observed in the primiparous group than multiparous buffaloes during the postpartum period.
There was a week effect on CBW (P = 0.003; C) and BCS (P = 0.006) during the prepartum period (). Also, a week effect was observed on urinary pH (P<0.001) and HR values (P = 0.009) during the postpartum period.
Milk yield and composition
There was an interaction effect between group and week for fat (P = 0.009), TDE (P = 0.008; C), expressed in percentage, and MUN (P<0.001) contents.
Figure 2. Milk yield (A), casein content (B), total dry extract production (TDE)(C), and milk fat content (D) in buffaloes. Primiparous, lactation number = 1; Multiparous = lactation number > 1. *P < 0.05, difference between primiparous and Multiparous groups in the week.
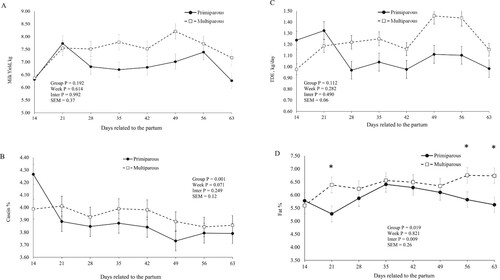
Multiparous group had higher FCM (P = 0.004), fat (P = 0.008, D), protein (P = 0.005), lactose (P = 0.005), and NFDE (P = 0.045) contents in the milk than primiparous group, expressed in Kg/day ().
Table 3. Milk yield and composition for the different buffaloes groups during the prepartum and postpartum periods (mean ± SEM).
Higher protein (P = 0.046), lactose (P<0.001), NFDE (P = 0.030) and casein (P = 0.001; B) contents, expressed in percentage, were observed in multiparous group than primiparous buffaloes ().
There was a week effect on protein (P = 0.013), lactose (P = 0.012) in percentage, and MUN contents (P<0.001)().
Complete blood count
There was an interaction effect between group and week for MCHC in the postpartum period (P = 0.026), whereas for lymphocytes (P = 0.012), it was noted in the prepartum period ().
Table 4. Weekly mean values of hemogram and leukogram for the different buffaloes groups during the prepartum and postpartum periods(mean ± SEM).
Higher hematocrit (P = 0.017), leukocytes (P<0.001), segmented neutrophils (P<0.001), and lymphocytes (P = 0.001) were observed in the primiparous group than multiparous buffaloes during the prepartum period (). Also, higher MCHC (P = 0.039), leukocytes (P = 0.033), and lymphocytes (P = 0.005) were observed in the primiparous group than multiparous, during the postpartum period ().
By contrast, the multiparous group had higher MCHC (P = 0.012), MCV (P<0.001) than primiparous buffaloes during the prepartum and, MCV (P<0.001) in the postpartum ().
There were a week effect on the hemoglobin (P = 0.046) and lymphocytes (P = 0.017) during the prepartum period. Also, a week effect was observed on the erythrocytes (P = 0.001), hematocrit (P = 0.014), MCV (P<0.001), red blood cell (P = 0.001) in the postpartum period ().
Blood metabolites
Higher glucose (P = 0.014), cholesterol (P = 0.013), triglycerides (P = 0.041) and calcium (P = 0.001) concentrations were observed in primiparous group than multiparous buffaloes during the prepartum period (). Also, higher cholesterol (P = 0.019) and calcium (P<0.001) concentrations were noted in the primiparous group than multiparous buffaloes during the postpartum period ().
Table 5. Weekly mean values of blood metabolites for the different buffaloes groups during the prepartum and postpartum periods (mean ± SEM).
Multiparous buffaloes had higher total protein (P = 0.044) and globulin (P = 0.025) concentrations during the prepartum period (). Similarly, higher total protein (P = 0.044), globulin (P = 0.019) and urea (P = 0.016) concentrations were observed in multiparous buffaloes than primiparous during the postpartum period.
In addition, there was a week effect on triglycerides (P = 0.041) during the prepartum period and, on glucose (P = 0.049), urea (P = 0.008) and total cholesterol (P = 0.007) concentrations in the postpartum period ().
Urinary metabolites
There was an interaction effect for urea content during the postpartum period (P = 0.016)().
Table 6. Urinary metabolites concentrations for the different buffaloes groups during the prepartum and postpartum periods (mean ± SEM).
Higher S and urea contents were observed in the primiparous group during the prepartum (P<0.001 and P<0.001, respectively), and during the postpartum (P<0.001 and P<0.001, respectively) periods than multiparous buffaloes (). In contrast, the multiparous group had higher K content (P<0.001) during the postpartum period than primiparous buffaloes.
Discussion
Performance and physiological parameters
During the pre- and postpartum periods, the multiparous group showed higher body weight and body condition score (BCS) than primiparous buffaloes. According to Roche (Citation2007) and Anitha et al. (Citation2011), these results observed can be attributed to the method used and the correlation that occurs between BW and BCS and maturity stage. We observed alteration on the body weight loss and the BCS (46.5 kg and 0.79 points for primiparous vs. 49.2 kg and 0.70 points for multiparous) of the groups during the transition between the pregnant non-lactating and lactating non-pregnant physiological stages.
In this study, there was a loss of initial body weight in the prepartum period in both groups evaluated. Therefore, the primiparous group lost 8.1% of their initial body weight, whereas the multiparous animals lost 7.3% of their weight. Despite evaluating buffaloes of different ages, it is necessary to emphasize that the recommended BCS at calving for dairy cows may be different for dairy buffaloes due to differences in metabolism between the species (Serrapica et al. Citation2020). We observed higher BCS from the multiparous group than the primiparous group in the prepartum (4.25 unit vs. 3.84 unit, respectively) and postpartum (3.55 unit vs. 3.05 unit, respectively). This result can be justified due to the relationship between BCS and calving order because adipose tissue has higher insulin resistance in multiparous cows (Karis et al. Citation2020).
As mentioned by Bauman (Citation2000), there is an alteration in the endocrine profiles, and lipolysis and lipogenesis are controlled to improve the lipid reserves through the pregnancy period. Furthermore, Roche et al. (Citation2009) reported that lipid metabolism is regulated by homeostatic and homeorhetic mechanisms. At the beginning of their first lactation, the mammary gland's competing demands are thus superimposed on the growth requirements (Wathes et al. Citation2007).
Some physiologic differences have been related in the literature between primiparous and multiparous cows. Wathes et al. (Citation2007) and Moyes (Citation2004) reported higher concentrations of IGF-I in primiparous cows than multiparous, and similarly, lower concentrations of insulin were described in primiparous than multiparous cows. These conditions can make cows more susceptible and vulnerable to variations in metabolic status. In female beef cattle, blood somatropin concentrations tend to be lower, and the insulin concentration is higher when compared with dairy-purpose breeds.
We measured some clinical parameters and blood metabolites associated with variations in the buffaloes’ metabolic status during the change in the physiological stage. From the results obtained, we found that the multiparous group had a higher rectal temperature in comparison to the primiparous group in the prepartum period. Rectal temperature is an essential measurement in the evaluation of physiological parameters, and this result can be attributed to the higher thermogenesis for the multiparous group compared to the primiparous group; so it is associated with variations of the metabolic status of the animals and albumin production (Celi et al. Citation2008; Ganaie et al. Citation2013).
Dandona et al. (Citation2004) suggested two mechanisms that might be linked to obesity and oxidative stress. First, the excessive macronutrient intake, and second, the increased secretion of proinflammatory cytokines (interleukin-6 and tumour necrosis factor - α) by adipose tissue in obese individuals. In addition to the temperature rectal, this study was carried out to assess the animal's frequency heart. In this way, indirectly, the entire lipidogram (cholesterol, triglycerides, non-esterified fatty acids, beta-hydroxybutyrate) can be indicators of oxidative stress. The alteration of oxidative status after calving might be related to the reduction of plasma and erythrocyte concentration (Keaney and Frei Citation1994). Albumin is exclusively synthesized by the liver, and it is the primary source of plasma thiol groups (SH). Glutathione is mainly synthesized again within the liver (Jefferies et al. Citation2003). The reduction of liver function that is usually observed in the early postpartum might explain lower plasma and erythrocyte thiol groups levels.
Milk yield and composition
No differences were detected in milk yield between both the groups. Despite this, the higher yield of fat-corrected milk, fat, protein, lactose, and non-fat dry extract and higher milk contents of fat, protein, lactose, total dry extract production, and the non-fat dry extract was noticed in the multiparous group. Some factors may be related to these results observed, such as the stage of maturity and age. The group of multiparous buffaloes has a higher capacity of dry matter intake and a higher capacity of adaptation to the metabolic changes. Thus, the multiparous buffaloes have returned to a positive balance about one week postpartum (C) later than the primiparous buffaloes. This group showed a capacity for higher solids production without damage to return for the weight recovery. Thus, this result represents a higher adaptation to physiological changes.
Besides, the body condition score (BSC) is an additional factor that can explain these modifications in the composition and production of solids. This result possibly can be associated with the mobilization of body reserves, since it may be higher for multiparous cows with high BCS. However, there was no difference on change of body condition score (CBCS) between groups. According to Bell (Citation1995), animals with a higher BCS have a higher mobilization of body fat (NEFA) from the adipose tissue into the bloodstream. This alteration, also according to the authors, can contribute to increasing the group of fatty acids that form the milk fat, consequently favouring the capture of long-chain fatty acids from the blood to the mammary gland, which promotes higher incorporation of the milk. Anitha et al. (Citation2011) evaluated a BCS classification system in Murrah buffaloes in different body score condition (BSC) groups and reported that BCS at calving influenced the milk composition.
The body protein that comes from catabolism and the deamination of the additional dietary protein can contribute to the blood urea nitrogen pool. As the blood is secreted from the mammary gland, urea is diffused into and out of the gland, coming into balance with the blood urea. Thus, this process enables milk urea nitrogen to be an outstanding predictor of the urea in the blood and the urinary nitrogen. However, in this study, there was no difference for CBW in the evaluated groups, and thus only this result can not explain this difference in the MUN content. We attributed the higher MUN content to the increase in protein consumption that may have occurred due to the multiparous group's higher consumption capacity (Kauffman and St-Pierre Citation2001).
Complete blood count and metabolites analyses
At the prepartum period, it was observed that the primiparous group showed a higher urine pH value than the multiparous group. Monitoring urine pH is a feasible method for determining the animals’ response to dietary anions because it generally reflects the acid–base status of an animal. Our objective in this study was to monitor urine pH values to determine possible variations in values according to the physiological phase and discuss another metabolic status, including immunological status for buffaloes.
The changes in urinary pH can be attributed to the higher urinary concentration of calcium, and also due to the metabolic acidosis might have improved the calcium reabsorption of bones and intestines; the Ca absorption has been attributed an increase in the synthesis of 1,25-dihydroxy vitamin D3 (1,25 (OH)2 D3)(Roche et al. Citation2009). However, in this study, no differences were detected in Ca excretion in the urine between the groups.
Higher concentrations of hematocrit, leukocyte, segmented neutrophil, and lymphocytes were noticed in the primiparous group at the prepartum period. This result indicates that primiparous females arrived at delivery with an improved physiological condition that multiparous. The amounts of leukocytes and neutrophils targeted in the postpartum period were higher for primiparous. This result indicates that the defense system naturally anticipated metabolic stress reducing the risk of infection and physiological changes because that is an animal at the growth period. Physiologically, at the end of pregnancy, the number of red blood cells rises due to the erythropoietic effect of the chorionic placental somatotropin, and also because of progesterone, and prolactin (Roche et al. Citation2009). The erythropoietic effect or erythropoiesis is the process that produces red blood cells, white blood cells (lymphocytes, monocytes, eosinophils, granulocytes, neutrophils, and basophils) and platelets. Besides, the increased blood volume is a reaction to the placental uterine circulation and fetal development, keeping the oxygenation of the tissue and blood pressure at acceptable levels. However, the nutritional condition can modify the blood volume, the erythropoietic effect, and milk yield. In agreement with Brun-Hansen et al. (Citation2006), high-producing animals show inferior concentrations of blood erythrocytes.
During the postpartum period, concentrations of mean corpuscular hemoglobin concentration, leukocytes, and lymphocytes and higher concentrations of mean corpuscular volume were inferior for the multiparous group than the primiparous buffaloes. This result can be attributed to the higher inflammatory status in the prepartum period. Another reason is the glucocorticoid hormone effects in the primiparous group that increased the concentration of lymphocytes (Brun-Hansen et al. Citation2006).
Given that mean corpuscular volume measures the size of the hemaceae, it is possible to infer that lower values are related to reduced hemaceae. Leukocytes participate in the protection of the host against the pathogen and in the monitoring and removal of non-self antigens. The increased blood leukocyte concentrations may be assigned to the lower nutritional condition of the primiparous group at calving. Primiparous buffaloes are animals with lesser behaviour to be affected by oxidative stress than multiparous buffaloes. So, the physiological changes before parturition such as colostrum production and adaptation to the milking parlour may have caused in the primiparous buffaloes higher stress during collections and at the pre-delivery management, verified by an increase in heart rate (P<0.001) in the 3 weeks before delivery (D). Thus, a more intense immune response occurred.
As abovementioned, the trend observed in the current study occurs because an efficient immune response is based on the interaction and the balance between different types of cells and their products. As the calving date approaches, the total number of leukocytes increases, primarily due to the absolute growth in the number of neutrophils (Graugnard et al. Citation2012).
In the prepartum period, it was observed high neutrophil levels in the primiparous group. Although the BCS has not been low for both groups the decay in nutritional status accompanied by the improves in the changes of metabolic status can increase the neutrophil concentration. So, it is elucidated by the fact that the phagocytosis of the microorganisms given that is the primary function of neutrophils (Graugnard et al. Citation2012). This result denotes one of the main lines of defense of the host against pathogens; leukocytes, predominantly, are often produced on a large scale in hosts with the bacterial load.
In agreement with the authors above mentioned, in a study carried out with dairy cows, Bernabucci et al. (Citation2005) concluded that dairy cows during the transition period showed some variations of the oxidative status related to metabolic status. When reactive oxygen metabolites (ROM) are produced faster than they can be neutralized by antioxidant mechanisms, oxidative stress can result. After calving, cows with high BCS at calving and high lipid mobilization have a more pronounced alteration of oxidative status. These conditions can make cows more sensitive to oxidative stress. Our results highlight the need to further investigate the possible role of oxidative stress in determining disorders related to obesity in transition dairy cows.
The multiparous group had a higher K content in the postpartum period (Lager and Jordan Citation2012). On the other hand, were verified higher S content during the pre- and postpartum periods and higher urea content during the pre- and postpartum periods in the primiparous group. No differences were observed in the concentrations of urinary metabolites between both groups in the present experiment, except for K, S, and urea, which differed between the groups in the pre and postpartum. The K and Cl concentrations are required to keep the osmotic pressure and acid–base regulation.
In the peripartum, as described by Alvarenga et al. (Citation2015), there is a positive correlation between the concentrations of calcium, phosphorus, and albumin in the blood. The primiparous group showed higher total plasma glucose, total cholesterol, and calcium in the prepartum period. The primiparous buffaloes have smaller metabolic challenges than the multiparous buffaloes, so there were higher blood glucose concentrations of the primiparous group (Coffey et al. Citation2006). The higher plasma total cholesterol for the primiparous group can be attributed to the central mobilization of body fuel, specifically fat. Thus, the interrelationships between metabolic traits and productive performance were positive for differences in metabolic status and the highest productive performance for the multiparous group.
During the transition period, buffaloes display some variations in oxidative status due to their metabolic status, which can be assessed through the rectal temperature and animals’ heart rate and indirectly by the whole lipidogram (cholesterol, triglycerides urea, erythrocyte, albumin). So, they can be relevant parameter indicators of variations of metabolic status and, consequently, oxidative stress, as previously explained.
According to the results of this study, multiparous buffaloes during the transition period, especially in the prepartum period, display the smallest variations in the hemogram than the primiparous group due to physiologic maturity decreasing the defense system and white cells. Most productive performance results indicate that the multiparous buffaloes showed more stability, which increased the production of the solids in the milk.
Conclusions
After calving, primiparous buffaloes during the transition period show negative interrelationships between metabolic traits and productive performance variations of their metabolic status. These conditions may indicate that multiparous buffaloes are fewer sensitive to variations of metabolic status during the transition period.
Acknowledgments
The authors are thankful to Mr. Urbano Antônio Souza Filho, the dairy company Bufalissima, and the Natal Farm for their technical assistance and provided the animals to conduct the research. We also thank the Coordination for the Improvement of Higher Education Personnel (CAPES) for the project's financial support of the project.
Disclosure Statement
No potential conflict of interest was reported by the author(s).
References
- Al Ibrahim RM, Kelly AK, O’Grady L, Gath VP, McCarney C, Mulligan FJ. 2010. The effect of body condition score at calving and supplementation with saccharomyces cerevisiae on milk production, metabolic status, and rumen fermentation of dairy cows in early lactation. J Dairy Sci. 93:5318–5328. doi:10.3168/jds.2010-3201.
- Alvarenga EA, Moreira GHFA, Facury Filho EJ, Leme FOP, Coelho SG, Molina LR, Lima AM, Carvalho AU. 2015. Evaluation of the metabolic profile of Holstein cows during the transition period. Rev Bras Zootec. 35:281–290. doi:10.1590/S0100-736X2015000300012.
- Anitha A, Rao KS, Suresh J, Moorthy PS, Reddy YK. 2011. A body condition score (BCS) system in Murrah buffalos. Buffalo Bull. 30:79–99.
- AOAC. 1999. Official Methods of Analysis (16th ed.). Arlington, VA, USA: Association of Official Analytical Chemists.
- AOAC. 2000. Official Methods of Analysis (17th ed.). Gaithersburg, MD, USA: Association of Official Analytical Chemists.
- Araújo KB, Rangel AH, Fonseca FC, Aguiar EM, Simplício AA, Novaes LP, Júnior DML. 2012. Influence of the year and calving season on production, composition and mozzarella cheese yield of water buffalo in the State of Rio Grande do norte, Brazil. Ital J Anim Sci. 11:e16. doi:10.4081/ijas.2012.e16.
- Bauman DE. 2000. Regulation of nutrient partitioning during lactation: homeostasis and homeorhesis revisited. In: Cronje PB, editor. Ruminant physiology: digestion, metabolism, growth and reproduction. Wallingford, UK: CAB International; p. 311–328.
- Bell AW. 1995. Regulation of organic nutrient metabolism during transition from late pregnancy to early lactation. J Anim Sci. 73:2804–2819. doi:10.2527/1995.7392804x.
- Bernabucci U, Ronchi B, Lacetera N, Nardone A. 2005. Influence of body condition score on relationships between metabolic status and oxidative stress in periparturient dairy cows. J Dairy Sci. 88:2017–2026. doi:10.3168/jds.S0022-0302(05)72878-2.
- Berry DP, Lee JM, Macdonald KA, Roche JR. 2007. Body condition score and body weight effects on dystocia and stillbirths and consequent effects on postcalving performance. J Dairy Sci. 90:4201–4211. doi:10.3168/jds.2007-0023.
- Brun-Hansen HC, Kampen AH, Lund A. 2006. Hematologic values in calves during the first 6 months of life. Vet Clin Pathol. 35:182–187. doi:10.1111/j.1939-165X.2006.tb00111.x.
- Campanile G, Neglia G, DiPalo R, Gasparrini B, Pacelli C, D’Occhio MJ, Zicarelli L. 2006. Relationship of body condition score and blood urea and ammonia to pregnancy in Italian Mediterranean buffaloes. Reprod Nutr Dev. 46:57–62. doi:10.1051/rnd:2005066.
- Celi P, Di Trana A, Quaranta A. 2008. Metabolic profile and oxidative status in goats during the peripartum period. Aust J Exp Agric. 48:1004–1008. doi:10.1071/EA07410.
- Chen XB, Gomes MJ. 1992. Estimation of Microbial Protein Supply to Sheep and Cattle Based on Urinary Excretion of Purine Derivatives - An Overview of the Technical Details. Occasional Publication. International Feed Resources Unit, Rowett Research Institute, Aberdeen, UK.
- Coffey MP, Hickey J, Brotherstone S. 2006. Genetic aspects of growth of Holstein–Friesian dairy cows from birth to maturity. J Dairy Sci. 89:322–329. doi:10.3168/jds.S0022-0302(06)72097-5.
- Dandona P, Aljada A, Bandyopadhyay A. 2004. Inflammation: the link between insulin resistance, and obesity and diabetes. Trends Immunol. 25:4–7. doi:10.1016/j.it.2003.10.013.
- Delfino NC, Bulcão LFA, Alba HDR, Oliveira MXS, Queiroz FPS, Carvalho GGP, Rennó FP, Freitas Júnior JE. 2018. Influence of body condition score at calving on the metabolic status and production performance of Murrah buffaloes (Bubalus bubalis) during the transition period. Asian-Australas J Anim Sci. 31:1756–1765. doi:10.5713/ajas.17.0223.
- Di Palo R. 1992. Produzione lattea nella bufala con diete tradizionali e con impiego di acidi grassi [Ph.D. Thesis], University of Naples, Italy.
- Fiore E, Giambelluca S, Morgante M, Contiero B, Mazzotta E, Vecchio D, Vazzana I, Rossi P, Arfuso F, Piccione G, Gianesella M. 2017. Changes in some blood parameters, milk composition and yield of buffaloes (Bubalus bubalis) during the transition period. Anim Sci J. 88:2025–2032. doi:10.1111/asj.12872.
- Ganaie AH, Shanker G, Bumla NA, Ghasura RS, Mir NA. 2013. Biochemical and physiological changes during thermal stress in bovines. J Vet Sci Technol. 4:126–135. doi:10.4172/2157-7579.1000126.
- Graugnard DE, Bionaz M, Trevisi E, Moyes KM, Salak-Johnson JL, Wallace RL, Drackley JK, Bertoni G, Loor JJ. 2012. Blood immunometabolic indices and polymorphonuclear neutrophil function in peripartum dairy cows are altered by level of dietary energy prepartum. J Dairy Sci. 95:1749–1758. doi:10.3168/jds.2011-4579.
- Grummer RR, Mashek DG, Hayirli A. 2004. Dry matter intake and energy balance in the transition period. Vet Clin N Am: Food A. 20:447–470. doi:10.1016/j.cvfa.2004.06.013.
- Hall MB. 2000. Calculation of Non-structural carbohydrate content of feeds that contain Non-protein nitrogen. Gainesville, FL, USA: University of Florida. p. A-25 (Bulletin-339).
- Hansen HH, El-Bordeny NE, Ebeid HM. 2017. Response of primiparous and multiparous buffaloes to yeast culture supplementation during early and mid-lactation. Anim Nutr. 3:411–418. doi:10.1016/j.aninu.2017.08.005.
- Jefferies H, Coster J, Kahlil A, Bot J, McCauley RD, Hall JC. 2003. Glutathione. ANZ J Surg. 73:517–522. doi:10.1046/j.1445-1433.2003.02682.x.
- Karis P, Jaakson H, Ling K, Bruckmaier RM, Gross JJ, Pärn P, Kaart T, Ots M. 2020. Body condition and insulin resistance interactions with periparturient gene expression in adipose tissue and lipid metabolism in dairy cows. J Dairy Sci. 103:3708–3718. doi:10.3168/jds.2019-17373.
- Kauffman AJ, St-Pierre NR. 2001. The relationship of milk urea nitrogen to urine nitrogen excretion in Holstein and jersey cows. J Dairy Sci. 84:2284–2294. doi:10.3168/jds.S0022-0302(01)74675-9.
- Keaney JF, Frei B. 1994. Antioxidant protection of low-density lipoprotein and its role in the prevention of atherosclerotic vascular disease. In: B. Frei, editor. Natural antioxidants in human health and disease. San Diego, CA: Academic Press Ltd; p. 303–351.
- Khattab RM, El-Shamaa IS, Ibrahim MA, Darwish SA. 1996. Pubbery and post-pubertal seminal changes leading to sexual maturity of winter and summer born buffalo males. J Agric Anim Sci. 34:1563–1575.
- Lager K, Jordan E. 2012. The metabolic profile for the modern transition dairy cow. In: Jordan E, editor. Proceedings of the mid-south ruminant nutrition conference 2012. Grapevine, TX: Texas A and M University: College Station; p. 9–16.
- Mertens DR. 2002. Gravimetric determination of amylase-treated neutral detergent fiber feeds with refluxing in beakers or crucibles: collaborative study. J AOAC Int. 85:1217–1240. doi:10.1093/jaoac/85.6.1217.
- Moyes TE. 2004. Variation in concentrations of insulin-like growth factor-1 (IGF-1) in pasture-fed Holstein–Friesian cows [Ph.D. Thesis]. Australia: University of Melbourne.
- Paul SS, Lal D. 2010. Nutrient requirements of buffaloes. Azadpur: Satish Serial Publishing House.
- Plaizier JC, Martin A, Duffield T, Bagg R, Dick P, McBride BW. 2000. Effect of a prepartum administration of monensin in a controlled-release capsule on apparent digestibilities and nitrogen utilization in transition dairy cows. J Dairy Sci. 83:2918–2925. doi:10.3168/jds.S0022-0302(00)75192-7.
- Robinson HW, Price JW, Hogden CG. 1937. The estimation of albumin and globulin in blood serum. 1. A study of the errors involved in the filtration procedure. J Biol Chem. 120:481–498. doi:10.1016/S0021-9258(18)45109-5.
- Roche JR. 2007. Milk production responses to pre- and postpartum dry matter intake in grazing dairy cows. Livest Sci. 110:12–24. doi:10.1016/j.livsci.2006.08.016.
- Roche JR, Turner LR, Lee JM, Edmeades DC, Donaghy DJ, Macdonald KA, Penno JW, Berry DP. 2009. Weather, herbage quality and milk production in pastoral systems. 2. temporal patterns and intra-relationships in herbage quality and mineral concentration parameters. Anim Prod Sci. 49:200–210. doi:10.1071/EA07308.
- SAS. 2004. SAS Systems for windows SAS 9.0 procedures guide. Cary, NC, USA: SAS Institute Inc.
- Serrapica F, Masucci F, Romano R, Napolitano F, Sabia E, Aiello A, Francia AD. 2020. Effects of chickpea in substitution of Soybean meal on milk production, blood profile and reproductive response of primiparous buffaloes in early lactation. Animals (Basel). 10:515. doi:10.3390/ani10030515.
- Uzun P, Masucci F, Serrapica F, Napolitano F, Braghieri A, Romano R, Manzo N, Esposito G, Di Francia A. 2018. The inclusion of fresh forage in the lactating buffalo diet affects fatty acid and sensory profile of mozzarella cheese. J Dairy Sci. 101:6752–6761. doi:10.3168/jds.2018-14710.
- Uzun P, Serrapica F, Masucci F, Assunta BCM, Yildiz H, Grasso F, Di Francia A. 2020. Diversity of traditional caciocavallo cheeses produced in Italy. Int J Dairy Technol. 73:234–243. doi:10.1111/1471-0307.12640.
- Wathes DC, Cheng Z, Bourne N, Taylor VJ, Coffey MP, Brotherstone S. 2007. Differences between primiparous and multiparous dairy cows in the inter-relationships between metabolic traits, milk yield and body condition score in the periparturient period. Domest Anim Endocrinol. 33:203–225. doi:10.1016/j.domaniend.2006.05.004.
