ABSTRACT
The objective of this study was to investigate the effects of rumen-protected methionine (RPM) supplementation during early lactation on performance and metabolic parameters of dairy cows. Forty-two Holstein cows were blocked by parity (22 primiparous and 20 multiparous) and calving date, then randomly assigned to two groups, Control and RPM. From calving through 29 ± 8 d in milk, cows received an early lactation diet [1.59 Mcal/kg of DM, 10.7% rumen-degradable protein and 6.5% rumen-undegradable protein] with no added methionine (Control, n = 21) or with supplementation of Smartamine M (RPM, Adisseo Inc, n = 21). RPM cows were supplemented with 12 g/day Smartamine M (7.2 g of metabolizable methionine), individually top-dressed over the total mixed ration. Blood and milk samples were collected during the first two weeks of lactation and milk yield recorded until 30 days in milk. No differences in milk yield or blood metabolites were observed. Cows supplemented with RPM increased milk fat and total solids contents and fat-to-protein ratio by 0.48% units, 0.66% units, and 0.09 units, while tended to increase milk total protein content by 0.13% units, respectively. This study showed beneficial effects of early postpartum RPM supplementation on milk composition of dairy cows.
Highlights
Rumen-protected methionine supplementation has a positive effect on milk composition.
In a well-managed dairy herd, healthy postpartum cows supplemented with methionine prioritize their milk components, with no effect on blood metabolites.
Introduction
The transition period, as defined by Drackley (Citation1999), comprises the three weeks before to three weeks after parturition, and it is a critical period to health, production, and profitability of dairy cows. A typical characteristic of this period is the drastic increase in nutrient requirements, including energy, amino acids, glucose, calcium, and others (Overton and Waldron Citation2004). This increase in nutrient requirements is primarily due to fetal growth and lactogenesis. Additionally, during the prepartum cows commonly experience a decrease in dry matter intake (DMI). Although immediately after parturition there is a progressive increase, feed intake is generally insufficient in parallel with the nutrient requirements in early lactation, resulting in negative energy balance (NEB) and the consequent mobilization of energy from the adipose tissue (Contreras and Sordillo Citation2011). Metabolic and immune dysfunctions are frequently associated with the transition period in dairy cows, especially those related to the hepatic role during NEB, such as ketosis and lipidosis. In turn, these conditions have been correlated with other diseases such as hypocalcemia, retained placenta, displaced abomasum, and mastitis.
The supplementation of Met during the transition period has been studied with the purpose of minimizing metabolic disorders and to promote healthier and more productive cows as they transition from pregnancy to lactation (Osorio et al. Citation2013; Roche et al. Citation2013; Sordillo Citation2016). Although not unanimously stablished in literature, methionine has been proposed to have a lipotropic role in the liver by promoting the synthesis of apolipoprotein B-100, which is an essential component for very low-density lipoprotein (VLDL) synthesis, and consequently could lead to a reduction on postpartum triglyceride accumulation in hepatic tissue (Strzetelsk et al. Citation2009; Osorio et al. Citation2013). In addition, Met has a strong impact on the oxidative stress status because it is essential for the synthesis of glutathione, one of the most abundant antioxidants produced in the liver (Osorio et al. Citation2013).
Methionine also has an essential role in milk protein production. According to Schwab and Boucher (Citation2007), Met is usually the first limiting amino acid for milk protein production, followed by lysine and histidine. The authors emphasize that adequate diets for dairy cows must provide rumen-protected methionine forms in order to achieve an adequate proportion of Met and Lys. In compliance with the NRC (Citation2001), levels of Lys and Met needed in metabolizable protein (MP) to optimize milk protein concentration are 7.2 and 2.4% of MP, respectively. In contrast, due to the difficulty to achieve such high levels of Lys, especially in corn based diets, Schwab and Boucher (Citation2007) suggest practical target formulation levels of 6.6 Lys and 2.2 Met as a % of MP, resulting a 3:1 Lys:Met ratio. However, since there is an apparent higher demand for Met in early lactation cows, a 2.8:1 Lys:Met ratio has been suggested by Osorio et al. (Citation2013) as a better option. Based on the above, we hypothesized that the supplementation of RPM exclusively on the postpartum period would increase milk yield and milk protein content as well as improve the hepatic lipid metabolism of fresh dairy cows at a commercial farm. The objectives of this study were to evaluate the effects of RPM supplementation during early lactation on productive and metabolic parameters of dairy cows.
Materials and methods
Animal housing and care
The Comity of Ethics in the Use of Animals of the Federal University of Paraná approved all procedures for this study (protocol no. 053/2016). The experiment was conducted in a commercial dairy farm located in the State of Paraná, Southern Brazil, from January to February 2017. All cows were housed in a compost barn system during the prepartum period and in a free-stall system during the experimental postpartum period. Cows were fed once daily (1300 h) and milked thrice daily (0500, 1200, and 1900h).
Experimental design and treatments
Forty-two Holstein Friesian cows, 22 primiparous and 20 multiparous, were blocked according to parity and calving date. Cows within each block were randomly assigned to 1 of the 2 treatments. From calving to 29 ± 8 days in milk (DIM), cows received a basal control diet with no added methionine (Control, n = 21) or a basal control diet plus Smartamine M (RPM, Adisseo Inc, n = 21). The Control group received 50 g/cow/day of corn meal as a placebo, while the RPM group received a 12 g/cow/day supplementation of RPM (Smartamine M, Adisseo Inc.) plus 38 g/cow/day of corn meal as carrier. Smartamine M product contains 75% DL-methionine protected from ruminal microbiota degradation, with a bioavailability of 80% (Schwab Citation1995). Thus, for every 12 g of rumen-protected methionine, each animal was supplemented with a total of 7.2 g of metabolizable methionine.
During the close-up period (−21 d to parturition), all cows received the same diet (1.59 Mcal/kg of DM, 11.7% rumen-degradable protein, and 4.3% rumen-undegradable protein) with no RPM supplementation, while during the early lactation period, animals received a basal control diet (1.59 Mcal/kg of DM, 10.7% rumen-degradable protein, and 6.5% rumen-undegradable protein) plus treatments. After the second daily milking (1300 h), cows were locked up in head-gates and both treatments were individually top-dressed on fresh total mixed ration. The ingredient and nutrient compositions of the diets are shown on and , respectively. Diets were formulated to meet cow’s predicted requirements, according to NRC (Citation2001).
Table 1. Ingredient composition of diets fed during close-up (−21 d relative to calving) and early lactation (+29 d relative to calving) periods.
Table 2. Nutrient composition and evaluation of diets fed during the close-up (−21 d relative to calving) and early lactation (+29 d relative to calving) periods.
Milk and feed samples
Close-up and early lactation diets samples were collected once a week, while corn silage and grass hay samples were collected at the beginning and end of the experimental period. Samples were kept under refrigeration (−20 °C) throughout the trial and analyzed for dry matter at 100 °C (DM), crude protein (CP), ether extract (EE), neutral detergent fibre (NDF), acid detergent fibre (ADF), lignin, and ash by the Laboratory of Animal Nutrition of UFPR (Curitiba, Brazil). Calcium, phosphorus, and potassium concentrations were also estimated by wet chemistry at the same lab. Crude protein was determined by micro-Kjeldahl steam distillation, while NDF and ADF contents were determined according to the methodology by Van Soest et al. (Citation1991).
Milk yield was recorded daily during the first 30 DIM. Milk energy output was calculated based on the following equation: milk yield × [(0.0929 × fat %) + (0.0547 × crude protein %) + (0.0395 × lactose %)] (NRC Citation2001). During the first 2 wk of lactation, milk samples were collected once a week, during three consecutive milkings, and actual sampling days relative to parturition were 8.1 ± 3.1 and 13.8 ± 2.6 DIM. At each milking, around 40 mL of milk was stored in polyethylene pots with bronopol preservative. Milk samples were analyzed for fat, total protein, lactose, total solids, casein, and milk urea nitrogen (MUN) by a commercial laboratory (DHI lab at APCBRH, Curitiba, Brazil) in a Bentley NexGen equipment (Bentley Instruments®) through infrared spectrometry.
Blood samples
Blood samples were collected from the coccygeal vein or artery on 0, 1, 3, 5, 7, and 14 d relative to parturition. Samples were collected into evacuated serum tubes (Vacuette; Greiner Bio-One, Kremsmünster, Austria) containing clot activator to obtain serum. Blood samples were handled according to Stokol and Nydam (Citation2005) recommendations. Immediately after collections, samples were centrifuged at 1,300 × g for 10 min to separate the serum, stored in duplicates in microcentrifuge tubes, and frozen at −20°C until analysis by the Laboratory of Veterinary Clinical Pathology of UFPR (Curitiba, Brazil).
The following metabolites were analyzed in the serum samples: urea nitrogen, aspartate aminotransferase (AST), triglycerides, total cholesterol, glucose, total proteins, albumin, globulin, bilirubin, NEFA (non-esterified fatty acids), and BHBA (β-hydroxybutyrate). Concentrations of NEFA and BHBA were quantified by colorimetric enzymatic methodology using commercial standard reagents (FA115 Kit and RB 1007 Kit, Randox, Crumlin, UK). Regarding the other metabolites, analyses were performed in an automatic biochemical analyser (Mindray BS-200). Hemolyzed samples were discarded (Stokol and Nydam Citation2006).
Statistical analysis
Data were analyzed using PROC MIXED of SAS 9.4 (SAS Institute Cary NC, USA) with a model containing the effects of block, treatment, time, and treatment × time interaction as fixed effects and cow within treatment as a random effect. Individual animal was considered as the experimental unit. For each variable, the covariance structure was chosen among compound symmetry, first-order autoregressive structure, and unstructured based on goodness of fit (smaller AIC, AICC, and BIC). Blood metabolites at 0 d relative to parturition were used as covariables. Statistical significance was declared at p<0.05, and tendencies at p<0.10.
Results and discussion
Milk yield and composition
Main effects and interactions for milk yield, milk energy output, and milk composition are shown in and . No differences were observed for milk yield (p=0.51) or milk energy output (p=0.14) during the first 30 DIM when comparing treatments. Milk yield had an increase over time (p<0.01), which is expected in early lactation (A). Our results are in agreement with similar studies (e. g. Benefield et al. Citation2009; Ordway et al. Citation2009; Preynat et al. Citation2009), which did not detect significant improvements in milk yield when dairy cows were supplemented with RPM. According to Ordway et al. (Citation2009), when evaluating the effects of RPM on periparturient dairy cows, it appears that lower milk yield was accompanied by lower DMI for multiparous cows fed Smartamine M. A similar effect was observed by Socha et al. (Citation2005), who found that cows receiving Smartamine M consumed less feed when compared with cows receiving a basal died or a combination of rumen-protected Met and Lys. In contrast, Zhou et al. (Citation2016b) observed an increase in milk yield in RPM supplemented cows (40.4 vs 42.2 kg/day), mainly driven by greater DMI (13.2 vs 14.3 kg/day). In the current study, differences in DMI between experimental groups might have helped explain the absence of RPM supplementation effects on milk yield. However, individual DMI data collection in the commercial herd was not feasible.
Figure 1. Milk yield until 30 DIM (A), milk energy output (B), milk fat (C), milk protein (D), milk casein (E), milk fat-to-protein ratio (F), milk total solids (G), and milk urea nitrogen (H) in RPM-supplemented or Control cows. Values are means, with standard errors represented by vertical bars.
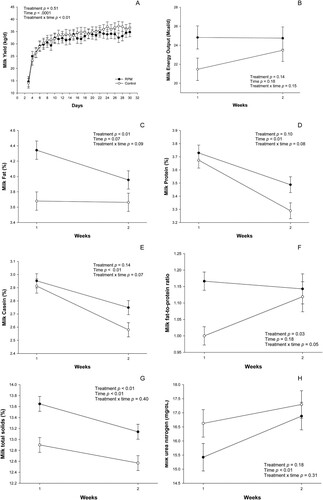
Table 3. Adjusted mean, respective standard error, and statistical significance of milk yield, milk energy output, and milk composition between experimental groups.
Milk fat content had a tendency to decrease (p=0.07) over time (C), which is expected as milk yield increases on early lactation. Animals supplemented with RPM produced milk with greater (p<0.01) fat content than animals of the Control group, a noticeable difference of 0.48% units. Others have also reported a positive effect of methionine supplementation on milk fat concentration (Overton et al. Citation1998; Samuelson et al. Citation2001; Chen et al. Citation2011; Osorio et al. Citation2013). Regarding the mechanism responsible for the increase in milk fat content observed in this study, Samuelson et al. (Citation2001) explains that as methionine plays a prominent role in the formation of VLDL precursors (e.g. choline and apolipoprotein B-100), it will help in the synthesis of this lipoprotein, which is likely limited in postpartal dairy cows. However, this mechanism cannot be confirmed since blood VLDL was not directly measured in the present study. Moreover, it is likely that higher blood NEFA on RPM-supplemented cows might have contribuitd to the increase in milk fat content.
Similarly to milk fat, milk total protein and milk casein content showed a reduction (p<0.01) over time (D and 1E, respectively). Our results show a trend (p=0.10) for greater milk total protein in RPM cows when compared to the Control, which was reflected in a difference of 0.13% units. Milk protein content has been observed to increase in response to RPM supplementation in other similar studies (e. g. Socha et al. Citation2005; Preynat et al. Citation2009; Chen et al. Citation2011). Osorio et al. (Citation2013) found an increase in milk protein percentage when cows were supplemented with two different RPM sources. In agreement with our findings, Overton et al. (Citation1998) reported that RPM tended to increase both milk protein and casein content. The increase in milk protein content in response to RPM supplementation has been consistently reported throughout the literature, highlighting the limiting factor of methionine for milk protein synthesis. Met is the only sulfur-containing EAA and is used as precursor of other sulfur-containing AA such as cysteine (Cys) through the transsulfuration pathway. Cysteine plays an important role in protein structure due to its ability to form intrachain disulfide bonds with other Cys residues (Brosnan and Brosnan Citation2006), which in case of milk proteins might help the selective aggregation of caseins in the secretory pathway of mammary epithelial cells, as suggested by Bouguyon et al. (Citation2006). Therefore, the results found in this trial were as expected.
In our study, milk urea nitrogen did not differ (p=0.41) between Control and RPM-supplemented cows. The Control and RPM-supplemented cows had 16.96 and 16.15 mg/dL MUN concentrations, respectively, which were higher than the suggested MUN values of 10–14 mg/dL for lactating cows (Rajala-Schultz and Saville Citation2003). Milk lactose content was similar (p=0.31) between treatments. Finally, milk total solids were greater (p<0.01) on RPM-supplemented cows than Control, with 13.39 and 12.73% total solids, respectively, a remarkable increase of 0.66% units.
Regarding the fat-to-protein ratio, there was a treatment × time interaction (p=0.05), in which RPM cows presented a greater ratio in the first week postpartum. A treatment effect also showed that RPM cows had a greater (p=0.03) ratio than Control. The fat-to-protein ratio is a suitable indicator of lipomobilization and NEB in postpartum dairy cows (de Vries and Veerkamp Citation2000; Buttchereit et al. Citation2011). According to Toni et al. (Citation2011), a fat-to-protein ratio outside the range between 1–2 is a strong predictor of a cow health event, early milk production performance, and survival in the current lactation. For instance, Heuer et al. (Citation1999) reported that cows with a postpartum fat-to-protein ratio greater than 1.5 had higher risks for ketosis, displaced abomasum, and mastitis. Taken together, these data suggest that the fat-to-protein ratio in both groups can be considered within a normal range, and the greater fat-to-protein ratio in RPM cows was mainly driven by the increase in milk fat content in wk 1 postpartum.
Blood metabolites
Main effects and interactions for blood metabolites are presented in and . Overall, results show a time effect (p<0.05) in all blood metabolites but NEFA (p=0.34). No significant treatment × time interaction (p<0.05) was observed for blood metabolites. In terms of liver function and energy balance biomarkers, RPM-supplemented cows presented greater NEFA concentration (p=0.03), and tented to have greater triglyceride (p=0.07) and AST (p=0.10) when compared to Control cows.
Figure 2. Serum urea (A), aspartate aminotransferase (B), triglycerides (C), cholesterol (D), glucose (E), total protein (F), albumin (G), calcium (H), total bilirubin (I), β–hydroxybutyrate (J), and non-esterified fatty acids (K) in RPM-supplemented or Control cows. Values are means, with standard errors represented by vertical bars.
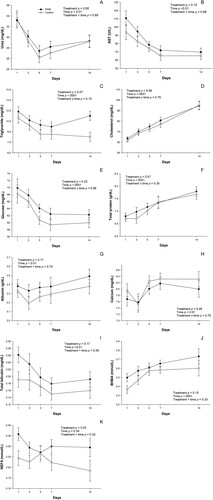
Table 4. Adjusted mean, respective standard error, and statistical significance of blood metabolites in the experimental groups.
Although RPM-supplemented cows presented greater NEFA, the concentration of this metabolite, together with BHBA concentrations, indicate that cows in this study were not under a high lipolytic state. Blood NEFA can be used as a marker of energy status during the transition period (Ospina et al. Citation2010; McArt et al. Citation2012). According to Adewuyi et al. (Citation2005), values greater than 0.7 mmol/L after calving indicate a severe NEB. In our study, RPM-supplemented and Control cows presented NEFA concentrations lower than 0.7 mmol/L until 14 d relative to parturition. Similar to NEFA, BHBA is a marker of energy status during the transition period, which could also indicate lipid β-oxidation capacity in the liver; in which values of BHBA ≥ 1.2 mmol/L are indicative of hyperketonemia (McArt et al. Citation2015). As explained by Ospina et al. (Citation2010), an increase in circulating NEFA and BHBA is expected during the transition period of dairy cows. This is due to their natural adaptation to high energy demands, which render cows in a high lipolytic state until energy intake catches up with the energy requirement to sustain milk production.
Regarding triglycerides serum concentrations, results could indicate that RPM cows might be able to export the triglycerides present in the liver more efficiently than Control cows after two weeks of supplementation. However, this mechanism is mostly unlikely since there is no treatment effect on blood cholesterol. Therefore, these results may indicate that RPM cows presented a greater DMI and the higher triglycerides concentration is primarily of dietary origin. In contrast, the AST concentration might indicate an impaired liver function in RPM-supplemented cows. Despite not being specific to the liver (Bionaz et al. Citation2007), AST is used as indicator of hepatic tissue damage, which might be related to a greater amount of NEFA arriving at the liver of RPM-supplemented cows.
Moreover, results demonstrate the absence (p>0.05) of dietary treatment effects on all other metabolic parameters evaluated. In agreement with our results, Zhou et al. (Citation2016a; Citation2016b) also did not find differences in cholesterol, glucose, total bilirubin, or BHBA concentrations of cows supplemented with RPM from −21 to 30 d relative to parturition. In contrast, the authors reported beneficial effects of RPM supplementation on liver function, inflammation, and oxidative stress biomarkers such as albumin, haptoglobin, and glutathione. Furthermore, Batistel et al. (Citation2018) found positive effects on cholesterol, paraoxonase, albumin, haptoglobin, and oxidative stress (FRAP, ROM, β-carotene, tocopherol and glutathione) biomarkers when supplementing cows with an ethyl-cellulose RPM from −28 to 60 d relative to parturition; however, no differences in AST or bilirubin were observed. It is worth mentioning that the blood metabolites evaluated in this study, such as glucose, urea, AST, cholesterol, NEFA, and total bilirubin suggest healthy postpartum cows regardless of treatment.
Conclusions
Our findings demonstrate beneficial effects on milk composition when dairy cows are supplemented with RPM during early lactation. The effect of methionine to increase milk protein content has been widely reported in the literature. However, RPM impact on milk fat content, as observed in this trial, requires further investigation in order to characterize its specific mechanism. The authors can speculate that the absence of positive effects of RPM on blood biomarkers may result from postpartum RPM supplementation only, while previous similar studies have evaluated RPM supplementation throughout the peripartum period. Overall, blood biomarkers related to energy metabolism indicated that cows in this study had a healthy transition into lactation, which might help explain why the overall positive effects of RPM were mainly associated with milk composition.
Acknowledgments
The authors thank the Laboratories of the Dairy Herd Analysis Service of APCBRH, the Laboratories of Veterinary Clinical Pathology and Animal Nutrition of the Federal University of Paraná, and specially, to the support of the Starmilk Farm where this trial was conducted.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Adewuyi AA, Gruysi E, van Eerdenburg FJCM. 2005. Non esterified fatty acids (NEFA) in dairy cattle. A review. Vet Q. 27:117–126.
- Batistel F, Arroyo JM, Garces CIM, Trevisi E, Parys C, Ballou MA, Cardoso FC, Loor JJ. 2018. Ethyl-cellulose rumen-protected methionine alleviates inflammation and oxidative stress and improves neutrophil function during the periparturient period and early lactation in Holstein dairy cows. J Dairy Sci. 101:480–490.
- Benefield BC, Patton RA, Stevenson MJ, Overton TR. 2009. Evaluation of rumen-protected methionine sources and period length on performance of lactating dairy cows within Latin squares. J Dairy Sci. 92:4448–4455.
- Bionaz M, Trevisi E, Calamari L, Librandi F, Ferrari A, Bertoni G. 2007. Plasma paraoxonase, health, inflammatory conditions, and liver function in transition dairy cows. J Dairy Sci. 90:1740–1750.
- Bouguyon E, Beauvallet C, Huet J, Chanat E. 2006. Disulphide bonds in casein micelle from milk. Biochem Biophys Res Commun. 343:450–458.
- Brosnan JT, Brosnan ME. 2006. The sulfur-containing amino acids: An overview. J Nutr. 136:1636–1640.
- Buttchereit N, Stamer E, Junge W, Thaller G. 2011. Short communication: genetic relationships among daily energy balance, feed intake, body condition score, and fat to protein ratio of milk in dairy cows. J Dairy Sci. 94:1586–1591.
- Chen ZH, Broderick GA, Luchini ND, Sloan BK, Devillard E. 2011. Effect of feeding different sources of rumen-protected methionine on milk production and N-utilization in lactating dairy cows. J Dairy Sci. 94:1978–1988.
- Contreras GA, Sordillo LM. 2011. Lipid mobilization and inflammatory response during the transition period of dairy cows. Comp Immunol Microbiol Infect Dis. 34:281–289.
- de Vries MJ, Veerkamp RF. 2000. Energy balance of dairy cattle in relation to milk production variables and fertility. J Dairy Sci. 83:62–69.
- Drackley JK. 1999. Biology of dairy cows during the transition period: the final frontier? J Dairy Sci. 82:2259–2273.
- Heuer C, Schukken YH, Dobbelaar P. 1999. Postpartum body condition score and results from the first test day milk as predictors of disease, fertility, yield, and culling in commercial dairy herds. J Dairy Sci. 82:295–304.
- McArt JAA, Nydam DV, Oetzel GR. 2012. Epidemiology of subclinical ketosis in early lactation dairy cattle. J Dairy Sci. 95:5056–5066.
- McArt JAA, Nydam DV, Overton MW. 2015. Hyperketonemia in early lactation dairy cattle: A deterministic estimate of component and total cost per case. J Dairy Sci. 98:2043–2054.
- NRC. 2001. Nutrient requirements of dairy cattle, 7th Revised Edition. Washington, D.C: Subcommittee on Dairy Cattle Nutrition, Committee on Animal Nutrition, Board on Agriculture and Natural Resources, National Research Council, National Academy Press.
- Ordway RS, Boucher SE, Whitehouse NL, Schwab CG, Sloan BK. 2009. Effects of providing two forms of supplemental methionine to periparturient Holstein dairy cows on feed intake and lactational performance. J Dairy Sci. 92:5154–5166.
- Osorio JS, Trevisi E, Ji P, Drackley JK, Luchini D, Bertoni G, Loor JJ. 2013. Supplemental Smartamine M or MetaSmart during the transition period benefits postpartal cow performance and blood neutrophil function. J Dairy Sci. 96:6248–6263.
- Ospina PA, Nydam DV, Stokol T, Overton TR. 2010. Evaluation of nonesterified fatty acids and β-hydroxybutyrate in transition dairy cattle in the northeastern United States: critical thresholds for prediction of clinical diseases. J Dairy Sci. 93:546–554.
- Overton TR, Emmert LS, Clark JH. 1998. Effects of source of carbohydrate and protein and rumen-protected methionine on performance of cows. J Dairy Sci. 81:221–228.
- Overton TR, Waldron MR. 2004. Nutritional management of transition dairy cows: strategies to optimize metabolic health. J Dairy Sci. 87:105–119.
- Preynat A, Lapierre H, Thivierge MC, Palin MF, Matte JJ, Desrochers A, Girard CL. 2009. Influence of methionine supply on the response of lactational performance of dairy cows to supplementary folic acid and vitamin B12. J Dairy Sci. 92:1685–1695.
- Rajala-Schultz PJ, Saville WJA. 2003. Sources of variation in milk urea nitrogen in Ohio dairy herds. J Dairy Sci. 86:1653–1661.
- Roche JR, Bell AW, Overton TR, Loor JJ. 2013. Nutritional management of the transition cow in the twenty-first century: A paradigm shift in thinking. Anim Prod Sci. 53:1000–1023.
- Samuelson DJ, Denise SK, Roffler R, Ax RL, Armstrong DV, Romagnolo DF. 2001. Response of holstein and brown Swiss cows fed alfalfa hay-based diets to supplemental methionine at two stages of lactation. J Dairy Sci. 84:917–928.
- SAS Institute Inc. 2014. SAS® 9.4 language reference: concepts, 3rd ed. Cary, NC: SAS Institute Inc.
- Schwab CG. 1995. Protected proteins and amino acids for ruminants. In: Biotechnology in animal feeds and animal feeding (eds R.J. Wallace and A. Chesson). Weinheim, Germany: Wiley-VCH Verlag GmbH; p. 115–141.
- Schwab CG, Boucher SE. 2007. Metabolizable protein and amino acid nutrition of the cow: Where are we in 2007? Proceedings of the 68th Annual Minnesota Nutrition Conference; Mankato – United States of America. p.121–138.
- Socha MT, Putnam DE, Garthwaite BD, Whitehouse NL, Kierstead NA, Schwab CG, Ducharme GA, Robert JC. 2005. Improving intestinal amino acid supply of pre- and postpartum dairy cows with rumen-protected methionine and lysine. J Dairy Sci. 88:1113–1126.
- Sordillo LM. 2016. Nutritional strategies to optimize dairy cattle immunity. J Dairy Sci. 99:4967–4982.
- Stokol T, Nydam DV. 2005. Effect of anticoagulant and storage conditions on bovine nonesterified fatty acid and β-hydroxybutyrate concentrations in blood. J Dairy Sci. 88:3139–3144.
- Stokol T, Nydam DV. 2006. Effect of hemolysis on nonesterified fatty acid and beta-hydroxybutyrate concentrations in bovine blood. J Vet Diagn Invest. 18:466–469.
- Strzetelsk JA, Kowalski ZM, Kowalczyk J, Borowiec F, Osieglowski S, Slusarczyk K. 2009. Protected methionine as a methyl-group donor for dairy cows fed diets with different starch sources in the transition period. J Anim Feed Sci. 18:28–41.
- Toni F, Vincenti L, Grigoletto L, Ricci A, Schukken YH. 2011. Early lactation ratio of fat and protein percentage in milk is associated with health, milk production, and survival. J Dairy Sci. 94:1772–1783.
- Van Soest PJ, Robertson JB, Lewis BA. 1991. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci. 74:3583–3597.
- Zhou Z, Bulgari O, Vailati-Riboni M, Trevisi E, Ballou MA, Cardoso FC, Luchini DN, Loor JJ. 2016a. Rumen-protected methionine compared with rumen-protected choline improves immunometabolic status in dairy cows during the peripartal period. J Dairy Sci. 99:8956–8969.
- Zhou Z, Vailati-Riboni M, Trevisi E, Drackley JK, Luchini DN, Loor JJ. 2016b. Better postpartal performance in dairy cows supplemented with rumen-protected methionine compared with choline during the peripartal period. J Dairy Sci. 99:1–17.
