ABSTRACT
The Marbled Eel (Anguilla marmorata) has a widespread distribution with a complex migration life cycle There were 350 individuals of sizes from 120 to 1137 mm were analysed on characteristic morphology at 4 developing stages: juvenile, fingerling, pre-adulthood and adulthood. Morphological indicators: total length (TL), head length (HL), dorsal fin origin (PD), anal fin origin (PA), distance from dorsal to anal fin (AD), distance from pectoral fins to dorsal fin (PDH), body length (TR), eye diameter (E), tail length (T) and eye distance (IO), colour characteristics and 10 characters were standardized against the LT or LH ratios determine 95.664% of morphological differences between individuals in the study population based on principal component analysis and hierarchical cluster analysis tools. Phylogenetic tree with five morphological clusters with distance > 2.0 built a high genetic diversity. The difference of clusters is related to the morphology at different stages. Cluster 1 corresponds to the morphological characteristics at the fingerling stage (TL = 260–552.0 mm); cluster 3 is the pre-adult stage (TL = 324.6–533.9); cluster 4 is the juvenile stage (TL = 120.0–255.0); and clusters 2 and 5 for pre-adult and adult at reproductive migration (TL > 400).
1. Introduction
Marbled Eel (Anguilla marmorata) of the genus Anguilla Schrank (1798) includes 16 species and 3 subspecies which have been identified (Ege Citation1939; Watanabe et al. Citation2004; Watanabe et al. Citation2005; Watanabe et al. Citation2006), A. marmorata is a large species (Jacoby and Gollock Citation2014), the body can reach 2 m in length (Pike et al. Citation2019). They have a complex migration life cycle between oceans and inland water bodies (Arai Citation2016) with distances from a few hundred to thousands of kilometres (Arai (Citation2014)). They are found in freshwater, brackish and saltwater environments (Lin et al. Citation2012). A. marmorata breeds in the sea and grows to adulthood in rivers, estuaries or the sea (Tsukamoto et al. Citation2011). Anguilla eels spawn at a depth of about 200 m during the new moon. The fertilized eggs slowly float to the surface (Tsukamoto Citation2009). About 24 h later, they hatch into tiny pre-larvae with 5 mm of total length, then transform into leptocephalus larva (Arai et al. Citation2001). Leptocephalus grows during migration along ocean currents to become glass eels when entering freshwater, estuarine or coastal growth habitats where they grow as yellow eels (Hagihara et al. Citation2012). The growth period (yellow eel) of Marbled Eel can be as short as 2–3 years in warm breeding environments, while as long as 6–20 years or more in northern latitudes (Pike et al. Citation2019). And then, they transform into silver eels that start migration to the ocean for spawning (Tesch and Thorpe Citation2003; Tsukamoto Citation2009; Pike et al. Citation2019). The metamorphosis of Anguilla eels during complex migrations (Arai and Chino Citation2018) and adaptation to habitats (Arai and Abdul Kadir Citation2017) has prompted studies concerning the morphological characteristics of the eels through different growth stages.
In Vietnam, five species of the Anguilla genus were identified, namely A. nebulosa (McClelland, 1844), A. japonica (Temminck & Schlegel, 1984), A. marmorata (Quoy & Gaimard, 1824), A. celebensis (Kaup, 1856) and A. bicolor pacifica (Schmidt, 1928)(Nguyen Citation2001). In the Central region, there are three species: A. marmorata, A. bicorlo pacifica and A. japonica (Nguyen et al. Citation2018). Thua Thien Hue located to the south of the North Central region, Vietnam, has been identified with the presence of two species of the Anguilla: Marbled Eel (A. marmorata) and Ebony Eel (A. bicolor) (Kieu et al. Citation2020) of which the Marbled Eel is more popular related to time of occurrence and productive (Nguyen Citation2015). In fact, Marbled Eel are being exploited to serve both commercial needs as well as fingerling needs of farms (Kieu and Vo Citation2015). The pressures of environmental changes during migration and factors affecting their natural habitats, such as environmental pollution, construction of lakes, dams and hydroelectricity have led to an increase in the risk of resource decline. A morphological classification system based on the morphology of the Eels was conducted by Ege (Citation1939), Watanabe (Citation2003), Watanabe et al. (Citation2004, Citation2005). Some information on the developmental stages of Marbled Eel was provided (Leander et al. Citation2012; Hagihara et al. Citation2012), morphological indices were also used to analyse the population structure and phylogenetics of the species (Watanabe et al. Citation2009). However, in Vietnam, information about Marbled Eel is still very limited, these studies only determine the species composition, there are no in-depth studies on the change in morphological characteristics of Eel related to their stages of growth. Recently, the analysis of the genetic structure of the Marbled Eel population in Thua Thien Hue by molecular markers has shown its close relationship with the Eel populations in the Indo-Pacific region (Kieu and Nguyen Citation2020; Arai et al. Citation2020), genetic variants were also found here (Arai and Hussein Citation2021). Therefore, Thua Thien Hue, Vietnam should be considered as an important conservation unit for the species in the region. The study was conducted with the aim of analysing the change and diversity of morphological characteristics of A. marmorata in Thua Thien Hue related to the growth stages of their life cycle, therefore, providing information on the adaptive characteristics of the species in Thua Thien Hue, Vietnam and as a basis for growth strategies for species development and conservation.
2. Material and methods
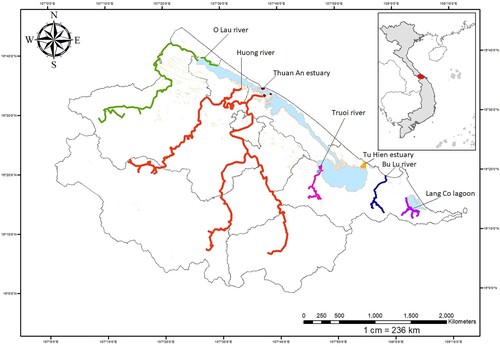
All morphological indicators were observed and measured on all 350 individual eels (A. marmorata Quoy & Gaimard, 1824) with sizes from 3.0 to 4500 g (120.0–1136.9 mm) collected from water bodies in Thua Thien Hue province ( and ) during the period from September 2017 to August 2018. Based on the examination of the colour of the pectoral fins and the body colouration according to the metal index stages described by Okamura et al. (Citation2007), and Hagihara et al. (Citation2012) and their migration, the growth stages of eels were determined to four stages: juvenile, fingerling, pre-adulthood and adulthood. The number of specimens of each period was collected based on the actual number in the study areas to show their distribution in the wild ().
Table 1. Number of Marbled Eel samples collected from study sites.
The colour and appearance of the A marmorata were observed and classified in the field. Eleven out of 16 indicators were measured using the left side of the body or the dorsal side of the head as described by Watanabe et al. (Citation2006), total length (TL), head length (LH), dorsal fin origin (PD), anal fin origin (PA), eye diameter (E) and eye distance (IO) were measured with a ruler with an accuracy of 1.0 mm and a calliper with an accuracy of 0.01 mm. Dorsal and anal (AD) distance, pectoral to dorsal fin distance (PDH), caudal fin length (T), body length (TR) are calculated from PA, PD, LH and LT. A total of 10 characters were standardized against the LT or LH ratios for comparison between the A. marmorata sample locations (Ege Citation1939; Watanabe et al. Citation2009). The collected data were processed according to the biological statistics method on Excell 2016.
SPSS Ver.20.0 software was used to analyse morphological data and morphological differences between groups with principal component analysis (PCA) and hierarchical cluster analysis (HCA) tools. PCA was used to describe and evaluate the variability of morphological features. A correlation matrix was used in the analysis of morphological features. Graphical depictions of specimen locations along the vector were surveyed for separate groups. The PCA technique derives the eigenvalues and variances from the correlation matrix of the original variables. The factors of the main components are performed by the factor extraction method. The main factor rotation is performed according to the original rotation method of factors to minimize the number of variables with large coefficients at the same factor (Vanimax with normal Kaiser) (Nguyen et al. Citation2017). In this study, all the relationships among parameters with correlation coefficients greater than 30% contribute to determining the characteristic morphological parameters of the study population. This method was chosen because it does not require any prior assumptions about the taxonomic identity of the specimen (Reyment and Joreskog Citation1993). HCA by Ward's method using the criterion of minimum variance was used to evaluate the relationship between study individuals. The nearest sequence algorithm is used to determine groups, which are temporally proportional to the size of the input distance matrix and spatially linear to the number of points analysed. The initial cluster distance in Ward's method of least variance is defined as the squared Euclidean distance between the points: dij = d({Xi}, {Xj}) = ||Xi–Xj||2, in which, Xi and Xj are the research points; dij is the square of the Eculid distance between the points Xi and Xj (Ward Citation1963).
3. Results
3.1. Description of morphological characteristics of the Marbled Eel A. marmorata
By observing 350 Marbled Eels with size 120–1136.9 mm (W = 3.0–4500.0 g) distributed in water bodies in Thua Thien Hue, we found that fish have the morphological characteristics as follows: long cylindrical body, tapering off afterward, smooth and scaleless. Dorsal and anal fins are soft, elongated and banded connected to caudal fin, without spines. Pectoral fins are small, almost circular, the number of rays is 18, the colour of the pectoral fins changes from almost transparent pale yellow to black, black to red according to development stages and habitat conditions. Body colour of the adult Marbled Eels is yellow with various spots from greenish-brown to black on the back, the belly is milky white close to the natural colour, the back of baby eels is slightly grey to yellow. The spots on the Marbled Eel's back appear more and more clearly along with the development of the fish. The results in show the values of the observed morphological indicators. Accordingly, the ratio of the indicators with the body length and head length is highly stable, including HL/TL: 11.2–16.6%, PD/TL: 21.2–29.3%, PA / TL: 37.5–48.1%, TR/TL: 22.7–34.3%, AD/TL: 11.9–21.3%, PDH/TL: 7.3–15.5%, T/TL: 51.9–62.5%, E/Hl: 5, 9–11.9 and IO/HL: 16.0–25.0.
Table 2. The body colour of the Marbled Eel in the population in Thua Thien Hue.
The change in morphology of the Marbled Eel occurs strongly when there is habitat change during the migration. In the glass eel stage when the Eels migrate from the sea to inland freshwater, their colour and morphology change rapidly. They are light yellow, brown banding on their backs, small spots, unspecified spots, almost transparent pale yellow fins, size from 120.0 to 228.5 mm (weight: 3.0–20.7 g) at this juvenile eel period accounting for 17.4% of the number of collected populations. After that, body colour of the Marbled Eel changes to yellow or grey with dark grey spots on back, yellowish-grey or black yellow on fins, and white belly. This stage is called the yellow eel. In which, fish with sizes between 187.0 and 410.0 mm with the yellow body colour, clear grey spots, yellow fins are often used as seed in commercial farms (29.7%) and called Fingerling eels; fish sized TL > 387 mm (W > 143.5 g) with brown back, clear spots with grey colour, grey -white belly, yellow-brown fins, can be used for food, called pre-adults (38.6%). After that, body colour of the Marbled Eel changes to yellow or grey with dark grey spots on back, yellowish-grey or black yellow on fins, and white belly. At this stage, they are called adult Marbled Eel (silver eel) accounting for 14.3% of the observed population ().
Table 3. The values of morphological indicators of the Marbled Eel distributed in Thua Thien Hue.
3.2. The Eel population structure in Thua Thien Hue based on morphological indicators
Key component analysis was used to evaluate the variability and contribution of traits to the degree of variation in studied individuals. The first five principal components (PC) have an eigenvalue greater than 1.0 and related parameters are shown in . The PC 1 account for 56.38% of the difference, including the morphological indicators: total length (TL), head length (HL), dorsal fin orgin (PD), anal fin orgin (PA), distance from dorsal to anal fin (AD), distance from pectoral fins to dorsal fins (PDH), body length (TR), eye diameter (E), T (tail length) and eye distance (IO) and colour characteristics, appearance. The PC 2 accounts for 18.72% of the difference, including the indicators PD/TL, PA/TL, TR/TL, PDH/TL and T/TL. The PC 3 accounts for 8.18% including two indicators: the ratios of eye diameter to head length (E/HL) and eye distance to head length (IO/HL). The PC 4 and PC 5 account for 7.60% and 4.78%, respectively, with the remaining two indicators including AD/TL and HL/TL.
Table 4. The PCA of the morphological characteristics of A. marmorata.
Hierarchical cluster analysis was used to determine the differences and genetic relationships among 350 individuals in the Marbled Eel population collected in Thua Thien Hue. The results show that the genetic similarity coefficients based on the Euclidean distance range from 0.1 to 25.0, indicating that the Marbled Eel's samples have a high genetic diversity (). Accordingly, at the position where the genetic distance is 25.0, the phylogenetic tree is divided into two main branches: branch I and branch II. In which, where the genetic distance is 3.0, branch I is divided into two cluster (cluster 4 and cluster 1); Branch II consists of 2 sub-branch (IIa and IIb) that are isolated at the genetic distance of 9.0. In which, sub-branch IIb, is divided into two subclusters (cluster 2 and cluster 5) with a genetic distance of 2.0. Cluster 3 is located on sub-branch IIa of branch II on phylogenetic tree. Morphological characteristics of the 5 clusters are shown in .
Figure 2. Phylogenetic tree of Marbled Eel population in Thua Thien Hue based on Euclidean distance using Ward method.
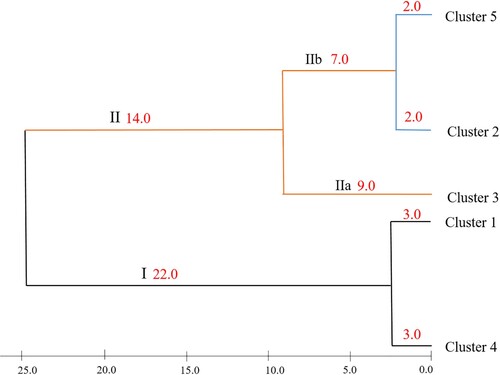
Table 5. Morphological characteristics of A. marmorata in five clusters using Ward method based on Euclidean distance.
4. Discussions
The first comprehensive morphological descriptions of Anguilla eels were made by Kaup (1856), Günther (1870) and Ege (Citation1939). According to the description of Ege (Citation1939), the Anguilla genus is divided into 16 species and 3 subspecies. According to Watanabe (Citation2003) and Watanabe et al. (Citation2004), Marbled Eel belongs to group 2 the classification systems with variegated body marking, narrow maxillary bands of teeth. At the dermal pigmented vitreous stage on the caudal fin of A. marmorata of group 3 together with A. luzonensis or A. celebesensis, there is a large patch of diffuse melanophores on the caudal bud, ADL/% TL > 13 (13.27–20.35) (Leander et al. Citation2012). Twenty-one morphological characteristics of 686 specimens (LT = 115–1,069 mm) were collected from 13 representatives within the geographic distribution areas of A. marmorata in the Pacific and Indian Oceans analysed by Watanabe et al. (Citation2009). The morphological values in of Marbled Eel collected in Thua Thien Hue are similar to these studies.
Based on characterization by the colourations of pectoral fins and ventral skin, Okamura et al. (Citation2007) and Hagihara et al. (Citation2012), described four stages for A. japonica and A. marmorata as follows: Y1 – yellow eel without a metallic at the base of pectoral fins, Y2 – late yellow eel with a metallic hue at the base of the pectoral fins but without melanization at the tip of pectoral fins, S1 – silver eel with complete melanization at the tip of pectoral fins but without full pigmented belly in black or dark brown, and S2 – late silver eel with black or dark brown belly. In this study, 4 stages have been proposed for A. marmorata population in Thua Thien Hue, Vietnam, namely: juvenile eel, fingerling eel, yellow eel (pre-adulthood) and silver eel (adulthood) (). In which, fingerling eel, pre-adulthood and adulthood stages of Marbled Eel population in Thua Thien Hue, Vietnam () are similar to Y1, Y2 and S1 described by Hagihara et al. (Citation2012).
During the migration, living and growth of Marble Eels in Thua Thien Hue water bodies, individual adaptations through changes in body colour and morphological indicators, but characters were standardized as a proportion of TL or HL did not change significantly when fish developed through different stages. It is shown in the analysis results in and with the value of indicators, including HL/TL, PD/TL, PA/TL, TR/TL, AD/TL, PDH/TL, T/TL, E/Hl and IO/HL, show their stability with the growth of eel and do not depend on the growth stages ( and ). The contribution of the morphological characteristics to the differences of the PCs () and the distribution of individuals in the clusters ( and ) indicated that there was homogeneity on these morphological characteristics related to growth stages of the Marbled Eel living in Thua Thien Hue, Vietnam. This result is consistent with PCA analysis for morphological indices to determine the degree of difference of morphological features between A. marmorata populations by Watanabe et al. (Citation2009) shown that there were no clear differences in 15 proportional and 6 vertebral characters using PC analysis.
The clustering on the phylogenetic tree based on HCA was characterized by the occurrence of a majority of the Marbled Eel samples in each growth stages. Specifically, cluster 4 was characterized of juvenile (71.8%); cluster 1 characterizes of fingerlings (67.9%); cluster 3 represents the pre-adulthood group (62.7%); Cluster 2 and cluster 5 characterizes pre-reproductive (pre-adulthood and pre-adulthood) eels. The relationships between clusters are also shown by the presence of more than one growth stage in each cluster ( and ) indicating overlap in morphological characteristics among individuals of different stages in the population. In which, cluster 3 correlated with the remaining clusters through the presence of individual eels in almost all growth stages. Cluster 4 and cluster 1 lying on the same branch had the genetic coefficient of 3.0 with the contribution of the Marbled Eel in fingerling stage (28.2% and 67.9%, respectively). Cluster 2 and cluster 5 also showed a close relationship with genetic distance of 2.0 with the presence of individuals in the two stages of pre-adulthood and adulthood. These results can be explained by slow metamorphosis between the growth stages of Marbled Eel (Hagihara et al. Citation2012), growth rate, migration and changes in habitat such as water temperature, surface cycle, etc. moon, wind and rain (Sudo et al. Citation2017), and unseasonable sexual maturity of fish of A. marmorata during the silver eel stage (Kita and Tachihara Citation2020).
Thus, the results of analysing the morphological characteristics of this study have shown a high phenotypic diversity in the structure of the Marbled Eel population distributed in Thua Thien Hue related to four growth stages: juvenile, fingerlings, pre-adulthood and adulthood. During growth and development in freshwater bodies, Marbled Eel have a slow (incomplete) metamorphosis between developmental stages. These are meaningful data for the development of research plans and strategies for conservation and development of Eel fish stocks in Thua Thien Hue, Vietnam.
Acknowledgements
Thank to staffs at communities and inhabitants in Thua Thien Hue province to support for collecting sample; Laboratories in the faculty of Fishery, Hue university of Agriculture and Forestry to support for sample analysis; and funding organizations.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data and materials are available in the manuscript.
Additional information
Funding
References
- Arai T. 2014. Evidence of local short-distance spawning migration of tropical freshwater eels, and implications for the evolution of freshwater eel migration. Ecol Evol. 4(19):3812–3819. https://doi.org/10.1002/ece3.1245.
- Arai T. 2016. Biology and ecology of Anguillid eels. In: Arai T (ed). Taxonomy and distribution. Vol. 1. CRC Press; p. 1–20. https://www.crcpress.com/Biology-and-Ecology-of-Anguillid-Eels/Arai/p/book/9781482255157.
- Arai T, Abdul Kadir SR. 2017. Diversity, distribution and different habitat use among the tropical freshwater eels of genus Anguilla. Sci Rep. 7:7593. https://doi.org/10.1038/s41598-017-07837-x.
- Arai T, Hussein T. 2021. Contrasting patterns of genetic population structure in tropical freshwater eels of genus Anguilla in the Indo-Pacific. Heliyon. 7(5):e07097. https://doi.org/10.1016/j.heliyon.2021.e07097.
- Arai T, Limbong D, Otake T, Tsukamoto K. 2001. Recruitment mechanisms of tropical eels, Anguilla spp. and implications for the evolution of oceanic migration in the genus Anguilla. Mar Ecol Prog Ser. 216:253–264. https://doi.org/10.3354/meps216253.
- Arai T, Taha H, Mohd-Riduan MN, Mokti SSA. 2020. Molecular and morphological evidence for the identity of the giant mottled eel, Anguilla marmorata in Southeast Asia. Trop Ecol. 61(3):429–436. https://doi.org/10.1007/s42965-020-00096-4.
- Arai T, Chino N. 2018. Opportunistic migration and habitat use of the Giant mottled eel Anguilla marmorata (Teleostei: Elopomorpha). Sci Rep. 8 (5666):1–10. https://doi.org/10.1038/s41598-018-24011-z.
- Ege V. 1939. A revision of the genus Anguilla Shaw, a systematic, phylogenetic and geographical study. Dana Rep. 16:1–256.
- Hagihara S, Aoyama J, Limbong D, Tsukamoto K. 2012. Morphological and physiological changes of female tropical eels, Anguilla celebesensis and Anguilla marmorata, in relation to downstream migration. J Fish Biol. 81(2):408–426. https://doi.org/10.1111/j.1095-8649.2012.03332.x.
- Jacoby D, Gollock M. 2014. Anguilla marmorata. The IUCN Red List of Threatened Species 2014, DOI:10.2305/IUCN.UK.
- Kieu TH, Nguyen QL. 2020. Phylogenetic analysis of Anguilla marmorata population in Thua Thien Hue, Vietnam based on the cytochrome C oxidase I (COI) gene fragments. AMB Express. 10(122). https://doi.org/10.1186/s13568-020-01059-7.
- Kieu TH, Vo DN, Tran NN, Truong VD, Vo VP, Tran QD, Nguyen QL. 2020. Using DNA barcodes based on mitochondrial COI and 16S rRNA genes to identify Anguilla eels in Thua Thien Hue province, Vietnam. Genet Mol Res. 19(4):gmr18722. https://doi.org/10.4238/gmr18722.
- Kieu TH, Vo VP. 2015. Assessment of distribution of Anguilla marmorata in Huong river system, Thua Thien Hue. J Sci Hue Univ. 104(5). http://jos.hueuni.edu.vn/index.php/TCKHDHH/article/view/1860 (inVietnamese).
- Kita T, Tachihara K. 2020. Age, growth, and gonadal condition of the Giant mottled eel, Anguilla marmorata, in Okinawa-Jima Island, Japan. Environ Biol Fishes. 103:927–938. https://doi.org/10.1007/s10641-015-0404-6.
- Leander NJ, Shen KN, Chen RT, Tzeng WN. 2012. Species composition and seasonal occurrence of recruiting glass eels (Anguilla spp.) in the Hsiukuluan river, Eastern Taiwan. Zool Stud. 51(1):59–71.
- Lin YJ, Jessop BM, Weyl OLF, Iizuka Y, Lin SH, Tzeng WN, Sun CL. 2012. Regional variation in otolith Sr:Ca ratios of African longfinned eel Anguilla mossambica and mottled eel Anguilla marmorata: A challenge to the classic tool for reconstructing migratory histories of fishes. J Fish Biol. 81(2):427–441. https://doi.org/10.1111/j.1095-8649.2012.03357.x.
- Nguyen AT, Tsukamoto K, Lokman PM. 2018. Composition and distribution of freshwater eels Anguilla spp. in Vietnam. Fish Sci. 84(8):987–994. https://doi.org/10.1007/s12562-018-1239-9.
- Nguyen DT. 2015. Data set on Anguilla Eels in Hương river system, Thua Thien Hue province, Vietnam. Hue Univ J Sci (HU JOS) Earth Environ Sci. 103(4). http://jos.hueuni.edu.vn/index.php/TCKHDHH/article/view/1597 (inVietnamese).
- Nguyen HA, Phan TKN, Hoang TTT, Phan NHN. 2017. Application of multivariate statistical analysis in the assessment of groundwater quality of Tan Thanh district, Ba Ria – Vung Tau province. Sci Technol Dev. 1(M2):66–72. https://doi.org/10.32508/stdjsee.v1iM2.446.
- Nguyen HP. 2001. Vietnamese Animals . Nguyen (ed). Vol. 10. Hanoi: Scientific and Technical Publisher.
- Okamura A, Yamada Y, Yokouchi K, Horie N, Mikawa N, Utoh T. 2007. A silvering index for the Japanese eel Anguilla japonica. Environ Biol Fishes. 80:77–89. https://doi.org/10.1007/s10641-006-9121-5.
- Pike C, Crook V, Gollock M, Jacoby D. 2019. Anguilla marmorata (Errata Version Published in 2020). The IUCN Red List of Threatened Species 2019. E.T166189A167699312. DOI: 10.2305/IUCN.UK.2019-3.RLTS.T166189A167699312.en.
- Reyment RA, Joreskog KH. 1993. Applied factor analysis in the natural sciences. Cambridge: Cambridge University Press; p. 1–4. https://doi.org/10.1017/CBO9780511524882.
- Sudo R, Okamura A, Fukuda N, Miller JM, Tsukamoto K. 2017. Environment factors affecting the onset of spawning migrations of Japanese eels (Anguilla japonica) in Mikawa bay Japan. Enviornment Biology of Fish. 100:237–249. https://doi.org/10.1007/s10641-017-0575-4.
- Tesch FW, Thorpe JE. 2003. The Eel. 3rd ed. Oxford: Blackwell Science Ltd. https://doi.org/10.1002/9780470995389.
- Tsukamoto K. 2009. Oceanic migration and spawning of Anguillid eels. J Fish Biol. 74(9):1833–1852. https://doi.org/10.1111/j.1095-8649.2009.02242.x.
- Tsukamoto K, Chow S, Otake T, Kurogi H, Mochioka N, Miller MJ, Shinoda A, Aoyama J, Kimura S, Watanabe S, et al. 2011. Oceanic spawning ecology of freshwater eels in the Western North Pacifica, Acific. Nat Commun. 2:179. https://doi.org/10.1038/ncomms1174.
- Ward JH. 1963. Hierarchical grouping to optimize an objective function. J Am Stat Assoc. 58(301):236–244.
- Watanabe S. 2003. Taxonomy of the freshwater eels, genus Anguilla Schrank, 1798. In: K. Aida, K. Tsukamoto, K. Yamauchi (eds.), Eel biology. Tokyo: Springer; p. 13–18. https://link.springer.com/chapter/10.1007%2F978-4-431-65907-5_1.
- Watanabe S, Aoyama J, Nishida M, Tsukamoto K. 2005. A molecular genetic evaluation of the taxonomy of eels of the genus Anguilla (Pisces: Anguilliformes). Bull Mar Sci 76(3):675–690. https://www.ingentaconnect.com/content/umrsmas/bullmar/2005/00000076/00000003/art00006.
- Watanabe S, Aoyama J, Nishida M, Tsukamoto K. 2006. Confirmation of morphological differences between Anguilla australis australis and A. australis Schmidtii. N Z J Mar Freshw Res 40(2):325–332. https://doi.org/10.1080/00288330.2006.9517424.
- Watanabe S, Aoyama J, Tsukamoto K. 2004. Reexamination of Ege's (1939) use of taxonomic characters of the genus Anguilla. Bull Mar Sci 74(2):337–351. https://www.ingentaconnect.com/content/umrsmas/bullmar/2004/00000074/00000002/art00006.
- Watanabe S, Miller MJ, Aoyama J, Tsukamoto K. 2009. Morphological and meristic evaluation of the population structure of Anguilla marmorata across its range. J Fish Biol. 74(9):2069–2093. https://doi.org/10.1111/j.1095-8649.2009.02297.x.