ABSTRACT
This study aimed to evaluate the effects of dietary guanidinoacetic acid (GAA) on performance, enzyme activity and biochemical variables of broilers with cold-induced ascites. A total of 640 day-old male broiler chicks (Cobb 500) were randomly assigned to four treatments (with 8 replicates of 20 birds) including a control and diets supplemented with 0.6, 1.2 and 1.8 g of GAA per kg of feed. On day 14, the temperature was reduced to 17°C during the daytime and 14°C at night. Dietary inclusion of 0.6 g/kg GAA decreased BWG in compared to those fed other levels in the grower period (P < 0.05). The ratio of right to total ventricle, mortality and relative weights of liver were lower in birds fed the diet with 1.2 g/kg GAA compared to bids fed other levels (P < 0.05). The plasma activity of creatine kinase (CK) increased in the birds fed with 1.2 and 1.8 g/kg GAA compared to chicks fed the control diet. It can be concluded that dietary supplemental 1.2 g/kg GAA would be a beneficial way to decrease the mortality rate of broiler reared under cold stress conditions. In addition, the blood levels of HDL-C increased in broilers fed the 1.2 g/kg GAA diet.
Introduction
During the past several years, the main objectives were to improve feed conversion ratio (FCR), mortality rate and carcass yield in fast-growing meat-type chicken (broiler) industry. Moreover, providing welfare is considered an essential factor in order to increase the animal production systems. Metabolic diseases increase compromised welfare and financial losses because of deterioration of production value on account of increased mortality rate, less efficient utilization of nutrients and condemnation of carcasses; also, both intensive, quantitative selection and improved diet and management have caused to improve the overall performance of broilers over the past 50 years (Kalmar et al. Citation2010). Ascites syndrome (AS) or pulmonary hypertension syndrome (PHs) is a metabolic disorder of meat-type lines/hybrids broilers which is accompanied by faulted FCR and rapid growth rate (GR) (Franciosini et al. Citation2012; Huchzermeyer Citation2012; Wideman et al. Citation2013; Fathi et al. Citation2016). On a worldwide basis, next to this financial cost, PHs are considered a main welfare concern as demonstrated by Zhang et al. (Citation2011). Additionally, PHs increase systemic venous pressure, valvular insufficiency and right ventricular hypertrophy (Wang et al. Citation2012). Stress is one of the basic factors in the aetiology of disease. Animals daily face a variety of environmental stressors, such as Cold stress which occurs in cold regions. cold stress dramatically affects the health and welfare of animals in cold regions (Tsutsayeva and Sevryukova Citation2000). Fast-growing broilers are severely susceptible to PHs. Thus, the proper environmental conditions and appropriate nutritional strategies are needed to prevent the development of PHs. It is essential to mention that the rearing costs in broiler chicken industry increase due to PHs. Research has addressed some nutritional factors including energy (Khajali and Fahimi Citation2010), protein (Izadinia et al. Citation2010; Behrooj et al. Citation2012; Saki et al. Citation2013), electrolytes (Khajali and Saedi Citation2011), feed texture (Baghbanzadeh and Decuypere Citation2008) and feed restriction (Özkan et al. Citation2010) on the development of PHs.
Arginine (Arg) is the fifth limiting amino acid in broiler chickens (Han et al. Citation1992; Fernandez et al. Citation1994; Waguespack et al. Citation2009), but is not commercially available. Arg may be needed for optimal growth because of increased growth rate of modern broilers (Havenstein et al. Citation2003), lack of de novo synthesis of Arg (Tamir and Ratner Citation1963), and the possibility of reduced protein quality in production diets (Han et al. Citation1992). It is estimated that the Arg requirement for maximal growth and preventing PHs was 20% higher than the nutrient requirements of poultry (NRC Citation1994) recommendation (13.2 vs 11.0 g/kg of diet) (Basoo et al. Citation2012). Guanidinoacetic acid (GAA) is a compound formed from Arg and glycine (Gly), which is produced via chemical synthesis from glycine cyanamide. GAA is capable to spare Arg that would otherwise be used for creatine (Cre) synthesis (Savage and O'dell Citation1960). Positive effects of supplemental GAA on broiler chicks and turkey performance have been demonstrated (Michiels et al. Citation2012; Dilger et al. Citation2013; Heger et al. Citation2014). Guanidinoacetic acid can spare Arg in Arg-deficient diets and also improve growth performance in Arg-adequate diets (Michiels et al. Citation2012). Also, Khajali et al. Citation2020, reported the effect of GAA on growth performance, energy utilization and muscle development (protein accretion).
If the cost of GAA is less expensive than, or equal to, commercially available Arg, then GAA supplementation would be more advantageous to supplement because of the improvement observed in Arg-adequate diets. Therefore, the poultry industry may use GAA as an Arg replacement, relieving the necessity for Arg supplementation in current poultry diets.
Based on the available literature, there is no study investigating the effect of dietary supplemental GAA on the performance of broiler chickens with induced ascites. It was hypothesized that supplemental GAA when included in Arg-adequate practical broiler diets would elicit a positive influence on alleviating the negative effects of ascites in broiler reared under cold-temperature conditions.
Materials and methods
The experiment was conducted at the animal husbandry station, Razi University, Kermanshah, western region of Iran positioned between 34◦18´N and 47◦03´E at a height of 1350 m from sea level.
Bird source and management
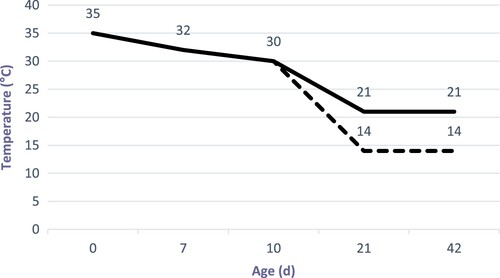
A total of 640 newly hatched Cobb male chicks were purchased from a commercial hatchery (male chicks were selected by vent sexing from a population of 1600, according to body weight; those with extreme weights were discarded). Chicks were randomly assigned into 4 groups, with eight replicates per treatment (20 birds per battery replicate cage). Birds were reared in battery cages (2.4 × 0.6 × 0.6 m) with a screen-wired floor. Each cage was equipped with a feeder to be manually filled daily. The temperature in the rearing chamber was 28–30°C in the first week and daily decreased by 1°C till 30°C on d 10. From d 11, all birds were exposed to a temperature cycle of 17°C during the daytime and 14°C at night in order to increase ascites susceptibility until the end of the experiment () (Shlosberg et al. Citation1992; Korte et al. Citation1999; Buyse et al. Citation2001). Except for the applied temperature schedule, the chickens were kept under conditions that closely resembled commercial practice. Continuous light was provided for 24 h for the first 3 days, and then, 23 L: 1D light was adopted for the rest of the trial period.
Feeding and experimental diets
The basal diet was free of animal by-products in order to avoid the contribution of creatine from an additional source. Prior to the experiments, the experimental diets were analysed for dry matter (DM), crude protein (CP) and amino acids (AAs) using near-infrared spectroscopy.Footnote1 Metabolizable energy (ME) contents of corn and soybean meal were estimated by using the regression models (NRC Citation1994). All dietary nutrients met or exceeded (Cobb Citation2008) recommendations (). The basal diet was supplemented with 0.0, 0.6, 1.2 or 1.8 g GAA per kg of feed. GAA was added in the form of CreAMINO®Footnote2 and supplied at the expense of corn. The birds had ad libitum access to feed and water throughout the trial period. All birds received a pre-starter diet from 1 to 10 d. Grower and finisher diets were provided from 11 to 21 d and 22 to 42d of age, respectively. The ME and CP contents of starter, grower and finisher diets were 2850, 3058 and 3130 Kcal/kg; 250, 225 and 215 g/kg, respectively. No type of medication was administered during the entire experimental period.
Table 1. Ingredients and composition of experimental diets supplemented with guanidinoacetic acid (GAA) (g/kg, as-fed basis).
Growth performance
Feed intake (FI) and body weight (BW) were recorded at 1, 10, 24, and 42 days of age, whereas mortality was recorded daily throughout the study. FI was corrected for mortality. From these data, average FI and body weight gain (BWG) and FCR were calculated per cage for different rearing periods.
Blood parameters
At 42 d of age, 3 ml of blood was collected from a brachial vein (eight birds per treatment). Sera were separated by centrifugation at 3000 × g for 10 min. Triglyceride (TG), cholesterol (CHOL), high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) in serum samples were determined using a corresponding reagent kit (Pars Azmoon Co., Tehran, Iran) and an automatic biochemical analyser (Clima, Ral. Co, Spain).
Thyroid hormones
The measurement of thyroid hormones was done by gama kit (Bio-source Europe Com.). In order to separate the serum from the samples, the clotted blood was centrifuged twice at 5000 rpm for two minutes. Then, 25 microliters of the sample was isolated for the 3, 5, 3´-triiodothy-ronine (T3) test, and 200 microliters of I125 iodine was added. After incubation for one hour in the shaker unit, it was perused by the gama kit. In order to evaluate the thyroxine (T4), 500 microliters of I125 iodine was added (Georgiou and Christofidis Citation1996). Then, the mixture was incubated in the shaker unit for one hour and finally read by the gama kit.
Enzyme activity
The activities of cytosolic creatine kinase (CK; EC 2.7.3.2) and lactate dehydrogenase (LDH; EC 1.1.1.27) enzymes were measured in heart tissue obtained from the mid-portion of the left ventricle free wall. Briefly, aliquots of 300 mg of frozen samples were homogenized in phosphate buffer (50 mM, pH 7.4) in the ratio of 100 mg tissue per 1 ml buffer in test tubes pre-cooled with ice. The homogenate was centrifuged at 2500 g for 10 min at 4 °C, and the suspension was further centrifuged at 12,000 g for 10 min. The supernatant was used for activity measurements of CK and LDH using a CK kit (Roche Diagnostics, Indianapolis, IN, USA) and LD-L10 (Sigma Diagnostics Inc., St. Louis, MO, USA), respectively. The final activity measurements were performed during the linear phase of responses pre-established during the validation phase. These measurements were performed in a single assay for each enzyme in order to avoid inter-assay variability (Nain Citation2008).
Organ index and ascites-related parameters
On day 42 of the trial, six chicks, close to the mean of herd weight, were selected and sequentially slaughtered with intervals of approximately 3 min between the slaughters of individuals. When the head, shanks and feathers were removed, the carcass was eviscerated by cutting around the vent to remove all of the viscera. Once eviscerated, the carcass without giblets was weighed and expressed as a percentage of live weight and considered as the carcass yield. Breasts were weighed and expressed as percentages of the live body weight. The weights of the abdominal fat, liver, heart and gastrointestinal sections (after removal of digesta) were also measured to the nearest 0.01 g and expressed as a percentage of the live BW. Moreover, after removing the intestinal contents, the lengths of duodenum (pancreatic loop), jejunum (from the pancreatic loop to Meckel’s diverticulum), ileum (from Meckel’s diverticulum to ileocecal junction), and caeca were recorded. Postmortem examinations were performed on all dead chickens during the experimental period in order to diagnose ascites. Moreover, on day 42, a total of 40 birds per each treatment (5 birds per each cage) were randomly selected, sacrificed, and used for the weight of right ventricular (RV) to total ventricular (TV) (RV/TV) determination. Ascites-related mortality was diagnosed when an accumulation of abdominal or pericardial fluid was present, and the RV/TV was higher than 0.29 (Julian Citation1987). In order to calculate the RV/TV, hearts were collected, and the pericardium, peripheral adipose tissues, and atria were removed. The left ventricle and RV were separated, and their individual weights were measured on an analytical balance (Scaltec SBA41, Germany; precision 10-13 g), and the RV/TV was calculated (Julian Citation2005).
Statistical analysis
The data were analysed based on a completely randomized design using the GLM procedure of SAS® (SAS Citation2008). The results are reported as means. The data were tested for linear and quadratic contrasts using the incremental dietary GAA treatments (0 or control diet, 0.6, 1.2 or 1.8 g GAA per kg of feed). Data were tested for distribution normality and homogeneity of variance. Variables with significant F-tests (P < 0.05) were compared using Duncan’s multiple range test. Results were considered as significant when P-values were less than 0.05. Mortality data were subjected to Chi-square analysis.
Results
Growth performance
The effects of diet inclusion of GAA on the growth performance of broilers are summarized in . During the starter period (0–10 days), FI was significantly higher (P < 0.05) in birds fed the control diet (138.55 g) compared to those fed the diets included with 0.6 g/kg GAA (96.62 g). It was no observed significant difference between the control group and those fed with FI in birds fed the diets included 1.2 (121.56 g) or 1.8 (106.92 g) g/kg GAA in the starter period. FI was significantly higher in 1.2 GAA in comparison to the 0.6 GAA group (P < 0.05) in grower and finisher periods. The birds in the control diet showed significantly higher BWG in comparison to the birds receiving diets including 0.6 g/kg GAA (P < 0.05) in grower and finisher periods (P < 0.05). No significant differences were observed (P > 0.05) in FCR among all dietary treatments during the starter period (0–10 d). At both, 11–24 and 25–42 days of age, chicks receiving the control diet showed lower (P < 0.05) FCR compared to the chicks receiving the 0.6 and 1.8 GAA-included diets. The ratio of right to total ventricle, mortality and relative weights of liver were significantly lower in birds fed the diet with 1.2 g/kg GAA compared to those in birds fed other levels (P < 0.05).
Table 2. Growth performance at different rearing periods, right ventricle to total ventricle ratio at day 42 of age, and mortality from 1 to 42 days of age in male broilers given guanidinoacetic acid (GAA)-supplemented diets.
As indicated in , RV/TV ratio was significantly lower in the birds fed diet included 1.2 g/kg GAA compared to other groups (P < 0.05). Also, quadratic responses to GAA levels were observed in terms of RV/TV ratio.`
Blood parameters
As it is shown in , the serum concentrations of TG, total CHOL and LDL-C were not significantly affected by dietary treatments (P > 0.05). Birds fed the diet supplemented with 1.2 g/kg GAA showed a significantly higher level of blood HDL-C (P < 0.05) in comparison to other groups.
Table 3. Biochemical parameters in serum 42 d in male broilers given guanidinoacetic acid (GAA)-supplemented diets.
Enzyme activity and Thyroid hormones
No significant effect of dietary treatments was found on serum levels of T3 and T4 (). In terms of enzyme activity, the activity of LDH was decreased in the birds fed the diet of 1.2 g/kg GAA in comparison to that of 1.2 g/kg GAA (). The activity of CK was lower in chickens fed control and 0.6 g/kg GAA diets compared to 1.2 and 1.8 g/kg GAA.
Table 4. Thyroid hormones, lactate dehydrogenase and creatine kinase activity in male broilers given guanidinoacetic acid (GAA)-supplemented diets.
Organ index and ascites-related parameters
Effects of dietary treatments on carcass traits are represented in . No significant impacts of supplemental GAA on breast meat, abdominal fat, heart, and relative length of duodenum and ileum were observed. The relative length of jejunum was significantly lower (P < 0.05) in the birds fed 0.6 and 1.2 g/kg GAA compared to birds fed the control diet (P < 0.05). The relative weight of the liver was significantly lower in the birds fed 1.2 g/kg GAA compared to other groups.
Table 5. Carcass characteristics at slaughter (d 42) in male broilers given guanidinoacetic acid (GAA)-supplemented diets.
Discussion
Growth performance
Based on our knowledge, there are no other reports evaluating the effect of in-feed supplementation of GAA on the performance of broiler chicks with induced ascites. Our findings for growth performance were conflicting. Significant decreased FI in the birds fed the diet included 0.6 g/kg GAA were observed in the starter period, which is in line with the previous reports in which 0.6 g/kg GAA and higher concentrations caused a significant decrease in FI and BWG (European Food Safety Authority Citation2009). However, other levels did show a significant difference for FI in the starter period. In contrast, broiler chicks fed with the level of 1.2 GAA consumed more FI in comparison to those fed level of 0.6 GAA. It cannot be certainly stated that decreased FI was attributed to higher levels of GAA because it was not observed significant between levels of 1.2 and 1.8 g/kg GAA in starter and grower periods. However, the differences between levels of 0.6 and 1.2 g/kg GAA may be attributed to voluntary FI in group 1.2 in comparison to 0.6 g/kg GAA (Mousavi et al. Citation2013; Heger et al. Citation2014). Based on a literature review, Michiels et al. (Citation2012) found slightly higher FI in chickens receiving 0.6 or 1.2 g/kg dietary supplemental GAA compared to the control birds. Another study did not show a significant effect of dietary supplemental GAA on FI in broilers (Ringel et al. Citation2007 and Khodambashi Emami et al. Citation2017). Guanidinoacetic acid not only spares Arg (Dilger et al. Citation2013) but it is also a precursor for the synthesis of nitric oxide (NO). Wang et al. (Citation2014) showed that dietary Arg may regulate appetite in ducks by conversion to NO. It can be argued that GAA may not increase NO, and thus, it does not improve NO. In the present study, increased FCR was detected in chicks receiving 0.6 and 1.8 g/kg GAA in the grower period and 1.2 g/kg supplemental GAA during the finishing period which is consistent with results reported by Michiels et al. (Citation2012). However, several studies have reported positive effects of GAA on FCR (Lemme et al. Citation2007; Ringel et al. Citation2008). Dilger et al. (Citation2013) reported that supplemental GAA to Arg-deficient diet improved in BWG and FCR. Tossenberger et al. (Citation2016) did not observe a significant effect of diet supplementation of 0.6 g/kg GAA on BWG, FI or FCR. Considering ascites, studies have shown reduced growth rate, higher FI and poorer FCR in chickens reared under low temperatures (Howlider and Rose Citation1987; Deaton et al. Citation1996; Pan et al. Citation2005). Deaton et al. (Citation1996) suggested that birds cannot compensate for reduced BWG caused by low temperature until market age. It can be stated that GAA in the different levels showed conflicting results which were not in agreement with most previous studies. The differences between our findings and others can be attributed to levels of GAA, type of birds and especially rearing conditions (stress vs normal condition).
RV/TV ratio is criteria in order to show ascites incidence (Balog et al. Citation2000; Julian Citation2005; Daneshyar et al. Citation2009; Izadinia et al. Citation2010). In broiler chicks, a RV/TV ratio higher than 0.25–0.30 is considered ascites (Julian Citation1987). Walton et al. (Citation2001) observed higher RV/TV value and increased ascites morbidity in the broilers with cold-induced ascetic. All the groups except group 1.2 g/kg GAA, other groups showed higher values for RV/TV. In addition, 1.2 g/kg GAA showed lower mortality. Supplementing GAA in vegetable-based diets could be an efficacious replacement for dietary Arg in young birds (Dilger et al. Citation2013). Regarding arginine, studies have reported that Arg supplementation in broiler diets significantly alleviated the adverse effect of cold stress on RV/TV (Tan et al. Citation2007; Khajali et al. Citation2014). Our findings showed that a level of 1.2 g/kg GAA showed better efficiency, while a level of 1.8 g/kg did not show such effects. The reason for conflicting results is unknown.
Blood parameters
In terms of serum lipid profiles, there were no significant effects of dietary treatments on serum TG, total CHOL and LDL-C. Conversely, other studies have shown that dietary supplement of GAA improves lipid metabolism in poultry (Daneshyar et al. Citation2009; Mohammadalipour et al. Citation2017) . HDL-C was increased in group 1.2 g/kg GAA which is parallel with findings for mortality. Increased HDL-C can be considered a health index. More studies would be needed to evaluate the GAA on blood parameters.
Thyroid hormones
Thyroid hormones (T3 and T4) are key metabolic hormones that are closely correlated with growth performance and energy metabolism in animals (Zhan et al. Citation2007). In the current study, although there were no significant differences among treatments, the birds fed diets with supplemental GAA showed a trend to increase the higher level of T3 in sera (), which is an indicator of increased metabolic rate and oxygen demand. Under these conditions, it seems that erythropoiesis enhances a physiological response to increase the oxygen transport capacity, increased red blood cell (RBC) counts and haemoglobin (Hb), and provide sufficient oxygen for the high metabolic rate. Luger et al. (Citation2001) reported increased plasma concentrations of T3 in response to cold-temperature exposure, accompanied by reduced plasma T4 one week before death in induced-ascetic broilers. The plasma concentration of thyroid hormones plays important role in increased metabolism in the birds and ascites incidence (Hassanzadeh et al. Citation2000; Scheele et al. Citation2003). It was found conflicting results for growth performance; thus, it is natural that the data for thyroid hormones are not significant.
Enzyme activity
Significant increase in activity of plasma CK and LDH enzymes in 1.8 g/kg GAA in the current study is suggesting the occurrence of necrotic damage to the myocardial membrane. Biopsy samples were taken from patients suffering from congestive heart failure also revealed higher activity of LDH (York et al. Citation1976; Schultheiss et al. Citation1980; Fathi et al. Citation2016). It was reported that LDH is released in the blood from the damaged heart, liver and pulmonary system (Jaffe et al. Citation1996). LDH activities were decreased in-feed GAA under cold-temperature conditions. In this situation, it is feasible that GAA prevents heart, liver and pulmonary system damage, probably due to their antioxidant property.
Organ index and ascites-related parameters
The relative length of the jejunum was increased by the inclusion of 0.6 and 1.2 g/kg GAA in the diets. The increased length of the jejunum in 0.6 and 1.2 g/kg GAA in the diets can be explained by the findings that dietary inclusion of Arg in the culture medium stimulates the growth of chicken intestinal epithelial cells (Yuan et al. Citation2015). Increased breast meat yield with an increasing tendency with GAA level in the diet has been previously reported (Lemme et al. Citation2007; Michiels et al. Citation2012). No differences among dietary treatments were observed in terms of breast meat yield. Similarly, Mousavi et al. (Citation2013) reported no significant effect of dietary supplemental GAA on breast meat yield which is in line with the results observed in the current experiment. The relative weight of the liver was significantly decreased in the 1.2 g/kg GAA group which may be due to lower metabolic activity.
5. Conclusion
Based on the results of the present investigation, it can be concluded that dietary supplemental 1.2 g/kg GAA would be a beneficial way to decrease the mortality rate of broiler reared under cold stress conditions. In addition, the increased blood level of HDL-C and the decreased relative weight of the liver were seen in broilers fed the 1.2 g/kg GAA-included diet. This level can be advised in order to alleviate the adverse effects of ascites. It will be needed in future studies with higher levels and/or compared with arginine in order to clear the exact mechanism.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Notes
1 Paya Amin Mehr, Tehran, Iran.
2 Evonik Degussa GmbH, Hanau-Wolfgang, Germany.
References
- Baghbanzadeh A, Decuypere E. 2008. Ascites syndrome in broilers: physiological and nutritional perspectives. Avian Pathol. 37:117–126. doi:10.1080/03079450801902062.
- Balog J, Huff G, Rath N, Huff W. 2000. Effect of dietary aspirin on ascites in broilers raised in a hypobaric chamber. Poult Sci. 79:1101–1105. doi:10.1093/ps/79.8.1101.
- Basoo H, Khajali F, Asadi Khoshoui E, Faraji MF, Wideman R. 2012. Re-evaluation of arginine requirements for broilers exposed to hypobaric condition during the 3-to 6-week period. J Poult Sci. 49:303–307. doi:10.2141/jpsa.0110133.
- Behrooj N, Khajali F, Hassanpour H. 2012. Feeding reduced-protein diets to broilers subjected to hypobaric hypoxia is associated with the development of pulmonary hypertension syndrome. Br Poult Sci. 53:658–664. doi:10.1080/00071668.2012.727082.
- Buyse J, Janssens G, Decuypere E. 2001. The effects of dietary L-carnitine supplementation on the performance, organ weights and circulating hormone and metabolite concentrations of broiler chickens reared under a normal or low temperature schedule. Br Poult Sci. 42:230–241.
- Cobb. 2008. Cobb Broiler Performance & Nutrition Supplement. Europe, Middle East, Africa version. Cobb-Vantress Inc., Siloam Springs, AR. Cobb 500. Accessed May 2011. http://www.cobbvantress.com/contactus/brochures/Cobb500_BPN_Supp_2008%28lbs%29pd.
- Daneshyar M, Kermanshahi H, Golian A. 2009. Changes of biochemical parameters and enzyme activities in broiler chickens with cold-induced ascites. Poult Sci. 88:106–110. doi:10.3382/ps.2008-00170.
- Deaton J, Branton S, Simmons J, Lott B. 1996. The effect of brooding temperature on broiler performance. Poult Sci. 75:1217–1220. doi:10.3382/ps.0751217.
- Dilger R, Bryant-Angeloni K, Payne R, Lemme A, Parsons C. 2013. Dietary guanidino acetic acid is an efficacious replacement for arginine for young chicks. Poult Sci. 92:171–177. doi:10.3382/ps.2012-02425.
- EFSA (European Food Safety Authority). 2009. Safety and efficiency of guanidino acetic acid as feed additive for chickens for fattening. The EFSA Journal. 988:1–30.
- Fathi M, Heidari M, Ahmadisefat AA, Habibian M, Moeini MM. 2016. Influence of dietary glutamine supplementation on performance, biochemical indices and enzyme activities in broilers with cold-induced ascites. Anim Prod Sci. 56(12):2047–2053. doi:10.1071/AN15300.
- Fernandez SR, Aoyagi S, Han Y, Parsons CM, Baker DH. 1994. Limiting order of amino acids in corn and soybean meal for growth of the chick. Poult Sci. 73:1887–1896. doi:10.3382/ps.0731887.
- Franciosini M, Tacconi G, Leonardi L. 2012. Ascites syndrome in broiler chickens. Vet Sci Res. 3:60–66.
- Georgiou S, Christofidis I. 1996. Radioimmunoassay of free thyroxine (T4) using 125I-labeled T4-IgG complex with very large molecular weight. Clin Chim Acta. 244(2):209–220, 31.
- Han Y, Suzuki H, Parsons CM, Baker DH. 1992. Amino acid fortification of a low-protein corn and soybean meal diet for chicks. Poult Sci. 71:1168–1178. doi:10.3382/ps.0711168.
- Hassanzadeh M, Bozorgmerifard M, Akbari A, Buyse J, Decuypere E. 2000. Effect of intermittent lighting schedules during the natural scotoperiod on T3-induced ascites in broiler chickens. Avian Pathol. 29:433–439. doi:10.1080/030794500750047180.
- Havenstein G, Ferket P, Qureshi M. 2003. Growth, livability, and feed conversion of 1957 versus 2001 broilers when fed representative 1957 and 2001 broiler diets. Poult Sci. 82:1500–1508.
- Heger J, Zelenka J, Machander V, de la Cruz C, Lešták M, Hampel D. 2014. Effects of guanidinoacetic acid supplementation to broiler diets with varying energy content. Acta Universitatis Agriculturae et Silviculturae Mendelianae Brunensis. 62:477–485. doi:10.11118/actaun201462030477.
- Howlider M, Rose S. 1987. Temperature and the growth of broilers. World's Poult Sci J. 43:228–237. doi:10.1079/WPS19870015.
- Huchzermeyer FW. 2012. Broiler ascites: a review of the ascites work done at the poultry section of the Onderstepoort Veterinary Institute 1981-1990. World's Poult Sci J. 68:41–50. doi:10.1017/S0043933912000050.
- Izadinia M, Nobakht M, Khajali F, Faraji M, Zamani F, Qujeqn D, Karimi KI. 2010. Pulmonary hypertension and ascites as affected by dietary protein source in broiler chickens reared in cool temperature at high altitudes. Anim Feed Sci Technol. 155:194–200. doi:10.1016/j.anifeedsci.2009.12.009.
- Jaffe AS, Landt Y, Parvin CA, Abendschein DR, Geltman EM, Ladenson JH. 1996. Comparative sensitivity of cardiac troponin I and lactate dehydrogenase isoenzymes for diagnosing acute myocardial infarction. Clin Chem. 42:1770–1776.
- Julian R. 1987. The effect of increased sodium in the drinking water on right ventricular hypertrophy, right ventricular failure and ascites in broiler chickens. Avian Pathol. 16:61–71. doi:10.1080/03079458708436353.
- Julian RJ. 2005. Production and growth related disorders and other metabolic diseases of poultry–a review. Vet J. 169:350–369. doi:10.1016/j.tvjl.2004.04.015.
- Kalmar I, Cools A, Buyse J, Roose P, Janssens G. 2010. Dietary N, N-dimethylglycine supplementation improves nutrient digestibility and attenuates pulmonary hypertension syndrome in broilers. J Anim Physiol Anim Nutr. 94:e339–e347. doi:10.1111/j.1439-0396.2010.01018.
- Khajali F, Fahimi S. 2010. Influence of dietary fat source and supplementary α-tocopheryl acetate on pulmonary hypertension and lipid peroxidation in broilers. J Anim Physiol Anim Nutr. 94:767–772. doi:10.1111/jpn.2010.94.issue-6.
- Khajali F, Saedi M. 2011. The effect of low chloride and high bicarbonate diets on growth, blood parameters, and pulmonary hypertensive response in broiler chickens reared at high altitude. Archiv für Geflügelkunde. 75:235–238.
- Khajali F, Moghaddam MH, Hassanpour H. 2014. An L-arginine supplement improves broiler hypertensive response and gut function in broiler chickens reared at high altitude. Int J Biometeorol. 58:1175–1179. doi:10.1007/s00484-013-0710-7.
- Khajali F, Lemme A, Rademacher-Heilshorn M. 2020. Guanidinoacetic acid as a feed supplement for poultry. World's Poult Sci J. doi:10.1080/00439339.2020.1716651.
- Khodambashi Emami N, Golian A, Rhoads D, Danesh Mesgaran M. 2017. Interactive effects of temperature and dietary supplementation of arginine or guanidinoacetic acid on nutritional and physiological responses in male broiler chickens. Br Poult Sci. 58:87–94. doi:10.1080/00071668.
- Korte S, Sgoifo A, Ruesink W, Kwakernaak C, Van Voorst S, Scheele C, Blokhuis H. 1999. High carbon dioxide tension (PCO2) and the incidence of cardiac arrhythmias in rapidly growing broiler chickens. Vet Record. 145:40–43.
- Lemme A, Ravindran V, Bryden W. 2004. Ileal digestibility of amino acids in feed ingredients for broilers. World's Poult Sci J. 60:423–438.
- Lemme A, Ringel J, Rostagno H, Redshaw M. 2007. Supplemental guanidino acetic acid improved feed conversion, weight gain, and breast meat yield in male and female broilers. Proceedings of the 16th European Symposium on Poultry Nutrition.
- Luger D, Shinder D, Rzepakovsky V, Rusal M, Yahav S. 2001. Association between weight gain, blood parameters, and thyroid hormones and the development of ascites syndrome in broiler chickens. Poult Sci. 80:965–971. doi:10.1093/ps/80.7.965.
- Michiels J, Maertens L, Buyse J, Lemme A, Rademacher M, Dierick N, De Smet S. 2012. Supplementation of guanidinoacetic acid to broiler diets: effects on performance, carcass characteristics, meat quality, and energy metabolism. Poult Sci. 91:402–412. doi:10.3382/ps.2011-01585.
- Mohammadalipour R, Rahmani H, Jahanian R, Riasi A, Mohammadalipour M, Nili N. 2017. Effect of early feed restriction on physiological responses, performance and ascites incidence in broiler chickens raised in normal or cold environment. Animal. 2: 219–226.
- Mousavi S, Afsar A, Lotfollahian H. 2013. Effects of guanidinoacetic acid supplementation to broiler diets with varying energy contents. J Appl Poult Res. 22:47–54. doi:10.3382/japr.2012-00575.
- Nain S. 2008. Study on dietary factors pertinent to the pathogenesis of heart failure in fast-growing commercial broilers [dissertion], University of Saskatchewan, Saskatoon, Canada.
- NRC, 1994. Nutrient Requirements of Poultry. 9th rev. ed. National Academy Press, Washington, DC.
- Özkan S, Takma C, Yahav S, Söğüt B, Türkmut L, Erturun H, Cahaner A. 2010. The effects of feed restriction and ambient temperature on growth and ascites mortality of broilers reared at high altitude. Poult Sci. 89:974–985. doi:10.3382/ps.2009-00293.
- Pan J-Q, Tan X, Li J-C, Sun W-D, Wang X-L. 2005. Effects of early feed restriction and cold temperature on lipid peroxidation, pulmonary vascular remodelling and ascites morbidity in broilers under normal and cold temperature. Br Poult Sci. 46:374–381. doi:10.1080/00071660500098152.
- Ringel J, Lemme A, Knox A, McNab J, Redshaw M. 2007. Effects of graded levels of creatine and guanidino acetic acid in vegetable-based diets on performance and biochemical parameters in muscle tissue. Proceedings of the 16th Europ Symp Poult Nut.
- Ringel J, Lemme A, Araujo L. 2008. ‘The effect of supplemental guanidino acetic acid in Brazilian type broiler diets at summer conditions, Poult Sci.’ (POULTRY SCIENCE ASSOC INC 1111 N DUNLAP AVE, SAVOY, IL 61874-9604 USA.
- Saki A, Haghighat M, Khajali F. 2013. Supplemental arginine administered in ovo or in the feed reduces the susceptibility of broilers to pulmonary hypertension syndrome. Br Poult Sci. 54:575–580. doi:10.1080/00071668.2013.811716.
- SAS. 2008. Institute SAS/ETS® 9.2 user’s guide. Cary: SAS Institute, Inc.
- Savage J, O'dell B. 1960. Arginine requirement of the chick and the argininesparing value of related compounds. J Nutr. 70:129–134.
- Scheele C, Van Der Klis J, Kwakernaak C, Buys N, Decuypere E. 2003. Haematological characteristics predicting susceptibility for ascites. 2. high haematocrit values in juvenile chickens. Br Poult Sci. 44:484–489. doi:10.1080/00071660310001598300.
- Schultheiss H, Bolte H, Fischer S, Cyran J. 1980. Enzymatic analysis and collagen content in endomyocardial biopsy samples of patients with congestive cardiomyopathy of unknown etiology. Clin Cardiol. 3:329–334. doi:10.1002/clc.4960030407.
- Shlosberg A, Zadikov I, Bendheim U, Handji V, Berman E. 1992. The effects of poor ventilation, low temperatures, type of feed and sex of bird on the development of ascites in broilers. physiopathological factors. Avian Pathol. 21:369–382. doi:10.1080/03079459208418855.
- Tamir H, Ratner S. 1963. Enzymes of arginine metabolism in chicks. Arch Biochem Biophys. 102:249–258. doi:10.1016/0003-9861(63)90178-4.
- Tan X, Sun W-D, Li J-C, Pan J-Q, Liu Y-J, Wang J-Y, Wang X-L. 2007. L-arginine prevents reduced expression of endothelial nitric oxide synthase (NOS) in pulmonary arterioles of broilers exposed to cool temperatures. Vet J. 173:151–157. doi:10.1016/j.tvjl.2005.08.004.
- Tossenberger J, Rademacher M, Németh K, Halas V, Lemme A. 2016. Digestibility and metabolism of dietary guanidino acetic acid fed to broilers. Poult Sci. pew083. doi:10.3382/ps/pew083.
- Tsutsayeva A, Sevryukova L. 2000. Effect of cold exposure on survival and stress protein expression of drosophila melanogaster at different development stages. Cryo Lett. 22:145–150.
- Waguespack A, Powell S, Bidner T, Payne R, Southern L. 2009. Effect of incremental levels of L-lysine and determination of the limiting amino acids in low crude protein corn-soybean meal diets for broilers. Poult Sci. 88:1216–1226. doi:10.3382/ps.2008-00452.
- Walton J, Julian R, Squires E. 2001. The effects of dietary flax oil and antioxidants on ascites and pulmonary hypertension in broilers using a low temperature model. Br Poult Sci. 42:123–129.
- Wang C, Hou SS, Huang W, Xu TS, Rong GH, Xie M. 2014. Arginine affects appetite via nitric oxide in ducks. Poult Sci. 93:2048–2053. doi:10.3382/ps.2013-03812.
- Wang Y, Guo Y, Ning D, Peng Y, Cai H, Tan J, Yang Y, Liu D. 2012. Changes of hepatic biochemical parameters and proteomics in broilers with cold-induced ascites. J Anim Sci Biotechnol. 3:1. doi:10.1186/2049-1891-3-41.
- Wideman R, Rhoads D, Erf G, Anthony N. 2013. Pulmonary arterial hypertension (ascites syndrome) in broilers: A review. Poult Sci. 92:64–83. doi:10.3382/ps.2012-02745.
- York JW, Penney DG, Weeks TA, Stagno PA. 1976. Lactate dehydrogenase changes following several cardiac hypertrophic stresses. J Appl Physiol. 40:923–926.
- Yuan C, Ding Y, He Q, Azzam M, Lu J, Zou X. 2015. L-arginine upregulates the gene expression of target of rapamycin signaling pathway and stimulates protein synthesis in chicken intestinal epithelial cells. Poult Sci. 94:1043–1051.
- Zhan X, Wang M, Ren H, Zhao R, Li J, Tan Z. 2007. Effect of early feed restriction on metabolic programming and compensatory growth in broiler chickens. Poult Sci. 86:654–660. doi:10.1093/ps/86.4.654.
- Zhang J, Chen D, Yu B, Wang Y. 2011. Effect of dietary energy source on deposition and fatty acid synthesis in the liver of the laying hen. Br Poult Sci. 52:704–710. doi:10.1080/00071668.2010.5474.