ABSTRACT
Five hundred and sixty broilers were placed into seven groups. The first negative control (A) was fed the baseline diet (BD); the second positive control (B) was fed BD and exposed to clostridium infection; and the third and fourth groups (C, D) were the same as the positive control with 500 and 1000 g/ton of feed, respectively. The fifth group (E) served as a positive control for the addition of lincomycin, whereas the sixth and seventh groups (F, G) received the same designed diet as the E group but supplemented with HA at 500 and 1000 g/ton of feed, respectively. Birds infected with Clostridium perfringens had considerably decreased body weight. However, a dietary combination of HA and lincomycin resulted in a greater improvement in growth. Body weight increased after 35 days, but feed intake dropped, therefore HA and lincomycin supplementation enhanced feed conversion ratio. Supplementing with HA and lincomycin increased crude protein retention. Furthermore, these additions mitigated the detrimental effects of clostridial infection on the gut by reducing degenerative changes in intestinal villi and increasing villi length, particularly at higher HA/lincomycin doses. In conclusion, nutritional supplementation with humic acid and lincomycin improved blood biochemistry, and gut morphology in broilers infected with Clostridium difficile.
Introduction
To achieve the best profitability in poultry production, gut health is critical for accomplishing the optimum growth, digestion, and nutrient absorption and utilization. It's also essential for immune function and effective post-absorption metabolism. The healthy gut- acts as a barrier against pathogens, protects the bird against toxins and harmful metabolites, and supports the mucosal immune response. Challenges in the poultry industry as enteric disease outbreaks can cause poor feed efficiency, lower weight gain, and an overall increase in mortality, resulting in major economic impacts (Porter Citation1998; Dosoky et al. Citation2022). Necrotic enteritis caused by Clostridium perfringens is considered one of the most important enteric diseases reported by poultry producers worldwide (Paiva and McElroy Citation2014). It negatively affects the intestinal mucosa resulting in decreased digestion and absorption, reduced weight gain and increased feed conversion ratio, and significant economic losses (Elwinger et al. Citation1992; Kaldhusdal et al. Citation2001).
Antibiotic growth promoters have been used in poultry farms for decades to keep birds healthy and help them grow faster (Huyghebaert et al. Citation2011). There is a worldwide endeavour to decrease antibiotic use in poultry production, since a high level of microbial resistance to antibiotics, and residues of antibiotics in poultry products can be harmful to humans. Since 2006, the European Union has banned the use of antibiotics as feed additives in animal diets (Saleh et al. Citation2020). Lincomycin and bacitracin are two well-developed antibiotics that appear promising for veterinary use (Chan et al. Citation2015), either alone or in combination positively affect broiler chicken growth, immunological, and hematobiochemical parameters (Elkomy et al. Citation2019). Lincomycin is a naturally occurring lincosamide antibiotic derived from Streptomyces lincolnensis fermentation products. It works against Gram-positive bacteria as well as the majority of anaerobes (Greenwood et al. Citation2004), by preventing bacterial cells from synthesizing proteins (Ali et al. Citation2014). Antibiotics are used to both prevent proliferation and destroy bacteria. However, scientific evidence suggests that the enormous use of antibiotics can promote bacterial resistance in treated animals (Furtula et al. Citation2010; Forgetta et al. Citation2012).
Feed mills already play an important role in feed preservation (Brul et al. Citation2002). In addition to their preservative function in feed, organic acids have been shown to promote gut health; antimicrobials as well as acidifiers which affect the stomachs of monogastric animals like pigs (Aljumaah et al. Citation2020; Tugnoli et al. Citation2020). Once they diffuse through the cell membrane of the bacterial cell, the weak organic acid dissociates due to the higher pH of the bacterial cytoplasm, causing the cytoplasm pH to drop rapidly, resulting in the death of the bacteria (Dibner and Buttin Citation2002). Additionally, organic acids have other biological activities besides antimicrobial activity, such as improved intestinal health for efficient nutrient consumption and absorption, thus enhancing the overall health and efficiency of broilers (Khan et al. Citation2022).
Humic acid (HA) is a naturally occurring component of drinking water, soil, and lignite which is formed by the decomposition of organic matter, especially plants. Positive effects of HA addition in feed have been reported as improved broiler growth by increasing protein digestibility and trace element utilization,(Islam et al. Citation2005; Bahadori et al. Citation2017), improved gut health, allowing for better nutrient utilization and improved health status, influence digestion, immune response and general performance of broilers (Furtula et al. Citation2010). Humic acids have recently been used in the feed and water of poultry to promote their development (Rath et al. Citation2006; Abd El-Hack Citation2016; Fouda et al. Citation2021). Salah et al. (Citation2015) indicated that HA supplementation significantly increased broiler body weight gain and FCR.
To the best of our knowledge, no more research has been done to evaluate the impact of HA with lincomycin on broiler performance and other physiological functions when exposed to bacterial infection. Further research into the growth performance and other physiological traits of meat quality and fatty acids contents of commercial broilers fed diets supplement with humic acid and lincomycin under clostridium infection appears to be needed in light of these observations. The purpose of the present study was to evaluate the effects of dietary supplementation of humic acid and lincomycin single or combined on growth, internal organs, blood biochemistry, and gut morphology in broilers under clostridium infection
Material and methods
A total of 560 one-day-old male broilers (Ross 308) were housed in pens. Birds with approximately equal average body weight (BW) were randomly divided into seven experimental treatments with eight replicates/ group (10 birds/ replicate). The experimental diets were formulated with a three-phase feeding system (starter, 0–10 d; grower, 11–24 d; and finisher, 25–35 d). The first negative control group (A) was fed the basal diet (BD), the second positive control group (B) fed the BD and exposed to clostridium infection, and the third (C) and fourth (D) experimental groups of chicks were the same as group B and supplemented with HA, 500 and 1000 g/ton of feed, respectively. The fifth (E) group of chicks was group B and supplemented with lincomycin antibiotic (the lincomycin concentration is 10%), while the sixth (F) and seventh (G) groups received the same formulated diet used in the fifth group and supplemented with HA, 500 and 1000 g/ton of feed, respectively. The composition of the experimental basal diet, which was formulated to comply with the recommendations of the Ross 308 broiler chickens strain requirements (Aviagen, Citation2014), is presented in . Diets and water were provided to the bird's ad libitum; starter diets were in crumble form, while the grower and finisher diets were in a pelleted form. The feeding trial was conducted in a temperature-controlled room with an 18 h light and 6 h darkness cycle. The temperature started from 33 ± 1 and decreased by 1°C every three days until 24 ± 1°C which continued until the end of the experiment (35 days), and proportional humidity between 50% and 70%. Throughout the experimental phase, the health status, and mortalities were measured daily on a regular basis. On day 7 of chicks’ age, they were challenged with Clostridium perfringes (1 × 1010 bacteria/ ml) by oral administration. Each bird was inoculated with 1 ml of challenge inoculum into the lumen of the crop. Isolation and identification of Clostridium Perfringens: according to Dar et al. (Citation2017)
Table 1. Composition of the experimental starter, grower, and finisher diets.
For isolation of C. perfringens, samples were inoculated in cooked meat medium (Himedia) and incubated anaerobically at 37°C for 24 hrs in an anaerobic jar containing GasPak (Oxoid Limited, Thermo Fisher Scientific Inc., UK). Enriched samples were streaked on the 5% sheep blood agar (Oxoid®) with 70 µg/ml neomycin sulphate and were incubated anaerobically as above. The colonies were producing a characteristic double zone of hemolysis around them on blood agar. Suspected colonies were stained with Gram’s stain. Then streaked on egg yolk agar plates and incubated anaerobically for 24 hr; characteristic colonies were producing zone of opalescence around the colonies on egg yolk agar were tentatively identified as C. perfringens.
Bird BW was measured individually every week, while feed intake (FI) was measured daily (on a group basis per pen) throughout the experimental period. Body weight gain (WG) and feed conversion ratio (FCR) were calculated accordingly. At 32 days of the experiment, all birds were weighed individually and sorted from the smallest to the heavyweight. Then, these birds were slaughtered and dissected to gauge the weights of the carcass, breast muscle, thigh muscle, liver, gizzard, heart, spleen, and abdominal fat. All organs were weighed and described as a ratio of the BW. Blood samples were collected from the wing vein immediately before slaughtering, gathered into heparinized test tubes, and then rapidly centrifuged (3000 rpm for 20 min at 5°C) to separate the plasma. Plasma samples were stored at −20°C pending analysis of the blood biochemical parameters.
Creatinine, globulin, albumin, total protein, serum glutamate pyruvate transaminase (SGPT), serum glutamic oxaloacetic transaminase (SGOT), total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, and glucose were measured colorimetrically using commercial kits (Diamond Diagnostics, Egypt) according to the procedure outlined by the manufacturer Saleh (Citation2013)
For the final three experiment days, birds (n = 10 birds/treatment of the same BW) were housed individually in special metabolic cages (40 × 40 × 50 cm3) to determine nutrient digestibility. During this period, body weight and feed intake were measured daily. Excreted feces were also collected, weighed daily, then stored in a freezer. After the digestibility experimental period, all samples were dried in a drying oven at 60 °C for 24 h. The whole dried samples were then homogenized. Samples were used for analysis according to the Association of Official Analytical Chemists (AOAC Citation1996). The crude protein concentrations in the diet and excreta were measured to determine nitrogen retention using the Kjeldahl method (AOAC 945.38 F), while the crude fibres analysis method (CF, Method 932.09). The calculation was as follows: nitrogen retention (%) = (total nitrogen intake – total nitrogen excreted)/total nitrogen intake × 100 (Saleh Citation2016).
Intestinal content was collected under aseptic conditions for the determination of bacterial count. Total bacterial count (TBC) was recorded from plate count agar (Merck, 1.05463, Darmstadt, Germany) at 35°C for two days. Salmonella was assessed on xylose lysine deoxycholate agar plates (Merck) after incubation for one day at 37°C. Microbial counts (total bacterial count; lactobacilli count, Salmonella spp.) were analyzed following (Xia et al. Citation2004; Mahgoub et al. Citation2019). Clostridial P. colony count: One gram of each sample was diluted 1:9 (wt/vol) in sterile PBS and 10-fold serial dilution was made. The colony counting was performed following (Quinn et al. Citation1994) with some variation. In Brief, dilutions of the samples were inoculated in Thioglycollate medium (Oxoid) and incubated anaerobically at 37°C for one day.
Bird abdomen (n = 5/ treatment) was dissected and tissue specimens from the duodenum, jejunum, ileum, cecum, and liver were randomly selected directly after slaughtering. The samples from the intestine were fixed in Bouin's solution for 18 h while liver samples were fixed in 10% formaldehyde solution for 24 h. After fixation, the tissue samples were dehydrated by using ascending concentrations of ethyl alcohol (70% to absolute alcohol) then cleared in xylene and prepared for histological investigations. Sections of 4–5 µm thickness were stained with hematoxylin and eosin (H&E) for histopathological examination according to Bancroft and Gamble (Citation2007).
Using SPSS statistical software version 26 (IBM SPSS stats for Windows Armonk, NY: IBM Corp), the obtained data were analyzed according to a completely randomized design. The significance of all differences in means was checked using Tukey's multiple comparison test based on p ≤ 0.05.
Results
illustrates the effects of dietary supplementation of HA single or combined with lincomycin supplementation on final BW, FI, FCR, and mortality rate in broilers under clostridium infection over 35 days experimental period. Broiler chickens infected with clostridium perfringens showed poor (perfringes) poor performance signs including a significant decrease in BW, poor FCR, and a higher mortality rate. Dietary HA and lincomycin supplementation did not affect BWG and FI of the broilers during 7, 14, 21, and 28 days of age. The combination of HA and lincomycin showed a higher improvement (p < 0.05) in BWG and FCR values compared to the positive control group during 35 days of age. In addition, use crude protein retention and crude fibre digestibility were significantly improved (p < 0.05) by HA and lincomycin supplementation.
Table 2. Effect of humic acid and lincomycin supplementation on growth performance in broilers under clostridium infection.
Results concerning the effect of dietary supplementation of HA single or combined with lincomycin supplementation on internal organs weight in broilers under clostridium infection are demonstrated in . Non-significant differences were found in the carcass, breast, thigh and abdominal fat weights; however, liver, gizzard, heart, and spleen weights were significantly increased in groups supplemented with HA and lincomycin of each supplement compared to other treatment groups (p < 0.05). Birds fed 1000 g/ton of HA and lincomycin had the highest liver and spleen weight compared with other groups. However, the highest heart weight was recorded (p < 0.05) in birds that received 500 g/ton of HA and lincomycin.
Table 3. Effect of dietary supplementation of humic acid and lincomycin on internal organs weight in broilers under clostridium infection.
As presented in , dietary supplementation of HA single or combined with lincomycin in broilers chickens under clostridium infection did not alter plasma globulin, SGPT, SGOT, and LDL parameters. On the other hand, plasma creatinine, total cholesterol, and glucose values were lower (p < 0.05) in birds fed on HA and lincomycin compared to the positive control group (fed the BD and exposed to infection). Furthermore, plasma albumin, total protein, and HDL concentrations were significantly increased (p < 0.05) in birds who received HA and lincomycin supplementation compared to the positive control group (fed the BD and infected with clostridia).
Table 4. Effect of dietary supplementation of humic acid and lincomycin on blood biochemical analysis in broilers under clostridium infection.
The effects of dietary supplementation of HA single or combined with lincomycin on gut microbiology of broiler chickens under clostridium infection are presented in . HA single or combined with lincomycin supplementation altered the gut microbiology of broiler chickens under clostridium infection. Birds exposed to clostridium infection (positive control, B) showed a significant increase (p < 0.05) in C. perfringens and total bacterial count (TBC), while reducing the lactobacilli count in the intestinal content when compared with the negative control group (A). Feeding of the infected birds with HA single or combined with lincomycin significantly reduced (p < 0.05) C. perfringens and TBC especially with the higher concentration of HA (1000 g/ton feed) and increased the lactobacilli count when compared with the positive control birds (B). Non-significant difference (p > 0.05) was observed in the salmonella count among the different treatments.
Table 5. Effect of dietary supplementation of humic acid and lincomycin on bacteriological counts in broiler chickens under clostridium infection.
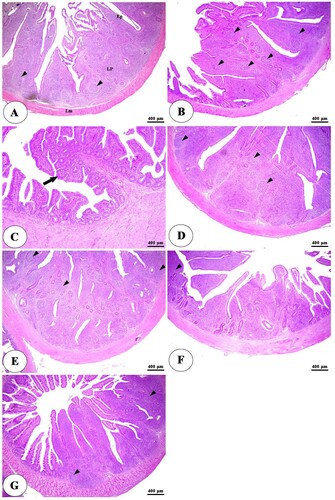
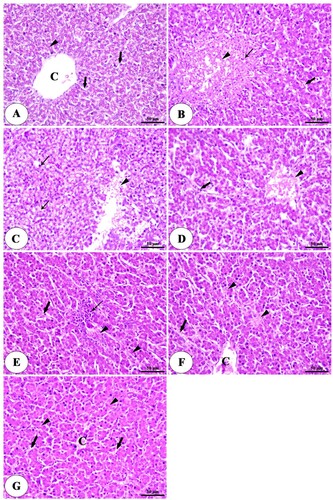
The histopathological examination of the intestine in clostridium perfringens infected group revealed significant changes in the tunica mucosa and lamina propria which ranged from degenerative changes in the apical part of intestinal villi in the ileum to the whole length of intestinal villi and thickening of some villi besides complete loss of villi in some areas in duodenum and jejunum in addition to massive inflammatory cells infiltration in the lamina propria compared with control negative group ((A,B), (A,B), (A,B)). The addition of HA either alone or with lincomycin showed an ameliorative effect on the intestine. It reduces degenerative changes of intestinal villi besides an increase in villi length, especially at higher concentrations with lincomycin ((C–G), (C–G), (C–G)). The cecum examination revealed an increase in the lymphatic element during infection but there were non-significant degenerative changes in its structure among all groups ((A–G)).
Figure 1. Photomicrograph of duodenum of negative control group (A) showing intact intestinal villi lined by simple columnar epithelium (Ep), lamina propria (Lp), and lamina muscularis (Lm). The positive control group (B) showing degeneration and necrosis of intestinal villi (thick arrows), thickening of some villi (black arrowheads), diffuse lymphocytic infiltration in the lamina propria (thin arrows), and lymphatic nodules (white arrowheads). The positive control group with humic acid 500 g/ton of feed (C) showing sloughing and degeneration of epithelial lining of intestinal villi along its whole length (arrows). The positive control group with humic acid 1000 g/ton of feed (D) showing sloughing and degeneration of the apical part of the intestinal villi (arrows). The positive control with lincomycin (E) showing desquamation of apical part of intestinal villi (arrows). The positive control with humic acid 500 g/ton of feed + lincomycin (F) and the positive control group with humic acid 1000 g/ton of feed+ lincomycin (G) showing long and intact intestinal villi (arrows). Stain H&E.
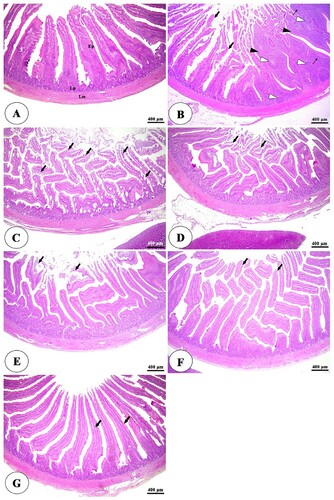
Figure 2. Photomicrograph of the jejunum of negative control group (A) showing intact intestinal villi lined by simple columnar epithelium (Ep), lamina propria (Lp), and lamina muscularis (Lm). The positive control group (B) showing degeneration and necrosis of intestinal villi (thin arrows), thickening of some villi (white arrowheads), complete loss of some intestinal villi (thick arrows), and diffuse lymphocytic infiltration in the lamina propria (black arrowheads). The positive control group with humic acid 500 g/ton of feed (C) showing mild degeneration of the small number of intestinal villi (thin arrows), thickening of some villi (white arrowheads), and lymphocytic infiltration in the lamina propria (black arrowheads). The positive control group with humic acid 1000 g/ton of feed (D) showing mild degeneration of the small number of intestinal villi (thin arrows) and lymphocytic infiltration in the lamina propria (black arrowheads). The positive control group with lincomycin (E) showing desquamation of apical part of intestinal villi (arrows) and massive lymphocytic infiltration in the lamina propria (black arrowheads). The positive control group with humic acid 1000 g/ton of feed + lincomycin (G) showing mild to moderate desquamation of apical part of intestinal villi (arrows) and lymphocytic infiltration in the lamina propria (black arrowheads). Stain H&E.
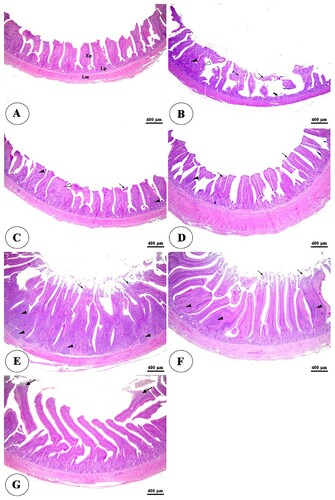
Figure 3. Photomicrograph of ileum of negative control group (A) showing intact intestinal villi lined by simple columnar epithelium (Ep), lamina propria (Lp), and lamina muscularis (Lm). The positive control group (B) showing degeneration of the stunted intestinal villi (arrowheads) and loss of some intestinal villi (arrows). The positive control group with humic acid 500 g/ton of feed (C) showing mild degeneration of the stunted intestinal villi (arrows). The control positive group with humic acid 1000 g/ton of feed (D) showing intact mucosa of intestinal villi (arrows). The control positive group with lincomycin (E) showing mild desquamation of the apical part of intestinal villi (arrows). The positive control group with humic acid 500 g/ton of feed+ lincomycin (F) and the positive control group with humic acid 1000 g/ton of feed+ lincomycin (G) showing intact, elongated, and branched intestinal villi (arrows). Stain H&E.
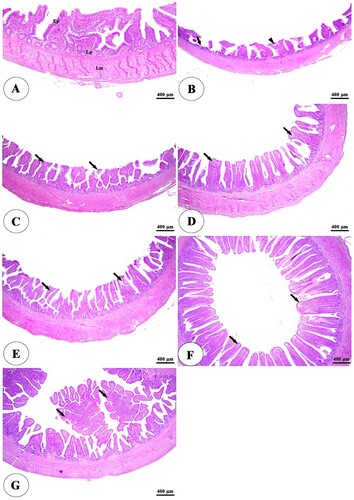
Figure 4. Photomicrograph of the cecum of negative control group (A) showing intact intestinal villi lined by simple columnar epithelium (Ep), lamina propria (Lp) filled with diffuse lymphatic tissue and lymphatic nodules (arrowheads), and lamina muscularis (Lm). The positive control group (B) showing an increase in the lymphatic elements in the lamina propria (arrowheads). The positive control group with humic acid 500 g/ton of feed (C), The positive control group with humic acid 1000 g/ton of feed (D), the positive control group with lincomycin (E), The positive control group with humic acid 500 g/ton of feed+ lincomycin (F) and the positive control group with humic acid 1000 g/ton of feed+ lincomycin (G) showing intact, branched intestinal mucosa (arrow) and lymphatic tissue in the lamina propria (arrowheads). Stain H&E.
On the other hand, the liver showed large focal areas of degeneration and necrosis with infiltration of inflammatory cells and congestion of hepatic blood vessels in the clostridium perfringens infected group ((B)) compared with the control negative group ((A)). The deleterious effects on the liver were significantly reduced with the high concentration of HA alone or combined with lincomycin ((C–G)).
Figure 5. Photomicrograph of liver of negative control group (A) intact central vein (C), polyhedral shaped hepatocytes (arrowheads) arranged in a cord-like pattern and separated by blood sinusoids (arrows). The positive control group (B) a large focal area of necrosis (arrowhead) surrounded by inflammatory cells (thin arrow) and congested blood sinusoids (thick arrow). The positive control group with humic acid 500 g/ton of feed. (C) A focal area of necrosis (arrowhead), vacuolar degeneration, and micro steatosis of hepatocytes (thin arrow). The positive control group with humic acid 1000 g/ton of feed. (D) A small area of necrosis (arrowhead), dilation, and congestion of blood sinusoids (thick arrow). The positive control group with lincomycin. (E) mild degenerative changes in hepatocytes (arrowheads), mild congestion of blood sinusoids (thick arrow), and infiltration of inflammatory cells (thin arrow). The positive control group with humic acid 500 g/ton of feed + lincomycin (F) mild congestion of central vein (C), necrosis of some hepatocytes (arrowhead), dilation, and congestion of blood sinusoids (arrow). The positive control group with humic acid 1000 g/ton of feed + lincomycin (G) normal hepatocytes (arrowhead) radiated from the central vein (C) and separated by blood sinusoids (arrows). Stain H&E.
Discussion
The current study showed that dietary supplementation of HA and lincomycin significantly improved broilers’ growth performance including BW, and FCR, and reduced mortality rate during the overall period of the experiment. Consistent and contradictory results have been documented regarding their supplementation and potential effects on the productive performance of broilers. Unlike the obtained results, Kaya and Tuncer (Citation2009) reported that there were no differences in broiler WG due to HA supplementation. On the other hand, previous reports agree with our findings (Ozturk et al. Citation2010; Saleh and Hayashi Citation2011, p. 1; Nagaraju et al. Citation2014; Ozturk et al. Citation2014; Pistová et al. Citation2016) who found that the use of HA daily showed a positive effect on broilers growth performance. In another experiment, Arif et al. (Citation2018) indicated that HA addition to the diet improved starter and finisher WG, BW and feed efficiency. Similarly, Avci et al. (Citation2007) and Salah et al. (Citation2015) reported that hamates supplemented with broiler diets improved WG and FCR. In support, lincomycin supplementation to broilers' diet significantly improved their performance (Sun et al. Citation2005; Khan and Nagra Citation2010; Dosoky et al. Citation2021). This improved response could be associated with the effect of HA as it helps in the stabilization of gut microflora which results in improved nutrient absorption and WG (Shermer et al. Citation1998; Saleh Citation2016). This improvement in FCR may be caused by the effects of the decrease in the total bacterial count, Salmonella, E. Coli, and Proteus by using HA. On the other hand, the improvement in the FCR with HA supplementation could be possibly due to better utilization of nutrients resulting in increased BW (Lala et al. Citation2016) which was supported in our study by the improved digestibility of nutrients (crude fibre digestibility and crude protein retention). Similarly, Son et al. (Citation2002) demonstrated that using organic acids in broiler diets decreases feed passage rate resulting in increased digestion time and therefore improved utilization of feed nutrients.
Changes in size and structure of internal organs can be indicative of the effect of diet and its components on the development and function of the organs. Dietary supplementation of HA without or with lincomycin in broilers exposed to clostridium infection did not affect the carcass, breast, thigh, and abdominal fat weights. However, liver, gizzard, heart, and spleen were significantly increased in groups treated with HA and lincomycin of each supplement. Our results are in agreement with Avci et al. (Citation2007) who reported that the breast weight of broilers was not affected by supplementation of HA and humates. Likewise, Kocabağli et al. (Citation2002) found that HA supplementing did not affect carcass yield or abdominal fat pad percentages in broilers. On the contrary, Aksu and Bozkurt (Citation2009) and Arif et al. (Citation2018) found that breast and thigh weights were increased with HA inclusion in broiler diets. The observed increase in weights of gizzards and spleen could be attributed to the trophic effect of HA in stimulating the proliferation of normal cells and tissues, enhancing healthy tissue turnover and maintenance (Li et al. Citation2016; Ivarsson and Wall Citation2017).
HA and lincomycin supplementation in broilers under clostridium infection significantly decreased the creatinine, total cholesterol, and glucose plasma concentration, while plasma albumin, total protein, and HDL concentrations were significantly increased. Previous reports have shown that using HA in the broiler diet reduced the cholesterol concentration of poultry (Ozturk et al. Citation2012; Jaďuttová et al. Citation2019). Moreover, Arif et al. (Citation2018) reported that HA as an organic feed additive could reduce the total and low-density lipoprotein cholesterol concentrations in broiler blood. However, Saleem et al. (Citation2020), found that total protein, globulin, HDL, and LDL concentrations showed non-significant differences (P > 0.05) among groups that received different organic acid treatments. The reduction in blood lipids and cholesterol concentrations might be attributed to the reductions in microbial intracellular pH (Abdo and Zeinb Citation2004). By inhibition of microbial enzymes, the bacterial cell membrane is forced to use energy to release acidic protons which lead to lower intracellular pH (Young and Foegeding Citation1993). Healthy broilers supplemented with HA showed significant increases in total proteins, globulin, coupled with significant decreases in the concentration of C. perfringens in intestinal content (Salah et al. Citation2015).
Gut microbiota is one of the important factors affecting poultry health. In the present study, dietary supplementation of HA and lincomycin decreased clostridium count in intestinal content. In the animal feed industry, organic acids are added to reduce the negative impacts of bacteria such as Escherichia coli, Campylobacter spp., and Salmonella spp. in contaminated feed (Andreopoulou et al. Citation2014). Abdel-Mageed (Citation2012) found that feeding on humic substances containing diets significantly reduced coliform, Escherichia coli, and Clostridium perfringes count in the intestinal content as well as intestinal pH. Likewise, Salah et al. (Citation2015) revealed that supplementing broilers’ diets with HA induces a reduction in the concentration of Clostridium perfringens in the intestine. The reported effect is attributed to the antibacterial activity of HA which has the potential to reduce pH to reduce the counts of pathogenic bacteria in the intestinal tract as well as it could reduce the growth of these pathogenic bacteria and consequently reduces the toxins level produced by these bacteria (Humin Citation2004; Taklimi et al. Citation2012). As well, lincomycin plays a potential activity against Gram-positive bacteria and most anaerobes (Greenwood et al. Citation2004). Lincomycin supplemented with broilers diets can inhibit the protein synthesis of bacterial cells (Ali et al. Citation2014). In addition, Wang et al. (Citation2015) found that the addition of lincomycin reduced caeca Clostridium. perfringens counts (p < 0.05). Also, the Antibacterial mechanism of lincomycin includes disruption of peptide chain elongation, genetic code misreading, blockage of a site of ribosomes and oligosaccharides side chain’s attachment to glycoprotein (Morar et al. Citation2009; Abudabos et al. Citation2019).
HA and lincomycin supplementation demonstrated an ameliorative effect on the intestine. It reduced the degenerative changes of intestinal villi besides increased the villi length, especially at higher concentrations (1000 g/ton of feed) with lincomycin. In support, Adil et al. (Citation2010, Citation2011) showed that organic acids improved the villus height in the small intestine and had a direct stimulatory effect on gastrointestinal cell proliferation, thus facilitating nutrient absorption and growth performance. In general, the increase in villus height and apparent villus surface area observed in the different segments of the small intestine of broilers may be attributed to a suppression of the growth of many pathogenic or non-pathogenic intestinal bacteria by the organic acids (Ghazalah et al. Citation2011; Saleh et al. Citation2021). Also, the intestinal morphological alterations enhanced by HA could have the effect of increasing the retention time of feed for digestion processes and enhancing mucosal permeability for efficient nutrient assimilation (Nagaraju et al. Citation2014; Ivarsson and Wall Citation2017; Saleh et al. Citation2019). Moreover, lincomycin may act independently of C. perfringens infection in improving intestinal health for the numerical improvement in the villus height over the uninfected control (Wang et al. Citation2015). Although villi growth generally depends on exposure to toxic substances, pH, and microflora in the intestine, the HA can reduce pH and the count of pathogenic bacteria in the intestine. Thus, HA could have a favourable impact on the performance of poultry via ecosystems in the gastrointestinal tract (Taklimi et al. Citation2012).
Conclusions
From the previously mentioned findings, humic acid and lincomycin supplementation combined improved growth performance by increased body weight and improved feed conversion ratio, blood biochemistry by increased total protein and HDL and decreased glucose and improved gut morphology in broilers with changed bacteriological counts. Therefore, the combination of humic acid and lincomycin could be an effective and beneficial growth promoter, with a recommended level of 1000 g/ton of humic acid in broilers' diet under clostridium infection.
Consent to participate
All the authors were aware regarding participation and publications.
Consent for publication
All the authors were aware regarding participation and publications.
Ethics approval
The study was approved by the Ethics Committee of the Local Experimental Animals Care Committee and conducted under the guidelines of Kafrelsheikh University, Egypt (Number 4/2016 EC).
Acknowledgments
The authors wish to acknowledge the helpful suggestions of members of the department of poultry production, faculty of agriculture, Kafrelsheikh University, Egypt.
Data availability statement
Data are available upon request.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Abd El-Hack ME. 2016. Impacts of dietary humic acid supplementation on growth performance, some blood metabolites and carcass traits of broiler chicks. Indian J Anim Sci. 86(9):1073–1078.
- Abdel-Mageed M. 2012. Effect of dietary humic substances supplementation on performance and immunity of Japanese quail. Egyptian Poult Sci J. 32(3):645–660.
- Abdo M, Zeinb A. 2004. Efficacy of acetic acid in improving the utilization of low protein-low energy broiler diets. Egypt Poult Sci. 24:123–141.
- Abudabos AM, Ali MH, Nassan MA, Saleh AA. 2019. Ameliorative effect of Bacillus subtilis on growth performance and intestinal architecture in broiler infected with salmonella. Animals. 9(1):190–197. doi:10.3390/ani9040190.
- Adil S, Banday MT, Bhat GA, Qureshi SD, Wani SA. 2011. Effect of supplemental organic acids on growth performance and gut microbial population of broiler chicken. Livest Res Rural Dev. 23(1):241–149.
- Adil S, Banday T, Bhat GA, Mir MS, Rehman M. 2010. Effect of dietary supplementation of organic acids on performance, intestinal histomorphology, and serum biochemistry of broiler chicken. Vet Med Int. 2010:1–7.
- Aksu T, Bozkurt SA. 2009. Effect of dietary essential oils and/or humic acids on broiler performance, microbial population of intestinal content and antibody titres in the summer season. Kafkas Univ Vet Fak Derg. 15:185–190.
- Ali SA, Hasan KA, Bin Asif H, Abbasi A. 2014. Environmental enterococci: I. Prevalence of virulence, antibiotic resistance and species distribution in poultry and its related environment in Karachi, Pakistan. Lett Appl Microbiol. 58(5):423–432.
- Aljumaah MR, Alkhulaifi MM, Abudabos AM, Alabdullatifb A, El-Mubarak AH, Al Suliman AR, Stanley D. 2020. Organic acid blend supplementation increases butyrate and acetate production in Salmonella enterica serovar typhimurium challenged broilers. PLoS One. 15(6):e0232831. doi:10.1371/journal.pone.0232831.
- Andreopoulou M, Tsiouris V, Georgopoulou I. 2014. Effects of organic acids on the gut ecosystem and on the performance of broiler chickens. J Hell Vet Med Soc. 65(4):289–302.
- AOAC International. 1996. International official methods of analysis, of the Association of Official Analytical Chemists. Arlington (VA): AOAC International.
- Arif M, Rehman A, Abd El-Hack ME, Saeed M, Khan F, Akhtar M, Swelum AA, Saadeldin IM, Alowaimer AN. 2018. Growth, carcass traits, cecal microbial counts, and blood chemistry of meat-type quail fed diets supplemented with humic acid and black cumin seeds. Asian Australas J Anim Sci. 31(12):1930–1938.
- Avci M, Denek N, Kaplan O. 2007. Effects of humic acid at different levels on growth performance, carcass yields and some biochemical parameters of quails. Humic Acid. 16:3.00–3.75.
- Aviagen R. 2014. Ross 308 nutrition specifications. Newbridge: Aviagen.
- Bahadori Z, Esmaielzadeh L, Karimi-Torshizi MA, Seidavi A, Olivares J, Rojas S, Salem AZ, Khusro A, López S. 2017. The effect of earthworm (Eisenia foetida) meal with vermi-humus on growth performance, hematology, immunity, intestinal microbiota, carcass characteristics, and meat quality of broiler chickens. Livest Sci. 202:74–81.
- Bancroft J, Gamble M. 2007. Theory and practice of histological techniques. Edinburgh: Churchill-Livingston, Elsevier.
- Brul S, Coote P, Oomes S, Mensonides F, Hellingwerf K, Klis F. 2002. Physiological actions of preservative agents: prospective of use of modern microbiological techniques in assessing microbial behaviour in food preservation. Int J Food Microbiol. 79(1–2):55–64.
- Chan G, Guthrie A, Sivaramalingam T, Wilson J, Vancraeynest D, Moody R, Clark S. 2015. A framework for assessing the efficacy of antimicrobials in the control of necrotic enteritis in broiler chickens. J Appl Poult Res. 24(2):246–256.
- Dar PS, Wani SA, Wani AH, Hussain I, Maqbool R, Ganaie MY, Kashoo ZA, Qureshi S. 2017. Isolation, identification and molecular characterization of clostridium perfringens from poultry in Kashmir valley, India. J Entomol Zool Stud. 5(5):409–414.
- Dibner J, Buttin P. 2002. Use of organic acids as a model to study the impact of gut microflora on nutrition and metabolism. J Appl Poult Res. 11(4):453–463.
- Dosoky WM, Al-Banna AA, Zahran SM, Farag SA, Abdelsalam NR, Khafaga AF. 2022. Zinc oxide nanoparticles induce dose-dependent toxicosis in broiler chickens reared in summer season. Environ Sci Pollut Res. doi:10.1007/s11356-022-19156-4
- Dosoky, W.M., Fouda, M.M.G., Alwan, A.B., Abdelsalam NR, Taha AE, Ghareeb RY, El-Aassar MR, Khafaga AF. 2021. Dietary supplementation of silver-silica nanoparticles promotes histological, immunological, ultrastructural, and performance parameters of broiler chickens. Sci Rep. 11:4166. doi:10.1038/s41598-021-83753-5
- Elkomy AA, Farag E, Elgharbawy EI, Elbadawy M. 2019. Comparative studies on the effects of lincomycin and bacitracin on hematobiochemical and immunological parameters in broiler chickens. Int J Pharmacol Toxicol. 7(1):1–5.
- Elwinger KA, Schneitz C, Berndtson E, Fossum O, Teglöf B, Engstöm B. 1992. Factors affecting the incidence of necrotic enteritis, caecal carriage of clostridium perfringens and bird performance in broiler chicks. Acta Vet Scand. 33(4):369–378.
- Forgetta V, Rempel H, Malouin F, Vaillancourt R Jr, Topp E, Dewar K, Diarra MS. 2012. Pathogenic and multidrug-resistant Escherichia fergusonii from broiler chicken. Poult Sci. 91(2):512–525.
- Fouda MMG, Dosoky WM, Radwan NS, Abdelsalam NR, Taha AE, Khafaga AF. 2021. Oral administration of silver nanoparticles-adorned starch as a growth promotor in poultry: immunological and histopathological study. Int J Biol Macromol. 187:830–839. doi:10.1016/j.ijbiomac.2021.07.157.
- Furtula V, Farrell EG, Diarrassouba F, Rempel H, Pritchard J, Diarra MS. 2010. Veterinary pharmaceuticals and antibiotic resistance of Escherichia coli isolates in poultry litter from commercial farms and controlled feeding trials. Poult Sci. 89(1):180–188.
- Ghazalah A, Atta AM, Elkloub K, Moustafa ME, Shata RF. 2011. Effect of dietary supplementation of organic acids on performance, nutrients digestibility and health of broiler chicks. Int J Poult Sci. 10(3):176–184.
- Greenwood D, Norrby SR, Whitley RJ. 2004. Antibiotic and chemotherapy: anti-infective agents and their use in therapy. Philadelphia (PA): Churchill Livingstone.
- Humin T. 2004. Humin animal feed supplements and veterinary medicine and humic acid based products. Vol. 189. Dusseldrof (Germany): Humintech-Humintech GmbH.
- Huyghebaert G, Ducatelle R, Van Immerseel F. 2011. An update on alternatives to antimicrobial growth promoters for broilers. Vet J. 187(2):182–188.
- Islam KMS, Schuhmacher A, Gropp J. 2005. Humic acid substances in animal agriculture. Pak J Nutr. 4(3):126–134.
- Ivarsson E, Wall H. 2017. Effects of toasting, inclusion levels and different enzyme supplementations of faba beans on growth performance of broiler chickens. J Appl Poult Res. 26(4):467–475.
- Jaďuttová I, Marcinčáková D, Bartkovský M, Semjon B, Harčárová M, Nagyová A, Váczi P, Marcinčák S. 2019. The effect of dietary humic substances on the fattening performance, carcass yield, blood biochemistry parameters and bone mineral profile of broiler chickens. Acta Vet Brno. 88(3):307–313.
- Kaldhusdal M, Schneitz C, Hofshagen M, Skjerve E. 2001. Reduced incidence of clostridium perfringens-associated lesions and improved performance in broiler chickens treated with normal intestinal bacteria from adult fowl. Avian Dis. 45:149–156.
- Kaya CA, Tuncer SD. 2009. The effects of humates on fattening performance, carcass quality and some blood parameters of broilers. J Animal Vet Adv. 8(2):281–284.
- Khan A, Nagra S. 2010. Performance of broiler chicks as influenced by feeding diets supplemented with organic acids. Indian J Poult Sci. 45(1):30–34.
- Khan RU, Naz S, Raziq F, Qudratullah Q, Khan NA, Laudadio V, Tufarelli V, Ragni M. 2022. Prospects of organic acids as safe alternative to antibiotics in broilerchickens diet. Environ Sci Pollut Res. doi:10.1007/s11356-022-19241-8
- Kocabağli N, Alp M, Acar N, Kahraman R. 2002. The effects of dietary humate supplementation on broiler growth and carcass yield. Poult Sci. 81(2):227–230.
- Lala A, Okwelum N, Bello KO, Famakinde NA, Alamu MO. 2016. Comparative study between ISA brown and fulani ecotype chickens supplemented with humic acid. Slovak J Anim Sci. 49(2):68–75.
- Li X-K, Wang JZ, Wang CQ, Zhang CH, Li X, Tang CH, Wei XL. 2016. Effect of dietary phosphorus levels on meat quality and lipid metabolism in broiler chickens. Food Chem. 205:289–296.
- Mahgoub SA, Abd El-Hack ME, Saadeldin IM, Hussein MA, Swelum AA, Alagawany M. 2019. Impact of rosmarinus officinalis cold-pressed oil on health, growth performance, intestinal bacterial populations, and immunocompetence of Japanese quail. Poult Sci. 98(5):2139–2149.
- Morar M, Bhullar K, Hughes DW, Junop M, Wright GD. 2009. Structure and mechanism of the lincosamide antibiotic adenylyltransferase LinB. Structure. 17(12):1649–1659.
- Nagaraju R, Reddy BS, Gloridoss R, Suresh BN, Ramesh C. 2014. Effect of dietary supplementation of humic acids on performance of broilers. Indian J Anim Sci. 84(4):447–452.
- Ozturk E, Coskun I, Ocak N, Erener G, Dervisoglu M, Turhan S. 2014. Performance, meat quality, meat mineral contents and caecal microbial population responses to humic substances administered in drinking water in broilers. Br Poult Sci. 55(5):668–674.
- Ozturk E, Ocak N, Coskun I, Turhan S, Erener G. 2010. Effects of humic substances supplementation provided through drinking water on performance, carcass traits and meat quality of broilers. J Anim Physiol Anim Nutr. 94(1):78–85.
- Ozturk E, Ocak N, Turan A, Erener G, Altop A, Cankaya S. 2012. Performance, carcass, gastrointestinal tract and meat quality traits, and selected blood parameters of broilers fed diets supplemented with humic substances. J Sci Food Agric. 92(1):59–65.
- Paiva D, McElroy A. 2014. Necrotic enteritis: applications for the poultry industry. J Appl Poult Res. 23(3):557–566.
- Pistová V, Arpášová H, Hrnčár C, Kačániová M, Haščík P. 2016. The effect of the humic acid and herbal additive supplement on production parameters of broiler chicken. Sci Pap Anim Sci Biotechnol/Lucr Stiint Zooteh Biotehnol. 49(2):166–169.
- Porter RE Jr. 1998. Bacterial enteritides of poultry. Poult Sci. 77(8):1159–1165.
- Quinn P, Carter ME, Markey BK, Carter GR. 1994. Clostridium species. Clin Vet Microbiol. 2:191–208.
- Rath N, Huff W, Huff G. 2006. Effects of humic acid on broiler chickens. Poult Sci. 85(3):410–414.
- Salah H, Mansour E, Abd El Hamid ES. 2015. Study on the effect of humic acid on growth performance, immunological, some blood parameters and control intestinal closterdium in broiler chickens. Zagazig Vet J. 43(1):102–109.
- Saleem K, Rahman A, Pasha TN, Mahmud A, Hayat Z. 2020. Effects of dietary organic acids on performance, cecal microbiota, and gut morphology in broilers. Trop Anim Health Prod. 52(6):3589–3596.
- Saleh AA. 2013. Effects of fish oil on the production performances, polyunsaturated fattyacids and cholesterol levels of yolk in hens. Emir J Food Agric. 25:605–612.
- Saleh AA. 2016. Effect of low-protein in Iso-energetic diets on performance, carcass characteristics, digestibilities and plasma lipids of broiler chickens. Egyptain Poult Sci. 36(I):251–262.
- Saleh AA, Ahmed EAM, Ebeid TA. 2019. The impact of phytoestrogen sources supplementation on reproductive performance, plasma profile, yolk fatty acids and antioxidative status in aged laying hens. Reprod Domest Anim. 54(6):846–854.
- Saleh AA, Amber K, Mohammed AA. 2020. Dietary supplementation with avilamycin and lactobacillus acidophilus effects growth performance and the expression of growth-related genes in broilers. Anim Prod Sci. 60(14):1704–1710.
- Saleh AA, Hayashi K. 2011. Aspergillus niger reduces skeletal muscle protein breakdown and stimulates growth in broilers. Res Opin Anim Vet Sci. 1(4):209–212.
- Saleh AA, Shukry M, Farrag F, Soliman MM, Abdel-Moneim AM. 2021. Effect of feeding wet feed or wet feed fermented by Bacillus licheniformis on growth performance, histopathology and growth and lipid metabolism marker genes in broiler chicken. Animals. 11(1):83–89.
- Shermer C, Maciorowski KG, Bailey CA, Byers FM, Ricke SC. 1998. Caecal metabolites and microbial populations in chickens consuming diets containing a mined humate compound. J Sci Food Agric. 77(4):479–486.
- Son J, Ragland D, Adeola O. 2002. Quantification of digesta flow into the caeca. Br Poult Sci. 43(2):322–324.
- Sun X, McElroy A, Webb KE Jr, Sefton AE, Novak C. 2005. Broiler performance and intestinal alterations when fed drug-free diets. Poult Sci. 84(8):1294–1302.
- Taklimi S, Ghahri H, Isakan MA. 2012. Influence of different levels of humic acid and esterified glucomannan on growth performance and intestinal morphology of broiler chickens. Agric Sci. 3:663–668.
- Tugnoli B, Giovagnoni G, Piva A, Grilli E. 2020. From acidifiers to intestinal health enhancers: how organic acids can improve growth efficiency of pigs. Animals. 10(1):134.
- Wang S, Zeng XF, Wang QW, Zhu JL, Peng Q, Hou CL, Thacker P, Qiao SY. 2015. The antimicrobial peptide sublancin ameliorates necrotic enteritis induced by clostridium perfringens in broilers. J Anim Sci. 93(10):4750–4760.
- Xia M, Hu C, Xu Z. 2004. Effects of copper-bearing montmorillonite on growth performance, digestive enzyme activities, and intestinal microflora and morphology of male broilers. Poult Sci. 83(11):1868–1875.
- Young K, Foegeding PM. 1993. Foegeding, acetic, lactic and citric acids and pH inhibition of listeria monocytogenes scott A and the effect on intracellular pH. J Appl Bacteriol. 74(5):515–520.
