 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This study explored the palatability of dog feed in response to different levels of Astragalus polysaccharide (APS), and the impact of APS on blood biochemistry and immunity in growing Beagles. A total of three APS treatments were manufactured: a control diet (CD, 0 mg/kg APS), a low APS concentration diet (LD, 400 mg/kg) and a high APS concentration diet (HD, 800 mg/kg). Based on our findings, APS did not have an influence on the first-choice ratio and consume ratio. In feeding trial, weight gain and feed efficiency were decreased in dogs fed the HD. APS increased the white blood cell count and serum total protein in Beagles. However, red blood cell count and haematocrit values followed an opposite trend. Serum levels of antibodies against canine distemper virus and rabies virus were improved by APS addition. Furthermore, C-reactive protein level was decreased in dogs fed the HD. As it can be concluded, this study indicated that APS could be safely and beneficially used in dog foods without compromising palatability. Further research is needed to investigate the feasibility of APS in coping with obesity and study the detailed mechanisms by which dietary APS improves immunity of Beagles.
Introduction
In pet nutrition field, feed additives with health-beneficial effects have attracted considerable interest (Pawar et al. Citation2017; Bruni et al. Citation2020). Herbal polysaccharides from natural resources have great potential as functional additives because of their significant bio-activities (Yu et al. Citation2018). Astragalus polysaccharide (APS), a major active ingredient of the roots of Astragalus membranaceus, possesses immunomodulatory, antioxidant, anti-inflammatory, anti-tumor and antiviral activities (Zheng et al. Citation2020). The application of APS in livestock animals has verified the benefits of dietary APS in promotion of growth, feed utilization, immunity, gut health and antioxidant status (Zahran et al. Citation2014; Wu Citation2018; Song et al. Citation2019; Yang et al. Citation2019). However, research on the function of APS in pet foods is limited, which warrants study. When introducing a new ingredient in pet foods, palatability is a crucial attribute for success. Ingredients with antioxidant properties could increase feed palatability by preventing oxidation and providing an odour that animals prefer (Chen et al. Citation2016). In addition, herb additives might be able to directly improve the odour of feed, resulting in an increased feed intake (Lei et al. Citation2018). The feed intake improving effect of dietary APS has been reported in Nile tilapia (Oreochromis niloticus) (Zahran et al. Citation2014) and lambs (Zhong et al. Citation2012). However, dogs have superior sense of smell. Whether diets containing APS would be well accepted by dogs is still unknown, a fact that awaits further investigation.
Generally, pet owners are willing to provide foods that can improve health of their animals without any safety loopholes. The safety of dietary APS has been demonstrated in livestock animals (Zahran et al. Citation2014; Wu Citation2018; Song et al. Citation2019; Yang et al. Citation2019). Furthermore, the intravenous injection of Astragalus membranaceus extract had no negative influence on Beagles (Yu et al. Citation2007). However, the safety of dietary addition of APS, which might be different from that of intravenous injection, has not been verified in dogs. To assess the physiological changes of animals in response to new dietary ingredients, the haematological and serum biochemical parameters are commonly determined. According to Zhong et al. (Citation2012), dietary APS had no negative impact on blood biochemistry of lambs. Besides, the aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activities were not affected by dietary APS addition in weaned piglets (Yang et al. Citation2019). At the same time, dietary APS did not have an effect on plasma haematology of chickens that were exposed to immunological stress (Liu et al. Citation2015). However, an active ingredient may induce various reactions in physiological status in different animal species (Onasanya et al. Citation2015). Hence, it is necessary to investigate the impact of dietary addition of APS on blood biochemistry of dogs.
Among the various claims promoted for functional feed additives, immune enhancing effect stands out as being of great importance. In laboratory animals, APS has been demonstrated possessing strong immune-potentiating effects, including stimulation of T- and B-cell proliferation, promoting the production of antibodies, macrophages activation, cytokines expression regulation and induction of surface antigen expressions on lymphocytes (Zheng et al. Citation2020). Besides, the immune-modulating activity of APS has also been demonstrated in livestock animals (Zhong et al. Citation2012; Liu et al. Citation2015; Wu Citation2018). Furthermore, studies showed that the intravenous injection of APS improved the immune response in dogs, such as increasing the antibody titres against rabies (Qiu et al. Citation2010; Liu et al. Citation2012). However, no information is available concerning immune responses of dogs to APS that is provided through dietary route.
This study was carried out to assess palatability of dog feed in response to variations in APS addition, and it also examined the impacts of dietary APS on blood biochemistry and immune responses in growing Beagles. The present study aimed to evaluate the feasibility, safety and benefit of APS as a functional additive in canine foods.
Material and methods
Protocols used here were all in accordance with the guidelines issued by the Animal Care Advisory Committee of the Institute of Laboratory Animals of Sichuan Academy of Medical Sciences (AE2020009).
Animals and facilities
Twenty adult dogs were used in the palatability trial, being six Beagles (13.1 ± 1.2 kg), two Labradors (27.2 ± 2.8 kg), four Pekingese (4.3 ± 0.8 kg); four Siberian Huskies (21.8 ± 2.3 kg) and four Pomeranian (2.3 ± 0.4 kg). Eighteen Beagle puppies (10 wk old; male) were used in the feeding trial. In a temperature-controlled room (21°C ± 2.5), all dogs were individually housed in kennels (1.4 × 1.4 m) (Dayan et al. Citation1998). Fresh water was available ad libitum.
Experimental diets
APS (with a purity of 80%) used in this study was a commercial product purchased from Shaanxi Ciyuan Biotechnology Co., Ltd (China), which was mainly composed of glucose, mannose, arabinose, xylose, galactose, rhamnose and ribose. The composition and the analysis of the basal diet are shown in . The nutrient requirements for the basal diet were based on the Association of American Feed Control Officials (AAFCO Citation2004). The low APS concentration diet (LD) and high APS concentration diet (HD) were prepared by adding APS into the basal diet to obtain final APS dose at 400 and 800 mg/kg, respectively. The control diet (CD) was the basal diet. Pellets were produced and stored in vacuum bags until they were used according to Hong et al. (Citation2020).
Table 1. Composition and nutritive value of the basal diet.
Palatability trial
Palatability was measured using the most common palatability assessment method: two-bowl test (Griffin Citation2003). Diets were paired-compared using two comparisons: CD vs LD and CD vs HD. Dogs were offered diets in separate bowls for 1 h per day (09:00) for five consecutive days (Chen et al. Citation2016). To ensure there would be leftovers, the amount of feed offered was 30% higher than the recommendation for the maintenance of adult dogs according to the guidelines of the NRC (Citation2006) and de Brito et al. (Citation2010). After 1 h, first-choice data were recorded, feed residues were weighed to calculate the consume ratio:
Feeding trial
Before the experimental procedures, Beagles were adapted to the experimental conditions for two weeks according to Hong et al. (Citation2020). During the acclimation period, all Beagles were vaccinated with a combined vaccine, which included canine parvovirus (CPV), canine parainfluenza virus (CPIV) canine adenovirus (CAV) and canine distemper virus (CDV) antigens as part of standard veterinary care. After the acclimation, six Beagles (3.80 ± 0.43 kg) were randomly allocated into one of the three treatment groups (CD, LD, HD). Beagles were fed twice per day for five weeks. Feed rations were adjusted weekly according to Dobenecker et al. (Citation2013). A rabies virus (RV) vaccine and another combined booster vaccine against CPIV, CAV, CPV and CDV were applied during the feeding trial (on day 16).
All Beagles were weighed every week. Total weight gain (WG) was calculated by subtraction the initial body weight from the observed weight at the end of the feeding trial. The feed efficiency (FE) was calculated using the following formula:
Sample collection and analysis
At the end of week two and five (on day 15 and 36), blood samples were obtained. After 12 h of fasting, blood were collected into two different types of tubes (containing EDTA-K2 or with no additive) from each dog via the jugular vein. Immediately after collection, tubes without additive were centrifuged to obtain serum.
Blood samples in tubes containing EDTA-K2 were evaluated using a haematology analyzer (KX-21, Sysmex Co. Ltd., Japan). The analysed parameters were: white blood cell (WBC) count, red blood cell (RBC) count, hemoglobin (HGB), haematocrit (HCT), platelets (PLT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH) and mean corpuscular hemoglobin concentration (MCHC). The following serum biochemical parameters were analysed by a biochemistry analyzer (BS-420, Shenzhen Mindray Bio-Medical Electronics Co. Ltd. China): AST, ALT, alkaline phosphatase (ALP), creatine kinase (CK), glucose (GLU), total protein (TP), albumin (ALB), globulin (GLOB), albumin - globulin (A/G) ratio, creatinine (CREA) and total cholesterol (TC).
Serum C-reactive protein (CRP) levels were detected using CRP test kits (Nanjing Jiancheng Bioengineer Institute, Nanjing, China). ELISA-test kits (Shenzhen Kejie Industrial Co., Ltd. China) were used to assess the serum antibody levels to the CPIV, CAV, CPV, CDV and RV vaccines.
Statistical analysis
The statistical significance of the palatability trial result was analysed by a two-tailed t-test. The results from the feeding trial were statistically evaluated by one-way analysis of variance (ANOVA) using SPSS (version 18.0: SPSS, Chicago).
Results
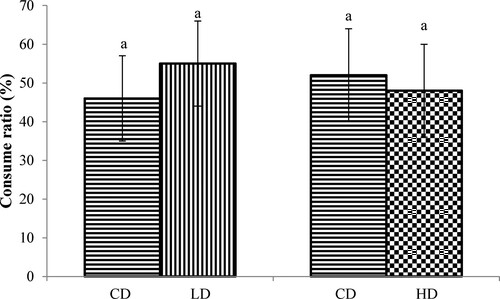
The palatability trial results are shown in and . In terms of first-choice ratio and consume ratio, significant differences occurred neither between the CD and the LD, nor between the CD and the HD (P > 0.05).
Figure 1. The first-choice ratios of the feed containing different levels of APS and control feed. CD: control diet; LD: low APS concentration diet; HD: high APS concentration diet; bars refer to mean values; different letters above bars indicate a significant difference (P < 0.05); error bars refer to standard deviation.
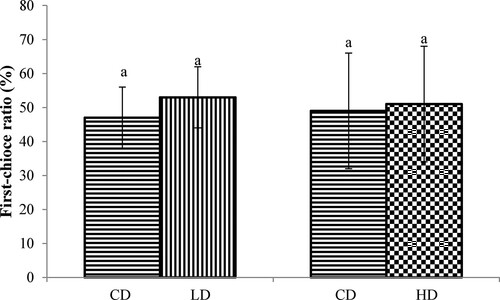
Figure 2. The consume ratios of the feed containing different levels of APS and control feed. CD: control diet; LD: low APS concentration diet; HD: high APS concentration diet; bars refer to mean values; different letters above bars indicate a significant difference (P < 0.05); error bars refer to standard deviation.
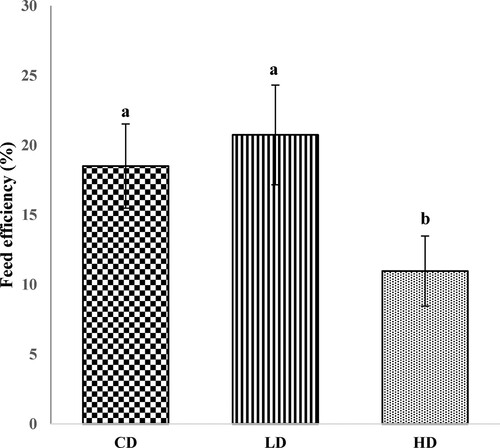
The body weight (BW) changes of Beagles in the feeding trial are shown in . In the first three weeks, there was no significant difference in BW among the groups (P > 0.05). However, at the end of fifth week, BW of Beagles fed the HD was significant lower than that of the LD and CD groups (P < 0.05). As described in , the FE was significantly lower in the HD group than in the other two groups (P < 0.05).
Figure 3. The body weight changes of Beagles fed the control and experimental diets. CD: control diet; LD: low APS concentration diet; HD: high APS concentration diet; bars refer to mean values; different letters above bars indicate a significant difference (P < 0.05); error bars refer to standard deviation.
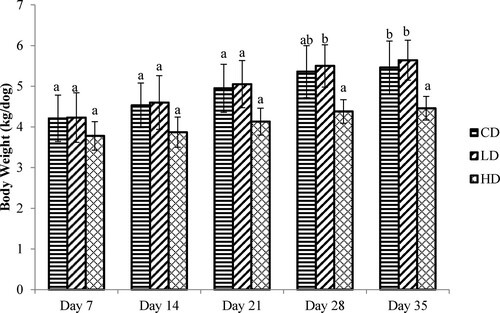
Figure 4. The feed efficiency of Beagles fed the control and experimental diets. CD: control diet; LD: low APS concentration diet; HD: high APS concentration diet; bars refer to mean values; different letters above bars indicate a significant difference (P < 0.05); error bars refer to standard deviation.
As presented in , after two weeks of dietary APS feeding, no significant differences were observed in haematology parameters among the three groups (P > 0.05). However, some significant differences in haematology parameters were observed after five weeks of dietary APS administration. Dogs fed the HD had a higher level of WBC (P < 0.05). At the same time, dietary APS addition significantly increased MCH and MCHC values (P < 0.05). However, RBC and HCT values exhibited an opposite trend (P < 0.05). As shown in , the majority of the serum biochemistry parameters were not significantly influenced by APS addition (P > 0.05), with the exception of serum TP concentrations. Dietary inclusion of APS at the level of 800 mg/kg significantly increased the TP concentration (P < 0.05).
Table 2. Selected blood haematology parameters of Beagles in each group.Table Footnote1
Table 3. Selected serum biochemistry parameters of Beagles in each group.Table Footnote1
Serum antibody responses and CRP levels are shown in . The levels of antibodies against CPIV and CAV were not significantly affected by dietary APS (P > 0.05). However, following APS administration for five weeks, the serum level of CPV antibody was increased in the HD fed Beagles (P < 0.05), while the CDV antibody level was improved in both LD and HD group (P < 0.05). Moreover, Beagles fed diets containing APS had a higher level of RV antibody (P < 0.05). Beagles in the HD group had a lower serum CRP level (P < 0.05).
Table 4. Levels of C-reactive protein (CRP), antibodies against parainfluenza virus (CPIV), canine adenovirus (CAV), canine parvovirus (CPV) and canine distemper (CDV) of Beagles in each group.Table Footnote1
Discussion
Palatability is an important criterion for introducing a new functional ingredient in pet foods. This study for the first time indicates the palatability of APS-containing feed in canine species. The two-bowl test is commonly used for pet food palatability assessments (Griffin Citation2003). The first-choice ratio, a key parameter in two-bowl test, shows the first feed tasted, reflecting the olfactory attractiveness (Tobie et al. Citation2015). On the other hand, the consume ratio compares how much of the two feeds provided simultaneously is consumed in a certain period of time (Tobie et al. Citation2015). In this study, both the first-choice ratio and consume ratio showed no significant differences between the groups. Thus, we can conclude that APS can be used as a functional additive in dog foods without any palatability-related drawbacks.
Safety assessment of dietary APS is another objective of this study. Functional ingredients should offer benefits to pets without any side effects. In our study, Beagles were all in a clinically healthy situation throughout the experimental period. The BW of Beagles fed LD, which was close to that observed in our previous study (Hong et al. Citation2020), showed no significance difference from that of dogs in the CD group. However, at the end of fifth week, the lower BW was observed in Beagles fed high levels of APS. This growth inhibitory effect of high dose APS has also been reported in large yellow croaker (Larimichthys crocea) (Liu et al. Citation2020), rats (Wang et al. Citation2009) and mice (Huang et al. Citation2017). Based on the correlation analysis, FE was positively correlated with BW in this study (r = +0.636, P = 0.066), implying that the poor growth may be partly due to the decreased FE caused by 800 mg/kg APS. Though the significant BW difference, the haematology and serum biochemical analysis revealed that Beagles in the HD group were as healthy as that of the other two groups. The blood biochemical parameters are useful in clinical chemistry, for which reference ranges are provided for assessment of animal physiological status. In the present study, ALP, CK, TP, ALB, CREA and TC were out of reference ranges according to Rørtveit et al. (Citation2015). This might be due to the age-related changes in blood biochemistry. Studies have demonstrated that the growth stage has a significant influence on blood biochemical parameters in animals (Jezek et al. Citation2006; Kiran et al. Citation2012). Meanwhile, the levels of these parameters were close to those of Beagles in other studies (Assarasakorn et al. Citation2006; Hong et al. Citation2020). These findings implied that the physiological status of the Beagles was not impaired by dietary APS with the dose up to 800 mg/kg. When comparing the haematology parameters among the groups, there were some changes caused after five weeks of dietary APS administration. WBC, which plays an important role in immune responses, was increased in the HD fed Beagles. This might be partly due to an improved proliferation of immune cells. Studies have demonstrated that APS could enhance the lymphocytes proliferation (Fan et al. Citation2012). Besides, for centuries, the root of Astragalus membranaceus has been used to promote haematopoiesis in China (Zhu and Zhu Citation2007). However, to our surprise, after five weeks of dietary APS administration, Beagles had lower RBC compared with the CD group. Furthermore, HCT, a parameter reflecting the fractional volume of a blood sample occupied by packed RBC (Kim et al. Citation2012), was also decreased. This unexpected WBC increasing and RBC decreasing effect of dietary APS has also been observed in lambs (Zhong et al. Citation2012). However, these results are inconsistent with the findings carried out on immunosuppressive dogs, which showed that with the increasing dose of injected APS, the density of RBC increased (Qiu et al. Citation2010). The reason for this phenomenon is still unknown, which is worthy of further investigation. MCH and MCHC are two useful parameters in the diagnosis of anaemia, which reflect content and concentration of HGB in RBC (Buttarello Citation2016). In this study, both MCH and MCHC values were increased after APS administration for five weeks, indicating an improved oxygen-carrying capacity of RBC. The elevated MCH and MCHC might be correlated with a change in blood iron level. There is a positive connection between MCH, MCHC and iron status in blood (Rusia et al. Citation1996). APS was reported to be able to regulate the blood iron level in mice (Ren et al. Citation2016). However, additional investigations are warranted to determine this hypothesis in dogs. Most of serum biochemical parameters were not significantly influenced by dietary APS, except for serum TP. Dogs fed the HD had a higher TP content. TP, an indicator of health and stress, usually varies in different physiological and pathological conditions (Li et al. Citation2018; Zhu W et al. Citation2019). The elected serum TP content in 800 mg/kg APS group might be due to an increase in content of blood proteins like antioxidant enzymes and bactericidal peptides (Zhu et al. Citation2019). The enhanced serum levels of these proteins as an effect of APS addition have been already demonstrated by previous studies (Zahran et al. Citation2014; Hamid et al. Citation2017; Zhao et al. Citation2018).
Because of the great importance of immunity in animal health, functional ingredients with immune-promoting properties have been attracting much attention in pet food industry. APS is a well-known immunostimulant (Zheng et al. Citation2020). Studies have demonstrated that APS exerted better preventive than therapeutic effects against pathogens (Ge et al. Citation2015), which makes APS be a great candidate in functional foods with health protective effects. For the first time, this study demonstrates that dietary administration of APS for five weeks enhanced immune responses in growing Beagles. Vaccine responses were used as markers for specific immune function in this study. We assessed the levels of antibodies against five specific vaccine antigens in the blood sample collected. CPV and CDV are two common and preventable infectious canine diseases, which are major causes of mortality in dogs (Litster et al. Citation2012). In this study, concentrations of antibodies against CPV and CDV were improved by dietary APS supplementation. RV, the agent of rabies, can cause a severe encephalomyelitis in carnivore populations (Wunner and Briggs Citation2010). The level of antibody against RV was also improved in APS treatment groups. The increased antibody levels here might be due to an enhanced activated status of B cells and macrophages. It is well known that antibodies are produced by activated B cells. The antibody production is also modulated by activated macrophages products (Dimitriu and Fauci Citation1978). In mice, APS was found to be able to activate B cells and macrophages (Shao et al. Citation2004). However, the levels of antibodies against CPIV and CAV here were not influenced by dietary APS, which suggested that dietary APS could target a specific antigen by improving corresponding antibody production. However, the mechanism of this phenomenon should be investigated in the future. In addition to the antibody responses, CRP, an acute-phase reactant synthesized by the liver in systemic response to inflammation (Kuribayashi et al. Citation2003), was also assessed. In this study, serum CRP levels in Beagles were all normal (0.8-18.9 µg/mL) (Kuribayashi et al. Citation2003; Satyaraj et al. Citation2013). Furthermore, dogs fed the HD for five weeks had a lower CRP level, indicating a better health status.
In conclusion, dietary inclusion of APS up to 800 mg/kg did not negatively affect palatability of dog feed. The growing Beagles could tolerate 800 mg/kg of dietary APS without adverse effects on blood biochemistry. Furthermore, dietary addition of APS influenced immune responses of Beagles by enhancing response to specific vaccines and alleviating inflammation. Considering the significant growth inhibitory effect of 800 mg/kg APS, 400 mg/kg APS addition dose might be appropriate for growing Beagles. The present work is the first study that demonstrated APS could be used as a beneficial functional additive in dog foods. This may be of great interest to those involved in pet nutrition research and the pet food industry. However, further research is needed to clarify the mechanism of the immunity improvements by dietary APS in Beagles. Besides, this study indicated that APS might be potentially used to cope with obesity in dogs, which would be worthy of investigation in the future.
Availability of data and materials
The data presented in this study are available on request from the corresponding author.
Author contributions
Guo-Qiang Cheng carried out the palatability studies, Han Dong, Jing Zhou and Ming-Ming Yuan participated in the feeding trial. Han Dong drafted the manuscript. Lei Zhang and Jie-Ying Xia participated in the design of the study and performed the statistical analysis. Yang Hong conceived of the study, and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- [AAFCO] Association of American Feed Control Officials. 2004. Dog and cat nutrient profiles: Official publication of the Association of American Feed Control Officials Incorporated. Oxford.
- Assarasakorn S, Niwetpathomwat A, Techangamsuwan S, Suvarnavibhaja S. 2006. A retrospective study of clinical hematology and biochemistry of canine hepatozoonosis on hospital populations in Bangkok, Thailand. Comp Clin Pathol. 15(2):107–109. doi: 10.1007/s00580-006-0606-6
- Bruni N, Martello E, Fusi E, Meineri G, Giardini A. 2020. Study of faecal parameters and body condition in dogs with a diet supplemented with Lactobacillus acidophilus D2/CSL (CECT 4529). Ital J Anim Sci. 19(1):704–711. doi: 10.1080/1828051X.2020.1783378
- Buttarello M. 2016. Laboratory diagnosis of anemia: are the old and new red cell parameters useful in classification and treatment, how? Int J Labor Hematol. 38:123–132. doi: 10.1111/ijlh.12500
- Chen M, Chen X, Cheng W, Li Y, Ma J, Zhong F. 2016. Quantitative optimization and assessments of supplemented tea polyphenols in dry dog food considering palatability, levels of serum oxidative stress biomarkers and fecal pathogenic bacteria. RSC Adv. 6(20):16802–16807. doi: 10.1039/C5RA22790A
- Dayan D, Kozlovsky A, Tal H, Kariv N, Shemesh M, Nyska A. 1998. Castration prevents calcium channel blocker-induced gingival hyperplasia in beagle dogs. Hum Exp Toxicol. 17(7):396–402. doi: 10.1177/096032719801700706
- de Brito CBM, Félix AP, de Jesus RM, de França MI, de Oliveira SG, Krabbe EL, Maiorka A. 2010. Digestibility and palatability of dog foods containing different moisture levels, and the inclusion of a mould inhibitor. Anim Feed Sci Technol. 159(3-4):150–155. doi: 10.1016/j.anifeedsci.2010.06.001
- Dimitriu A, Fauci AS. 1978. Activation of human B lymphocytes: IX. modulation of antibody production by products of activated macrophages. J Immunol. 120(6):1818–1823.
- Dobenecker B, Endres V, Kienzle E. 2013. Energy requirements of puppies of two different breeds for ideal growth from weaning to 28 weeks of age. J Anim Physiol Anim Nutr. 97(1):190–196. doi: 10.1111/j.1439-0396.2011.01257.x
- Fan Y, Hu Y, Wang D, Liu J, Zhang J, Zhao X, Liu X, Liu C, Yuan J, Ruan S. 2012. Effects of Astragalus polysaccharide liposome on lymphocyte proliferation in vitro and adjuvanticity in vivo. Carbohydr Polym. 88(1):68–74. doi: 10.1016/j.carbpol.2011.11.067
- Ge M, Zhang W, Shi G, Xiao C, Zhao X, Zhang R. 2015. Astragalus polysaccharide perseveres cytomembrane capacity against Newcastle disease virus infection. Pak Vet J. 35(3):382–394.
- Griffin R., Kvamme J, Phillips T. 2003. Palatability testing methods: parameters and analyses that influence test conditions. In: Petfood technology. 1st ed. Mt. Morris: Watt Publishing Co; p. 187–193.
- Hamid M, Liu D, Abdulrahim Y, Liu Y, Qian G, Khan A, Gan F, Huang K. 2017. Amelioration of CCl4-induced liver injury in rats by selenizing Astragalus polysaccharides: role of proinflammatory cytokines, oxidative stress and hepatic stellate cells. Res Vet Sci. 114:202–211. doi: 10.1016/j.rvsc.2017.05.002
- Hong Y, Zhou J, Yuan MM, Dong H, Cheng GQ, Wang YJ, Xia J-Y, Zhang L. 2020. Dietary supplementation with housefly (Musca domestica) maggot meal in growing beagles: hematology, serum biochemistry, immune responses and oxidative damage. Ann Anim Sci. 20(4):1351–1364. doi: 10.2478/aoas-2020-0045
- Huang YC, Tsay HJ, Lu MK, Lin CH, Yeh CW, Liu HK, Shiao YJ. 2017. Astragalus membranaceus-polysaccharides ameliorates obesity, hepatic steatosis, neuroinflammation and cognition impairment without affecting amyloid deposition in metabolically stressed APPswe/PS1dE9 mice. Int J Mol Sci. 18(12):2746–2758. doi: 10.3390/ijms18122746
- Jezek J, Klopcic M, Klinkon M. 2006. Influence of age on biochemical parameters in calves. Bull Vet Inst Pulawy. 50(2):113–119.
- Kim M, Kim A, Kim S, Yang S. 2012. Improvement of electrical blood hematocrit measurements under various plasma conditions using a novel hematocrit estimation parameter. Biosens Bioelectron. 35(1):416–420. doi: 10.1016/j.bios.2012.02.010
- Kiran S, Bhutta AM, Khan BA, Durrani S, Ali M, Iqbal F. 2012. Effect of age and gender on some blood biochemical parameters of apparently healthy small ruminants from Southern Punjab in Pakistan. Asian Pac J Trop Biomed. 2(4):304–306. doi: 10.1016/S2221-1691(12)60028-8
- Kuribayashi T, Shimada T, Matsumoto M, Kawato K, Honjyo T, Fukuyama M, Yamamoto Y, Yamamoto S. 2003. Determination of serum C-reactive protein (CRP) in healthy beagle dogs of various ages and pregnant beagle dogs. Exp Anim. 52(5):387–390. doi: 10.1538/expanim.52.387
- Lei XJ, Yun HM, Kim IH. 2018. Effects of dietary supplementation of natural and fermented herbs on growth performance, nutrient digestibility, blood parameters, meat quality and fatty acid composition in growing-finishing pigs. Ital J Anim Sci. 17(4):984–993. doi: 10.1080/1828051X.2018.1429955
- Li W, Pan X, Cheng W, Cheng Y, Yin Y, Chen J, Xu G, Xie L. 2018. Serum biochemistry, histology and transcriptomic profile analysis reflect liver inflammation and damage following dietary histamine supplementation in yellow catfish (Pelteobagrus fulvidraco). Fish Shellfish Immunol. 77:83–90. doi: 10.1016/j.fsi.2018.03.036
- Litster A, Nichols J, Volpe A. 2012. Prevalence of positive antibody test results for canine parvovirus (CPV) and canine distemper virus (CDV) and response to modified live vaccination against CPV and CDV in dogs entering animal shelters. Vet Microbiol. 157(1-2):86–90. doi: 10.1016/j.vetmic.2011.12.030
- Liu L, Shen J, Zhao C, Wang X, Yao J, Gong Y, Yang X. 2015. Dietary Astragalus polysaccharide alleviated immunological stress in broilers exposed to lipopolysaccharide. Int J Biol Macromol. 72:624–632. doi: 10.1016/j.ijbiomac.2014.08.057
- Liu Y, Miao Y, Xu N, Ding T, Cui K, Chen Q, Ai Q. 2020. Effects of dietary Astragalus polysaccharides (APS) on survival, growth performance, activities of digestive enzyme, antioxidant responses and intestinal development of large yellow croaker (Larimichthys crocea) larvae. Aquaculture. 517:734–752. doi: 10.1016/j.aquaculture.2019.734752
- Liu Y, Zhang S, Zhang F, Hu R. 2012. Adjuvant activity of Chinese herbal polysaccharides in inactivated veterinary rabies vaccines. Int J Biol Macromol. 50(3):598–602. doi: 10.1016/j.ijbiomac.2012.01.035
- NRC. 2006. Nutrient requirements of dogs. Washington, DC: Nat Acad Press. p. 329–335.
- Onasanya GO, Oke FO, Sanni TM, Muhammad AI. 2015. Parameters influencing haematological, serum and bio-chemical references in livestock animals under different management systems. Open J Vet Med. 5(8):181–189. doi: 10.4236/ojvm.2015.58025
- Pawar MM, Pattanaik AK, Sinha DK, Goswami TK, Sharma K. 2017. Effect of dietary mannanoligosaccharide supplementation on nutrient digestibility, hindgut fermentation, immune response and antioxidant indices in dogs. J Anim Sci Technol. 59(1):1–7. doi: 10.1186/s40781-017-0136-6
- Qiu H, Cheng G, Xu J, Zhang N, Liu F, Zhu X, Zhao J, Zhang Y. 2010. Effects of Astragalus polysaccharides on associated immune cells and cytokines in immunosuppressive dogs. Procedia Vaccinol. 2(1):26–33. doi: 10.1016/j.provac.2010.03.006
- Ren F, Qian X-H, Qian X-L. 2016. Astragalus polysaccharide upregulates hepcidin and reduces iron overload in mice via activation of p38 mitogen-activated protein kinase. Biochem Biophys Res Commun. 472(1):163–168. doi: 10.1016/j.bbrc.2016.02.088
- Rørtveit R, Sævik BK, Eggertsdóttir AV, Skancke E, Lingaas F, Thoresen SI, Jansen JH. 2015. Age-related changes in hematologic and serum biochemical variables in dogs aged 16–60 days. Vet Clin Pathol. 44(1):47–57. doi: 10.1111/vcp.12220
- Rusia U, Flowers C, Madan N, Agarwal N, Sood S, Sikkai M. 1996. Serum transferrin receptor levels in the evaluation of iron deficiency in the neonate. Pediatr Int. 38(5):455–459. doi: 10.1111/j.1442-200X.1996.tb03526.x
- Satyaraj E, Reynolds A, Pelker R, Labuda J, Zhang P, Sun P. 2013. Supplementation of diets with bovine colostrum influences immune function in dogs. Br J Nutr. 110(12):2216–2221. doi: 10.1017/S000711451300175X
- Shao BM, Xu W, Dai H, Tu P, Li Z, Gao XM. 2004. A study on the immune receptors for polysaccharides from the roots of Astragalus membranaceus, a Chinese medicinal herb. Biochem Biophys Res Commun. 320(4):1103–1111. doi: 10.1016/j.bbrc.2004.06.065
- Song X, Feng Z, Zhang Y, Zhu W. 2019. Regulation of dietary Astragalus polysaccharide (APS) supplementation on the non-specific immune response and intestinal microbiota of sea cucumber Apostichopus japonicus. Fish Shellfish Immunol. 94:517–524. doi: 10.1016/j.fsi.2019.09.049
- Tobie C, Péron F, Larose C. 2015. Assessing food preferences in dogs and cats: a review of the current methods. Animals (Basel). 5(1):126–137. doi: 10.3390/ani5010126
- Wang N, Zhang D, Mao X, Zou F, Jin H, Ouyang J. 2009. Astragalus polysaccharides decreased the expression of PTP1B through relieving ER stress induced activation of ATF6 in a rat model of type 2 diabetes. Mol Cell Endocrinol. 307(1-2):89–98. doi: 10.1016/j.mce.2009.03.001
- Wu S. 2018. Effect of dietary Astragalus membranaceus polysaccharide on the growth performance and immunity of juvenile broilers. Poult Sci. 97(10):3489–3493. doi: 10.3382/ps/pey220
- Wunner WH, Briggs DJ. 2010. Rabies in the 21st century. PLoS Negl Trop Dis. 4(3):e591. doi: 10.1371/journal.pntd.0000591
- Yang CM, Han QJ, Wang KL, Xu YL, Lan JH, Cao GT. 2019. Astragalus and ginseng polysaccharides improve developmental, intestinal morphological, and immune functional characters of weaned piglets. Front Physiol. 10:418–425. doi: 10.3389/fphys.2019.00418
- Yu SY, Ouyang HT, Yang JY, Huang XL, Yang T, Duan JP, Cheng JP, Chen YX, Yang YJ, Qiong P. 2007. Subchronic toxicity studies of Radix Astragali extract in rats and dogs. J Ethnopharmacol. 110(2):352–355. doi: 10.1016/j.jep.2006.09.024
- Yu Y, Shen M, Song Q, Xie J. 2018. Biological activities and pharmaceutical applications of polysaccharide from natural resources: a review. Carbohydr Polym. 183:91–101. doi: 10.1016/j.carbpol.2017.12.009
- Zahran E, Risha E, AbdelHamid F, Mahgoub HA, Ibrahim T. 2014. Effects of dietary Astragalus polysaccharides (APS) on growth performance, immunological parameters, digestive enzymes, and intestinal morphology of Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 38(1):149–157. doi: 10.1016/j.fsi.2014.03.002
- Zhao L, Tan S, Zhang H, Liu P, Tan YZ, Li JC, Jia D, Shen XF. 2018. Astragalus polysaccharides exerts anti-infective activity by inducing human cathelicidin antimicrobial peptide LL-37 in respiratory epithelial cells. Phytother Res. 32(8):1521–1529. doi: 10.1002/ptr.6080
- Zheng Y, Ren W, Zhang L, Zhang Y, Liu D, Liu Y. 2020. A review of the pharmacological action of Astragalus polysaccharide. Front Pharmacol. 11:349–362. doi: 10.3389/fphar.2020.00349
- Zhong RZ, Yu M, Liu HW, Sun HX, Cao Y, Zhou DW. 2012. Effects of dietary Astragalus polysaccharide and Astragalus membranaceus root supplementation on growth performance, rumen fermentation, immune responses, and antioxidant status of lambs. Anim Feed Sci Technol. 174(1-2):60–67. doi: 10.1016/j.anifeedsci.2012.02.013
- Zhu W, Zhang Y, Zhang J, Yuan G, Liu X, Ai T, Su J. 2019. Astragalus polysaccharides, chitosan and poly (I: C) obviously enhance inactivated Edwardsiella ictaluri vaccine potency in yellow catfish Pelteobagrus fulvidraco. Fish Shellfish Immunol. 87:379–385. doi: 10.1016/j.fsi.2019.01.033
- Zhu XL, Zhu BD. 2007. Mechanisms by which Astragalus membranaceus injection regulates hematopoiesis in myelosuppressed mice. Phytother Res. 21(7):663–667. doi: 10.1002/ptr.2144
