 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
We compared the outcomes of various concentrations of Cinnamomum verum bark powder (CNP), a natural product, to a conventional anticoccidial drug in induced Eimeria tenella infection in broilers aged 21 days. On day 21, 250 birds (Ross 308) were randomly assigned to one of 10 treatments, five of which had Eimeria tenella infection and five of which did not. Results 14 days post-infection (dpi) revealed that the treatments had a significant effect on body weight gain (BWG) and production efficiency factor (PEF). Although the 2 g CNP and Salinomycin (Sacox) treatments performed equally well, the 2 g CNP had the highest BWG and PEF under challenging conditions. Although the PEF improved satisfactorily at 14 dpi, the loss of BWG at 7 dpi did not compensate at 14 dpi. Challenged birds had longer and heavier small intestines, atrophiedceca, lower creatinine levels and higher eosinophils % than unchallenged birds. The elevation lesion score and oocyst secretion of challenged birds decreased with increasing cinnamon dosage. In conclusion, 6 g CNP as a natural product could be as effective as Sacox against occidiosis, with 2 g CNP performing best at 14 dpi. However, more research is required to fully understand its anticoccidial mechanisms.
Highlights
Cinnamomum verum bark may benefit broiler health by reducing Eimeria tenella oocysts.
Under coccidial challenge, Cinnamomum verum bark at 2 g/kg diet may improve broiler body weight gain and production efficiency at 34 days of age (14 days post-infection).
The lesion score and oocyst secretion of challenged birds decreased less as cinnamon dosage was increased.
Introduction
In intensive farming, the spread of avian coccidiosis oocysts in faeces is a risk factor for the prevalence of infectious coccidiosis disease (Wondimu et al. Citation2019). In addition, infected birds are easily susceptible to secondary bacterial infection due to coccidial lesions act as a predisposing factor for Necrotic enteritis (Arczewska-Włosek and Świątkiewicz Citation2013; Mustafa et al. Citation2021). Global economic losses associated with Necrotic enteritis in broilers, such as increased condemnations and decreased growth performance, are estimated to be $6 billion (Wade and Keyburn Citation2015). Infection with sporulated Eimeria tenella oocysts destroys the epithelial cells of the cecum, causing caecum lesions, malabsorption, dehydration and bloody diarrhea, as well as high mortality and low growth, and poor feed-to-meat conversion in broiler chickens (Lan et al. Citation2016; Abdisa et al. Citation2019; Yang et al. Citation2019a). The Eimeria tenella stage multiplied endogenously and exogenously, and the developed oocysts were excreted in the faeces (El-Shazly et al. Citation2020). Coccidiosis infection is generally controlled by the use of coccidiostats and vaccines (Lei et al. Citation2022). In commercial broiler industry, coccidiostats and live coccidia vaccines have been used successfully (Li et al. Citation2005; Mustafa et al. Citation2021). However, their use is associated with drug resistance and significant economic losses due to side effects such as vaccines causing localized inflammation by causing trauma to the intestinal epithelium and, as a result, reduced growth performance (Li et al. Citation2005). Besides, drug residues in meat have been noted, posing a further threat to consumer health (Noack et al. Citation2019). In recent years, there has been a strong push to replace coccidiostats in poultry production with effective natural product strategies. The use of natural dietary supplements provides a new domain for eimeriosis control, possibly due to their origin, wide range of doses, side effects and stimulation of the brain's appetite centre (Arczewska-Włosek and Świątkiewicz Citation2013). Natural products such as garlic, ginger (Ali et al. Citation2019), coconut oil (Hafeez et al. Citation2020), grape seed (Chand et al. Citation2021), mananoligosacharide (Chand et al. Citation2016), wild rue (Tanweer et al. Citation2014) alleviate experimentally induced coccidia in broilers. In addition, as a natural effective compound, tea tree is used to control coccidiosis in Japanese quails (Khan et al. Citation2022).
Cinnamomum verum bark, a member of the Lauraceae family, can be used in poultry rations as a phytogenic feed additive (El-Hack et al. Citation2020). Cinnamon is regarded as hypocholesterolemic agents, antioxidants, antimicrobials, antifungals, digestive enzyme stimulants and growth promoter (Chowdhury et al. Citation2018a; El-Hack et al. Citation2020). Cinnamon's potential activities can be attributed primarily to its high volatile component content (primarily cinnamaldehyde, cinnamyl acetate, eugenol, linalool and carvacrol) (Chowdhury et al. Citation2018b; Ahmadi et al. Citation2021; Syafiq et al. Citation2021). Cinnamon contains a variety of nutrients such as sodium, carbohydrates, sugar, fatty acids, amino acids and so on.
The application of cinnamon powder to broiler diets to identify value-added phytogenic alternatives to traditional antibiotics and anticoccidials in broiler production has not been thoroughly investigated. Some research has looked at the growth and health parameters of broilers fed cinnamon alone (Khafaji Citation2018; Chowlu et al. Citation2019; El-Hack et al. Citation2020; Adedeji et al. Citation2021), or in combination with another phytogenic, such as turmeric (Kanani et al. Citation2017), garlic (Valavi et al. Citation2016), ginger (Gaikwad et al. Citation2019) and citric acid (Krauze et al. Citation2021). Cinnamon bark supplementation has rarely been studied in broilers challenged with coccidia. As a result, the study's hypothesis states that the coccidia challenge would cause intestinal damage, increase oocysts output and reduce performance. This study aimed to compare the effect of Cinnamomum verum bark powder supplementing broiler feed within broiler feed to the standard anticoccidial drug on productive efficiency, carcass variables, intestinal morphometric, cecal lesion scores, leukogram and serum biochemical indicators of broilers exposed and not exposed to an experimental Eimeria tenella oocyst challenge.
Materials and methods
The bioactive constituents of CNP (cinnamaldehyde and other 25 volatile compounds) were detected in our previous pre-starter and starter study that presented HPLC and GCMS analysis (Qaid et al. Citation2021). In addition, total phenolics content in the ethanolic extract of cinnamon barks was 258.18 mg Gallic acid equivalent (GAE)/g dry weight for CNP. Furthermore, proximity analysis showed that the macronutrients of this plant are present in different ratios (Qaid et al. Citation2021).
Ethical approval
The experimental protocols were approved by the King Saud University's local animal care and welfare committee and met Saudi Arabia's animal use standards (KSU-SE-20-44).
Birds and experimental diets
The research was carried out at the poultry production experimental rooms of King Saud University, Riyadh, Saudi Arabia.
A total of 250 one-day-old Ross 308 mixed-sex broiler chicks were obtained from hatchery of AL-Khumasia Feed And Animal Products company and divided into five dietary treatment; each treatment had 10 replicates (cages). Each five birds was housed in a cage measuring 50 × 60 × 36 cm (length × width × depth).
At 21 days of age, birds of half cages (five replicates of each treatment group) were infected with live Eimeria tenella oocysts. In a 2 (Coccidial challenge or not) × 5 (dietary treatments: 0, 2, 4, 6 g cinnamon powder; CNP/kg diet and 66 mg Sacox (Salinomycin)/kg diet factorial arrangement design, five pens were randomly assigned to one of 10 treatments: (1) Negative control (NC). (2) Coccidial challenge (positive control, PC). (3) Sacox at 66 mg /kg, without coccidiosis challenge. Sacox as a standard anticoccidial ionophore drug. (4) Sacox at 66 mg /kg, with coccidiosis challenge (positive Sacox). (5) 2 g CNP/kg, without coccidiosis challenge (negative 2 g CNP). (6) 2 g CNP/kg, with coccidiosis challenge (positive 2 g CNP). (7) 4 g CNP/kg, without coccidiosis challenge (negative 4 g CNP). (8) 4 g CNP/kg, with coccidiosis challenge (positive 4 g CNP). (9) 6 g CNP/kg, without coccidiosis challenge (negative 6 g CNP). (10) 6 g CNP/kg, with coccidiosis challenge (positive 6 g CNP).
The feeding schedule was formulated as broiler diet (). The broiler diet met or exceeded the requirements of the National Research Council (NRC Citation1994). Throughout the experiment, feed and water were available ad libitum. On the first day of the chick's life, the environmental heat was set to 33°C. It then gradually drops to around 0.5°C per day until it reaches 22°C at age 21 and stays there for the rest of the growing cycle at the poultry house (Maiorka et al. Citation2006; Zahraa, Citation2008). During the experimental period, the average outdoor temperature was around 26.4°C, and it was slightly to moderately humid. The relative humidity ranged between 65 and 85%. As the photoperiod, a lighting programme (23L:1D) ‘23 h on:1 h off’ was used.
Table 1. Ingredients and calculated nutrients of broilers diet.
Cinnamomum verum dried bark was bought from a supermarket in Riyadh, Saudi Arabia. When it arrived, the bark was crushed and ground in a nutrition laboratory into a fine powder with a blender (particle size: 0.25–0.30 mm). The CNP was mixed into the diets for 8 min with Rice bran oil as a carrier.
Inoculation of Eimeria oocysts
Eimeria tenella oocysts were floated on 2.5% sodium dichromate and washed three times with distilled water. It was generously provided by parasitology laboratory staff at King Saud University's Zoology Department in Saudi Arabia. On day 21 (day 1 of infection), a 1 mL suspension of distilled water containing an oral infective dose of Eimeria tenella 4*104 sporulated oocysts/chicken was injected directly into the pharynx with a long injector pipette (Lee et al. Citation2012; Al-Shaibani et al. Citation2020). The positive control chickens (n = 25) were given distilled water via the same route.
Blood sampling, white blood cells differential count and safety assessment through biochemical analysis
On day 27 of age, blood samples were taken from one chick per replicate at 7th dpi. Blood samples were drawn from the wing vein and placed in clean, non-heparinized vacutainer tubes. For the determination of heterophils and lymphocytes, one drop of fresh blood was prepared, smeared and stained with Giemsa stain. The differential count of white blood cells determines the percentage of each type of white blood cell present in bird blood, and the heterophil: lymphocyte ratio is calculated. The sera were separated for biochemical analysis by centrifugation (3000 rpm at 30°C) for 15 min. The serum was aspirated with a pipette and placed in sterile Eppendorf tubes, which were kept at −20°C until spectrophotometer analysis for total protein, albumin, glucose, total cholesterol, aspirate aminotransferase (AST), Alanine aminotransferase (ALT) and creatinine using Randox reagent kits from London, U.K. Total globulin was determined (Globulin = Total protein – Albumin) (Alqhtani et al. Citation2022).
Oocysts number per gram of excreta and Coccidial lesion
Coccidial lesions in the cecal region of the birds were scored as proposed by Abudabos et al. (Citation2017). Lesion severity was graded as 0, 1, 2, 3, or 4 from mild to severe. In pooled faecal specimens taken on 7 dpi, the number of oocysts per gram of excreta (OPG) was counted in the McMaster chamber. The prepared excreta suspension in 10% w/v salt solution was mixed with 9 mL of salt solution.
Slaughter variables and small intestine evaluation
Five 27-day-old chickens from each treatment (one bird per pen) were randomly selected for slaughter by cervical dislocation. Slaughter weight and carcass weight (CW; after removal of the head, feathers and internal organs) were recorded to calculate carcass yields. Then the carcass of birds was opened, and the lymphoid organ (thymus, bursa and spleen) was carefully removed and separately weighed. The various organs were calculated as a percentage of live body weight.
The duodenum, jejunum, ileum and total small intestine, as well as the ceca, were all measured in length and weight. The relative intestinal weight (g) to total small intestinal weight (g) ratio was computed.
Growth performance evaluation
The body weight (BW; g) was measured on the first, seventh and fourteenth days of the initial experimental coccidial challenge. Daily weight gain (DWG) and daily feed intake (DFI) were calculated by measuring feeders and birds in pens over 7 days. The feed conversion ratio (FCR) and European performance efficiency factor (PEF) were then calculated.
Statistical analysis
The data from the experiment were analysed with ANOVA for a complete randomized block design (RCBD) using the Statistical Analysis System software's general linear model (GLM) procedure (SAS Citation2012), which included five dietary groups (5 levels) and challenge (2 levels), as well as their interactions. The model equation was described as follows:
where Yij denotes the individual observation; μ is the general experimental mean; Ti denotes the effect of ith treatment; Cj denotes the effect of
th challenge; TCij denotes the effect of treatment by challenge interaction; eijk is a random error with a mean of zero and a variance of σ2ε ijk ∼ N (0, σ2). Duncan's multiple range test with a 5% probability was used to compare the means of groups when they were significant.
Results
Blood biochemical and leukogram measurement
presents the biochemical indices analysed in serum blood collected at 27 days of age (7th dpi). When compared to the control group, experimental treatments had a significant decreasing effect on total protein (TP) (P = 0.0003) and globulin (P = 0.0006) levels, resulting in an increase in albumin: globulin ratio (P = 0.01). The effect of diet on glucose concentration was statistically significant (P = 0.035), as administration of cinnamon at a level of 2 g CNP/kg diet resulted in a significant increase in serum glucose concentration, particularly under challenging conditions. The glucose concentration decreased significantly when the cinnamon dosage was increased, resulting in hypoglycemia at level 6 g CNP. Although there was a significant increasing effect of treatment receiving 2 g CNP/kg diet on ALT level compared to Sacox and control groups, it was within the normal range. When compared to unchallenged birds, challenged birds had lower creatinine levels. Furthermore, for TP, globulin and cholesterol, the treatment by challenge interaction was statistically significant. Diet by challenge interaction was observed as TP and globulin concentrations increased in challenged birds in the control group. The cholesterol level in coccidial challenged birds was higher in the Sacox treated group and lower in the supplemental 6 g CNP group, indicating that diet affects cholesterol under challenge conditions.
Table 2. Serum biochemical parameters of Eimeria tenella-infected and uninfected broiler chickens fed diets containing varying levels of Cinnamomum verum bark powder at 27 days of age (at 7 days post inoculation).
A leukogram of chicken blood collected at 7 dpi revealed no significant differences in treatment, challenge, or their interaction, as shown in . However, Eosinophil levels were elevated in challenged birds and significantly affected between treatments, resulting in treatment by challenge interaction.
Table 3. Leukogram of blood samples collected at 27 days of age from Eimeria tenella-infected and uninfected broiler chickens fed experimental diets.
Coccidial lesion and oocysts number per gram of excreta
shows that natural feed additives, challenge and their interaction had a significant effect on OPG output and lesion scores in the ceca of birds at 7 dpi. Uninfected birds (control, unchallenged) were generally free of lesions, so no Eimeria tenella oocysts were observed in faeces. The coccidial challenge had an effect (P < 0.001) on lesion score at ceca at 7 dpi. Lesions in the ceca section of infected birds were greater in the challenged-untreated group than in the challenged-treated group. The results showed that natural feed additives performed better than controls to a degree that was close to or equal to Sacox.
Table 4. Cecal lesion score (0-4) and faecal oocyst output (Log10 per g excreta) (at 7 days post infection) from Eimeria tenella-infected and uninfected broiler chickens fed experimental diets.
Slaughter variables
As shown in , the treatments, the challenge and their interactions had no significant (P > 0.05) effect on broiler chicken carcass yield and internal components at 27 days of age (7 days post-inoculation). However, treatments significantly affected the proventriculus, thymus, breast and abdominal fat (P < 0.05). Unchallenged birds had the highest heart weight%, bursa weight%, thymus weight% and pancreas weight% compared to challenge birds. The highest weight percentage of gizzard was found in Sacox-treated birds with no coccidial challenge, while the lowest weight percentage was found in 6 g CNP-treated birds with no coccidial challenge, indicating a significant treatment challenge interaction.
Table 5. Relative carcasses weights of Eimeria tenella-infected and uninfected broiler chickens fed diets containing varying levels of Cinnamomum verum bark powder at 27 days of age (at 7 days post inoculation).
Small intestine evaluation
shows the morphometric indicators of the intestinal measurements at 27 days (7 days after inoculation). There were no significant differences in intestinal percentage length, intestinal percentage weight, or relative intestine weight (IRW) due to treatment or the interaction of treatment and challenge (P > 0.05). The challenge, on the other hand, had an effect on the total length and weight of the small intestine and the percentage of duodenum, jejunum and cecal length (P < 0.01). When compared to unchallenged birds, challenged birds had longer and heavier total small intestines, a higher percentage of jejunum length, and a shorter duodenum length. The percentage of cecal weight was also affected by the challenge (P < 0.05), with unchallenged birds having a higher percentage of cecal weight than challenged birds. When compared to unchallenged birds, challenged birds had higher IRW. When compared to unchallenged birds, challenged birds had higher IRW. Birds given 4 g CNP had a higher cecal length percentage than those given control or Sacox (P < 0.05) but were comparable to those given 2 and 6 g CNP. The unchallenged birds in the 6 g CNP group had the shortest small intestine, while the challenged birds in the PC group had the longest.
Table 6. Total and partial lengths and weights of the small intestine and intestinal relative weight of Eimeria tenella-infected and uninfected broiler chickens fed diets containing varying levels of Cinnamomum verum bark powder at 27 days of age (at 7 days post inoculation) (% of small intestine).
Performance variables
Performance indicators at the 7th and 14th days post-infection (ages 21–27 and 28–34 days, respectively) are presented in and and . The results for the seventh period revealed that the main effect of treatment and challenge, as well as the two-way interaction of treatment and challenge, were significant for the performance parameter (P < 0.05). Differences in DWG and PEF, however, were not significant as a result of diet (P > 0.05), and differences in DFI were not significant as a result of the two-way interaction of treatment and challenge (P > 0.05). For the 14th dpi, the main effect of treatment and challenge, as well as the two-way interaction of treatment and challenge, were not significant (P > 0.05) for FCR but significant (P < 0.05) for PEF. In addition, for DFI and DWG, the two-way interaction of treatment and challenge during the second week of the challenge was significant (P < 0.05). The birds that received the coccidial challenge and 6 g CNP/kg gained less body weight at both time points, resulting in a significant treatment and challenge interaction at 14th dpi. At 7 dpi, all performance parameters suffered as a result of the challenge. Compared to challenged birds, unchallenged birds consumed more feed, gained more weight, converted feed more efficiently, and had higher PEF. However, after a week of infection, the negative effects of the challenge on BWG, DFI and FCR faded to no effect, but this improved in challenged birds, which obviously had higher FEF. The challenged chicks who received a 2 g CNP/kg diet had the lowest performance at the 7th day post-infection, comparable to the PC group, but gained more weight and had higher PEF, comparable to or exceeding the other challenged and unchallenged groups.
Figure 1. The effect of dietary treatments and coccidial challenge on body weight gain of broiler chickens at 7th and 14th days post-infection. a-dDifferent superscript letters indicate statistical significant differences (P < 0.05) between groups.
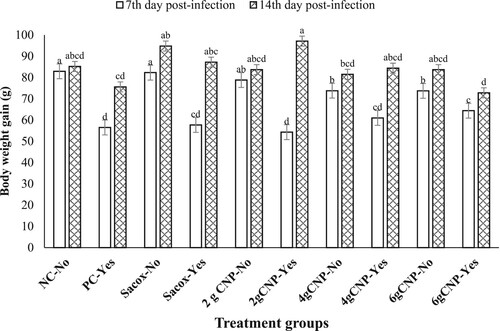
Figure 2. The effect of dietary treatments and coccidial challenge on production efficiency factor of broiler chickens at 7th and 14th days post-infection. a-eDifferent superscript letters indicate statistical significant differences between groups (P < 0.05).
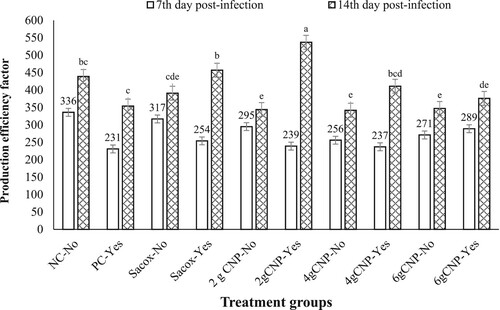
Table 7. Daily weight gain (DWG), daily feed intake (DFI), feed conversion ratio (FCR) and production efficiency factor (PEF) of Eimeria tenella-infected and uninfected broiler chickens fed diets containing varying levels of Cinnamomum verum bark powder at 27 and 34 days of age (at 7 and 14 days post inoculation ‘dpi’).
Discussion
The search for alternatives to synthetic anticoccidial drugs such as the ionophore Sacox for coccidiosis control has recently become an exciting and critical area of research (Lan et al. Citation2016; Alhotan and Abudabos Citation2019; Al-Quraishy et al. Citation2020; Al-Shaibani et al. Citation2020; Balta et al. Citation2021 ) . After looking at a variety of herbs, we focused on Cinnamomum verum bark. Cinnamon spice is high in vital compounds with numerous biological activities, including cinnamaldehyde, cinnamate, cinnamic acid, alkaloids, flavonoids, and some steroids, fatty acids, peptides, and essential oils (El-Hack et al. Citation2020; Ali et al. Citation2021; Rakasivi and Chin Citation2022). There had previously been little research on the effects of CNP on selected parameters of birds facing a coccidial challenge. In addition, previous studies, as far as we know, used a wide range of CNP concentrations ranging from 1 g (Hussein et al. Citation2016) to 70 g (Safa Eltazi Citation2014) to look at it from this angle in this study.
Many studies have found that coccidial challenge has a negative impact on broiler performance (Alhotan and Abudabos Citation2019; Al-Shaibani et al. Citation2020; Balta et al. Citation2021). In terms of body weight gain and feed efficiency factor, the positive 2 g CNP group had a similar adverse effect as the positive control. This loss in 2 g CNP group was compensated to a degree equal to negative control at the 14th dpi. The negative effect of low cinnamon levels on bird performance at 7th dpi could be attributed to the infectiveness of low doses as anticoccidial on oocyst reduction and reduced coccidiosis symptoms. While within age, the lowest cinnamon level (2 g/kg diet) in infected birds at the 14th dpi reduced body weight gain losses induced by coccidial challenge was recovered to a degree similar to that of birds treated with anticoccidial drugs and NC, and improved production efficiency to a degree more significant than that of the NC and Sacox treated groups. Eimeria infection caused significant loss of BW and decreased FI in animals (Mehlhorn Citation2014), leading to poor nutrient absorption and a weakened immune response, resulting in intestinal damage (Adhikari et al. Citation2020). The effectiveness of dietary cinnamon reduces the detrimental effects of coccidial infection on broiler growth (Song et al. Citation2020). The NC and Sacox treated groups gained the most weight on the seventh day after infection with the protozoan Eimeria tenella, followed by CNP at level 2 g, while the infected unmedicated group (PC) gained the least. Prior literature has also reported improved performance in response to anticoccidials and the ability of natural products to reduce the induced weight loss due to bird infection, which supports our findings that the birds recovered more quickly than the negative control in the second week after infection (Alhotan and Abudabos Citation2019; Habibi et al. Citation2022). According to Habibi et al. (Citation2022), in 17-day-old domestic chickens experimentally infected with the protozoan Eimeria, the uninfected unmedicated group gained the most weight, followed by medicinal treated groups. The observed improvement in growth performance by including 2 g CNP/kg in broiler diets could be attributed to the effect of some active components found in the cinnamon plant (cinnamaldehyde and eugenol). These substances are considered digestion stimulating factors because they stimulate the secretion of endogenous digestive enzymes, protect the intestinal villi through intercellular antioxidant activity, and improve nutrient absorption (Jamroz et al. Citation2006). The current study's findings are consistent with those of Saied et al. (Citation2022), who found that birds fed cinnamon oil had higher body weight, weight gain and feed conversion ratios than the control group. According to Toghyani et al. (Citation2011), the BW in broiler chickens at days 28 and 42 was higher in birds fed 2 g cinnamon powder/kg than in control birds. In contrast, Symeon et al. (Citation2014) found no significant effects of different cinnamon oil levels (0.5 or 1.0 ml/kg diet) on broiler chicken growth performance. Because of the increased weight gain and the highest PEF in the second week indicated that the 2 g CNP/kg level is the best choice under coccidial challenge conditions. Except for one bird in PC, there was no mortality in any experimental groups. Thus, the mortality rate in the PC group was 2%, while it was 0.08% in all experimental groups.
Except for proventriculus percentage and pancreas, which increased in the CNP supplementation group compared to the Sacox treated group, the CNP treatments had no effect on carcass traits. This increase in proventriculus percentages may be attributed to cinnamon properties, stimulating broiler appetite, digestive system, and digestion, increasing pancreatic digestive enzymes, and improving liver function (Kumar et al. Citation2014). The current study's findings are consistent with those reported by others (Symeon et al. Citation2014; Gomathi et al. Citation2018; Saied et al. Citation2022), who observed that the majority of carcass characteristics did not change when different cinnamon levels were added. The findings of Sang-Oh et al. (Citation2013) found that dietary supplementation with CNP (5.0% CNP) resulted in higher levels of immunoglobulins against most avian diseases associated with heavier lymphoid organs than non-supplemented birds. The relative weight of lymphoid organs can be used to assess the state of the immune system; typically, the greater weight of these organs is associated with strong immune functions (Ravis et al. Citation1988). Here, when compared to the control, CNP treatments resulted in significantly higher thymus relative weight and numerical bursa of Fabricius. Here, neither coccidial challenge nor medications had an effect on the spleen index, but they did in the thymus. Furthermore, Yang et al. (Citation2019b) observed that diets supplemented with cinnamon oil or mixed with bamboo leaf had no effect on lymphoid organ relative weights. As expected, the results of this study revealed that the challenge negatively affected the heart percentage, relative lymphoid organ weights of the bursa and thymus, and pancreatic gland when compared to unchallenged birds, resulting in an insignificantly lower dressing yield percentage. Because CNP positively stimulated lymphoid organ weight in our study with a lower dose of CNP as natural herbal feed supplements, immune responses were expected to be boosted, and CNP may have broad antimicrobial activity.
In line with Abudabos et al. (Citation2017) the current study found that infected birds’ intestine relative weight increased significantly, demonstrating the infection's harmful effect.
The biochemical parameters in serum can reveal information about the body's physiological state and nutrient metabolism. Despite differences in total protein and globulin levels between groups, blood protein levels were within the normal range. Hussein (Citation2018) indicated that the addition of 3% cinnamon to broilers’ diets reduced plasma total protein, albumin, and globulin at day 42 of age. The increase in serum total proteins, albumin, and globulin of broilers fed higher cinnamon rates could be attributed to the fact that cinnamon improves protein digestion and absorption, allowing for better protein use in broilers and, consequently, an improvement in weight gain (Bento et al. Citation2013; Krishan and Narang Citation2014). Other studies, however, found that feed supplements containing 2 and 4 g cinnamon had no effect on serum albumin or the albumin-to-globulin ratio (Toghyani et al. Citation2011). In this study, the infection had no effect on bird blood proteins.
In this study, 6g of cinnamon reduced cholesterol in challenged birds compared to unchallenged birds. According to studies, cinnamon has hypoglycemic effects in broilers (Al-Kassie Citation2009; Faghani et al. Citation2014; Hussein et al. Citation2016). Cinnamon has been shown in numerous studies to lower cholesterol levels (Al-Kassie Citation2009; Faghani et al. Citation2014; Hussein et al. Citation2016; Krauze et al. Citation2021). On the other hand, Hussein (Citation2018) found no evidence of a cinnamon effect on cholesterol levels. Cholesterol levels in all groups were within or nearest the normal range of 100–200 mg/dL (Thaxton et al. Citation2006). Several active compounds, including cinnamaldehyde, cinnamic acid, and some of its synthetic derivatives, as well as carvacrol and thymol can cause serum hypocholesterolemia by inhibiting the hepatic enzyme regulating cholesterol synthesis, 3-hydroxy-3-methylglutaryl coenzyme A reductase (Bahr et al. Citation2021). The dietary additives had no negative effects on the safety profiles of chickens in this study. In terms of liver and kidney function parameters, the current coccidial challenge has no effect on ALT and AST, despite the fact that except for 6 g CNP, the ALT levels increased in treatments under challenging conditions but remained within normal limits, indicating no liver or muscle damage. This means that up to 6 g CNP per kg diet can be added to the diet of broiler chickens with no adverse effect. The addition of cinnamon powder to broiler diets had no effect on blood AST and ALT levels (Koochaksaraie et al. Citation2011; Saied et al. Citation2022). Contrarily, cinnamon reduced AST in growing Japanese quails (Ahmed et al. Citation2019) and layer chickens (Abo Ghanima et al. Citation2020) compared to both the control and antibiotic-supplemented groups, while creatinine levels remained unchanged. Furthermore, Hussein (Citation2018) found a significant increase in blood AST levels in broiler chicks fed a diet supplemented with 3% cinnamon powder but not in ALT levels. In the current study, a coccidial challenge reduced creatinine levels. Under normal conditions, adding cinnamon powder to broiler diets had no effect on blood creatinine levels (Kanani et al. Citation2016; Saied et al. Citation2022). In contrast to Habibi et al. (Citation2022), who found that coccidial challenge reduced total protein and globulin levels while increasing serum ALT, and serum cholesterol levels compared to the unchallenged unmedicated, the challenged supplemented with diclazuril (1 ppm) and the challenged supplemented with ZnO-NPs (20 ppm) groups were comparable.
Infected chickens had more eosinophils than unchallenged unmedicated chickens, which is consistent with the findings of Ahmad et al. (Citation2020) but not with the findings of Nahed et al. (Citation2020) and El-Maddawy et al. (Citation2022), where there was no change in the percentage of eosinophils. However, contrary to Ahmad et al. (Citation2020), the infection increased monocytes while decreasing heterophils. The percentage of basophils remained unchanged. In contrast to the findings of Habibi et al. (Citation2022), reported that it was significantly higher in control groups than in all other groups. In contrast to the findings of others (Nahed et al. Citation2020; El-Maddawy et al. Citation2022), they observed that infected chickens had a higher heterophil-to-lymphocyte ratio than the unchallenged unmedicated group, indicating stress following parasitic or viral infections.
Our findings support Dubey (Citation2019) observations of typical clinical signs of avian coccidiosis. After induced-infection in broilers, oocyst shedding and lesion score of the small intestines improved in ginger and garlic supplemented birds (Ali et al. Citation2019). This study confirms and supports the previously reported findings that CNP reduces the number of OPG output and lesions in the cecum of cinnamon-treated birds to a greater or lesser extent than Sacox-treated birds, implying that CNP may play an important role in controlling large-scale avian coccidiosis outbreaks in poultry farms. The presence of biologically active compounds that alter microflora modulation, reduce Eimeria tenella oocyst shedding, decrease intestinal inflammation, enhance immunity and improve antioxidant status has been linked to the positive effects of anticoccidial cinnamon criterion use (Rao and Gan Citation2014; Abbasi et al. Citation2020; Khater et al. Citation2020; Yang et al. Citation2020; Adarsh et al. Citation2020b; Habibi et al. Citation2022). Furthermore, due to its organ-protective properties, cinnamon may play an essential role in improving the conditions of diseased birds (Yang et al. Citation2020). In general, our findings in this study confirmed that the powder of cinnamon bark herb exhibited anticoccidial activity that was dose CNP dependent.
CNP had a low crude protein content (4.43%) and a high carbohydrate content (64.58%) in this study, resulting in a higher nutritional value (4974.77 Kcal/100 g). Furthermore, HPLC detected only caffeine and seven unknown components in the CN extract. The unknown components were identified using GC-MS, which detected cinnamaldehyde as the major active polyphenol component and 25 volatile compounds in the methanolic CN bark extract. This discovery is supported by Hameed et al. (Citation2016), who discovered the majority of the identified volatile components. According to Khan et al. (Citation2003), cinnamon contains high levels of cinnamaldehyde, followed by eugenol and carvacrol. Furthermore, these bioactive compounds are known to have antioxidant properties (Moreira and de Souza Dias Citation2018; Chowdhury et al. Citation2018b; Ahmadi et al. Citation2021; Syafiq et al. Citation2021). Cinnamaldehyde, eugenol and carvacrol, which are phenolic components of the cinnamon plant, have antimicrobial activity due to their antibacterial properties against pathogenic microorganisms (Chowdhury et al. Citation2018b; Ahmadi et al. Citation2021; Syafiq et al. Citation2021).
Secondary plant metabolites with an aromatic ring and one or more hydroxyl substituents are known as phenolic compounds. Folin-Ciocalteu reagent as Gallic acid equivalents was used to determine TPC. The cinnamon bark extract was found to be high in TPC, yielding 58.18 mg GAE per g dry weight. The dry powder of Cinnamon bark yielded a result of 57.7 mg GAE/g as determined by Georgieva and Mihaylova (Citation2014), which is very close to the value obtained by the study. Furthermore, according to (Muchuweti et al. Citation2007), cinnamon has a TPC of about 15 mg GAE/g of a sample. According to Wijewardhana et al. (Citation2019), the cinnamon bark extract has TPC yielding 18.94 mg GAE per 100g dry weight. The study's findings were consistent with previous research; however, variations in values could be due to differences in raw material origin under different geo-climatological conditions.
Many diseases, including parasitic diseases, are caused by oxidative stress, which occurs when the equilibrium between the processes of producing free radicals and removing them is disrupted, which is normally balanced under natural conditions when the production of free radicals by antioxidant compounds exceeds the process of removal (Georgieva et al. Citation2006). The antioxidant defense mechanism of natural herbs can be boosted by regulating and inducing the activity of antioxidant enzymes (Hsu and Liu Citation2004). Cinnamon phenolic compounds, such as Cinnamaldehyde, have antioxidant activity. Antioxidants are widely used as dietary supplements in the poultry industry to alleviate the oxidative stress caused by high oxidative free radical production during the immune response of host cells to Eimeria infestation (El-Maddawy et al. Citation2022). This could help in the fight against parasitic infections as well as the elimination of cytotoxicity and tissue damage.
CNP contributed positively as phytogenic by containing secondary metabolites such as phenols, flavonoids, saponins, alkaloids, and tannins, all of which have antioxidant, anti-parasitic, anti-inflammatory, and other medicinal properties (Rao and Gan Citation2014; Ahmadi and Shahri Citation2020; El-Hack et al. Citation2020; Adarsh et al. Citation2020a).
As a result, CNP's anticoccidial and growth performance enhancer should be considered in future prescriptions in both performance and health cases. More research needs to be done using a lower dose of Eimeria tenella oocysts or a higher drug dose to reverse the negative effects on performance. Furthermore, more research is needed, either alone or in combination with other herbal products, to see if it can reduce the consequences of coccidiosis symptoms and oocyst output and thus contribute to improved performance. Furthermore, the phytochemicals detected here should be investigated further in vivo or in vitro to determine whether the anticoccidial activity and mode of action, if any, are directly or indirectly related to the reduction of faecal oocyst counts in birds.
Conclusions
In conclusion, the current study concluded that cinnamon powder is very effective compared to an anticoccidial drug in preventing and controlling coccidiosis in broiler chickens at the levels tested. In coccidia-infected broilers, dietary CNP supplementation reduced oocysts per gram of excreta and cecal lesion score.
The current study found that supplementing 2 g Cinnamomum verum bark/kg diet produced positive results in broiler growth performance during the coccidial challenge.
Taking all of these factors into consideration, dietary CNP could be used as a potential health and growth promoter by alleviating coccidia effects and improving broiler growth performance.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Abbasi R, Abdi-Hachesoo B, Razavi SM, Namazi F, Nazifi S. 2020. In vitro and in vivo activity of cinnamaldehyde against Eimeria kofoidi in chukar partridge (Alectoris chukar). Exp Parasitol. 218:107978.
- Abdisa T, Hasen R, Tagesu T, Regea G, Tadese G. 2019. Poultry coccidiosis and its prevention. Control J Vet Ani Res. 2:101-107.
- Abo Ghanima MM, Elsadek MF, Taha AE, Abd El-Hack ME, Alagawany M, Ahmed BM, Elshafie MM, El-Sabrout K. 2020. Effect of housing system and rosemary and cinnamon essential oils on layers performance, egg quality, haematological traits, blood chemistry, immunity, and antioxidant. Animals (Basel). 10(2):245-261.
- Abudabos AM, Alyemni AH, Swilam EO, Al-Ghadi M. 2017. Comparative anticoccidial effect of some natural products against Eimeria spp. infection on performance traits, intestinal lesion and occyte number in broiler. Pak J Zool. 49(6):1989–1995.
- Adarsh A, Chettiyar B, Kanthesh B, Raghu N. 2020a. Phytochemical screening and antimicrobial activity of “Cinnamon zeylanicum”. 13:22–33.
- Adarsh A, Chettiyar B, Kanthesh B, Raghu N. 2020b. Phytochemical Screening and Antimicrobial Activity of “Cinnamon zeylanicum”. Int J Pharm Res aInnov. 13:22–33.
- Adedeji OS, Oyetoro BA, Oki HA. 2021. Effect of dietary cinnamon powder on the organolopetic properties of cockerel chickens. 11(3):157–162.
- Adhikari P, Kiess A, Adhikari R, Jha R. 2020. An approach to alternative strategies to control avian coccidiosis and necrotic enteritis. J Appl Poult Res. 29(2):515–534.
- Ahmad Z, Hafeez A, Ullah Q, Naz S, Khan RU. 2020. Protective effect of Aloe vera on growth performance, leucocyte count and intestinal injury in broiler chicken infected with coccidiosis. J Appl Anim Res. 48(1):252–256.
- Ahmadi E, Shahri MM. 2020. The antioxidant and anticoagulant effects of coumarin and quercetin from cinnamon methanolic extract, and the assessment of cinnamon powder effect on plasma parameters in diabetes, and the disinfectant activity in diabetic patients. Herb Med Jo. 4(3):103–110.
- Ahmadi S, Hivechi A, Bahrami SH, Milan PB, Ashraf SS. 2021. Cinnamon extract loaded electrospun chitosan/gelatin membrane with antibacterial activity. Int J Biol Macromol. 173:580–590.
- Ahmed EM, Attia A, Ibrahem ZA, El-Hack A. 2019. Effect of dietary ginger and cinnamon oils supplementation on growing Japanese quail performance. Zagazig J Agri Res. 46(6):2037–2046.
- Alhotan RA, Abudabos A. 2019. Anticoccidial and antioxidant effects of plants derived polyphenol in broilers exposed to induced coccidiosis. Environ Sci Pollut Res. 26(14):14194–14199.
- Ali A, Ponnampalam EN, Pushpakumara G, Cottrell JJ, Suleria HA, Dunshea FR. 2021. Cinnamon: a natural feed additive for poultry health and production—A review. Animals (Basel). 11(7):2026-2042.
- Ali M, Chand N, Khan RU, Naz S, Gul S. 2019. Anticoccidial effect of garlic (Allium sativum) and ginger (Zingiber officinale) against experimentally induced coccidiosis in broiler chickens. J Appl Anim Res. 47(1):79–84.
- Al-Kassie GA. 2009. Influence of two plant extracts derived from thyme and cinnamon on broiler performance. Pak Vet J. 29(4):169–173.
- Alqhtani AH, Qaid MM, Al-Garadi MA, AA A-A, Alharthi AS, Al-Mufarrej SI. 2022. Efficacy of Rumex nervosus leaves or Cinnamomum verum bark as natural growth promoters on the growth performance, immune responsiveness, and serum biochemical profile of broiler chickens. Ital Jo Anim Sci. 21(1):792–801.
- Al-Quraishy S, Qasem MA, Al-Shaebi EM, Murshed M, Mares MM, Dkhil MA. 2020. Rumex nervosus changed the oxidative status of chicken caecum infected with Eimeria tenella. J King Saud University-Science. 32(3): 2207-2211.
- Al-Shaibani I, Al-Khadher A, AlHibah A. 2020. Anticoccidial activity of Allium sativum and Punica granatum against experimentally induced Eimeria tenella infection in broiler chickens. Asian J Res Anim Vet Sci. 5(4):20–29.
- Arczewska-Włosek A, Świątkiewicz S. 2013. Improved performance due to dietary supplementation with selected herbal extracts of broiler chickens infected with Eimeria spp. J Anim Feed Sci. 22(3):257–263.
- Bahr T, Butler G, Rock C, Welburn K, Allred K, Rodriguez D. 2021. Cholesterol-lowering activity of natural mono-and sesquiterpenoid compounds in essential oils: a review and investigation of mechanisms using in silico protein–ligand docking. Phytother Res. 35(8):4215–4245.
- Balta I, Marcu A, Linton M, Kelly C, Stef L, Pet I, Ward P, Pircalabioru GG, Chifiriuc C, Gundogdu O. 2021. The in vitro and in vivo anti-virulent effect of organic acid mixtures against Eimeria tenella and Eimeria bovis. Sci Rep. 11(1):1–11.
- Bento M, Ouwehand A, Tiihonen K, Lahtinen S, Nurminen P, Saarinen M, Schulze H, Mygind T, Fischer J. 2013. Essential oils and their use in animal feeds for monogastric animals–Effects on feed quality, gut microbiota, growth performance and food safety: a review. Veterinární Medicína. 58(9):449–458.
- Chand N, Ali P, Alhidary IA, Abdelrahman MA, Albadani H, Khan MA, Seidavi A, Laudadio V, Tufarelli V, Khan RU. 2021. Protective effect of grape (Vitis vinifera) seed powder and zinc-glycine complex on growth traits and gut health of broilers following Eimeria tenella challenge. Antibiotics. 10(2):186-194.
- Chand N, Faheem H, Khan RU, Qureshi MS, Alhidary IA, Abudabos AM. 2016. Anticoccidial effect of mananoligosacharide against experimentally induced coccidiosis in broiler. Environ Sci Pollut Res. 23(14):14414–14421.
- Chowdhury S, Mandal GP, Patra AK. 2018b. Different essential oils in diets of chickens: 1. Growth performance, nutrient utilisation, nitrogen excretion, carcass traits and chemical composition of meat. Anim Feed Sci Technol. 236:86–97.
- Chowdhury S, Mandal GP, Patra AK, Kumar P, Samanta I, Pradhan S, Samanta AK. 2018a. Different essential oils in diets of broiler chickens: 2. Gut microbes and morphology, immune response, and some blood profile and antioxidant enzymes. Anim Feed Sci Technol. 236:39–47.
- Chowlu H, Vidyarthi V, Zuyie R, Maiti C. 2019. Effect of dietary supplementation of cinnamon on the performance of broiler chicken. Livest Res Int. 7:83–87.
- Dubey JP. 2019. Coccidiosis in livestock, poultry, companion animals and humans. CRC Press, Taylor & Francis Group, p. 1–127.
- El-Hack MEA, Alagawany M, Abdel-Moneim A-ME, Mohammed NG, Khafaga AF, Bin-Jumah M, Othman SI, Allam AA, Elnesr SS. 2020. Cinnamon (Cinnamomum zeylanicum) oil as a potential alternative to antibiotics in poultry. Antibiotics. 9(5):210-222.
- El-Maddawy ZK, El-Sawy AE-SF, Ashoura NR, Aboelenin SM, Soliman MM, Ellakany HF, Elbestawy AR, El-Shall NA. 2022. Use of zinc oxide nanoparticles as anticoccidial agents in broiler chickens along with its impact on growth performance, antioxidant status, and hematobiochemical profile. Life. 12(1):74-91.
- El-Shazly KA, El-Latif AA, Abdo W, El-Morsey A, El-Aziz MIA, El-Mogazy H. 2020. The anticoccidial activity of the fluoroquinolone lomefloxacin against experimental Eimeria tenella infection in broiler chickens. Parasitol Res. 119:1955–1968.
- Faghani M, Rahimian Y, Rafiee A, Namjoo AR. 2014. Effect of garlic and cinnamon in comparison to virginiamycin on performance and some haematological parameters in broiler chicks. Res Opin Anim Vet Sci. 4(9): 504-507.
- Gaikwad D, Fulpagare Y, Bhoite U, Deokar D, Nimablkar C. 2019. Effect of dietary supplementation of ginger and cinnamon on growth performance and economics of broiler production. Int J Current Microbiol Appl Sci. 8(3):1849–1857.
- Georgieva L, Mihaylova D. 2014. Evaluation of the in vitro antioxidant potential of extracts obtained from Cinnamomum zeylanicum barks. Научни Трудове На Русенския Университет. 53:41–45.
- Georgieva N, Koinarski V, Gadjeva V. 2006. Antioxidant status during the course of Eimeria tenella infection in broiler chickens. Vet J. 172(3):488–492.
- Gomathi G, Senthilkumar S, Natarajan A, Amutha R, Purushothaman MR. 2018. Effect of dietary supplementation of cinnamon oil and sodium butyrate on carcass characteristics and meat quality of broiler chicken. Vet World. 11(7):959–964.
- Habibi H, Ghahtan N, Tohidi S, Zarrinfar A. 2022. Effect of composition of medicinal plants on growth performance, gut bacteria, hematological parameters, anticoccidial index, and optimum anticoccidial activity in domestic chicken. Comp Clin Path 1-9.
- Hafeez A, Ullah Z, Khan R, Ullah Q, Naz S. 2020. Effect of diet supplemented with coconut essential oil on performance and villus histomorphology in broiler exposed to avian coccidiosis. Trop Anim Health Prod. 52:2499–2504.
- Hameed IH, Altameme HJ, Mohammed GJ. 2016. Evaluation of antifungal and antibacterial activity and analysis of bioactive phytochemical compounds of Cinnamomum zeylanicum (Cinnamon bark) using gas chromatography-mass spectrometry. Orient J Chem. 32(4):1769-1788.
- Hsu D-Z, Liu M-Y. 2004. Sesame oil protects against lipopolysaccharide-stimulated oxidative stress in rats. Crit Care Med. 32(1):227–231.
- Hussein AH. 2018. Physiological responses and productive performance of broiler chicks fed diets supplemented with different levels of cinnamon powder. Egyp Poult Sci J. 38(4):1171–1184.
- Hussein TK, Hwaidi EH, Mohammad AH. 2016. The effects of cinnamon powder feeding on some blood aspects and performance of broiler chicken. Kufa J Vet Med Sci. 6(1):118-122.
- Jamroz D, Wertelecki T, Houszka M, Kamel C. 2006. Influence of diet type on the inclusion of plant origin active substances on morphological and histochemical characteristics of the stomach and jejunum walls in chicken. J Anim Physiol Anim Nutr. 90(5-6):255–268.
- Kanani PB, Daneshyar M, Aliakbarlu J, Hamian F. 2017. Effect of dietary turmeric and cinnamon powders on meat quality and lipid peroxidation of broiler chicken under heat stress condition. Vet Res Forum. 8(2): 163–169.
- Kanani PB, Daneshyar M, Najafi R. 2016. Effects of cinnamon (Cinnamomum zeylanicum) and turmeric (Curcuma longa) powders on performance, enzyme activity, and blood parameters of broiler chickens under heat stress. Poultry Sci J. 4(1):47–53.
- Khafaji S. 2018. Study the effect of ceylon cinnamon (cinnamomumzeylanicum) powder on some physiological parameters in broiler chicks. J Global Pharma Technol. 10(07):236–242.
- Khan A, Safdar M, Khan MMA, Khattak KN, Anderson RA. 2003. Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes Care. 26(12):3215–3218.
- Khan M, Chand N, Naz S, Khan RU. 2022. Dietary tea tree (Melaleuca alternifolia) essential oil as alternative to antibiotics alleviates experimentally induced Eimeria tenella challenge in Japanese quails. J Anim Physiol Anim Nutr. 8(2):163–169.
- Khater HF, Ziam H, Abbas A, Abbas RZ, Raza MA, Selim A. 2020. Avian coccidiosis: recent advances in alternative control strategies and vaccine development. Agrobiol Rec. 1:11–25.
- Koochaksaraie R, Irani M, Gharavysi S. 2011. The effects of cinnamon powder feeding on some blood metabolites in broiler chicks. Revista Brasileira de Ciência Avícola. 13(3):197–202.
- Krauze M, Cendrowska-Pinkosz M, Matuseviĉius P, Stępniowska A, Jurczak P, Ognik K. 2021. The effect of administration of a phytobiotic containing cinnamon oil and citric acid on the metabolism, immunity, and growth performance of broiler chickens. Animals (Basel). 11(2):399-416.
- Krishan G, Narang A. 2014. Use of essential oils in poultry nutrition: a new approach. J Adv Vet Anim Res. 1(4):156–162.
- Kumar M, Kumar V, Roy D, Kushwaha R, Vaiswani S. 2014. Application of Herbal Feed Additives in Animal Nutrition-A Review. Int J Livestock Res. 4(9):1–8.
- Lan L, Zuo B, Ding H, Huang Y, Chen X, Du A. 2016. Anticoccidial evaluation of a traditional Chinese medicine—Brucea javanica—in broilers. Poult Sci. 95(4):811–818.
- Lee H-A, Hong S, Chung Y-H, Song K-D, Kim O. 2012. Anticoccidial effects of Galla rhois extract on Eimeria tenella-infected chicken. Lab Anim Res. 28(3):193–197.
- Lei T, Wu D, Song Z, Ren Y, Yu Q, Qi C, Xiao P, Gong J. 2022. Research note: effects of different anticoccidial regimens on the growth performance, hematological parameters, immune response, and intestinal coccidial lesion scores of yellow-feathered broilers. Poult Sci. 101(10):102019-102023 .
- Li G, Kanu S, Xiao S, Xiang F. 2005. Responses of chickens vaccinated with a live attenuated multi-valent ionophore-tolerant Eimeria vaccine. Vet Parasitol. 129(3-4):179–186.
- Maiorka A, Dahlke F, Morgulis MSFA. 2006. Broiler adaptation to post-hatching period. Ciencia Rural. 36(2):701–708.
- Mehlhorn H. 2014. Encyclopedic reference of parasitology, 4th ed. Berlin: Springer.
- Moreira GC, de Souza Dias F. 2018. Mixture design and Doehlert matrix for optimization of the ultrasonic assisted extraction of caffeic acid, rutin, catechin and trans-cinnamic acid in Physalis angulata L. and determination by HPLC DAD. Microchem J. 141:247–252.
- Muchuweti M, Kativu E, Mupure CH, Chidewe C, Ndhlala AR, Benhura MAN. 2007. Phenolic composition and antioxidant properties of some spices M. Am J Food Technol. 2(5):414–420.
- Mustafa A, Bai S, Zeng Q, Ding X, Wang J, Xuan Y, Su Z, Zhang K. 2021. Effect of organic acids on growth performance, intestinal morphology, and immunity of broiler chickens with and without coccidial challenge. AMB Express. 11(1):1–18.
- Nahed A, Shewita RS, Abd El-Hack ME, AlKahtane A, Alarifi S, Alkahtani S, Abdel-Daim MM, Sedeik ME. 2020. Effect of essential oils on the immune response to some viral vaccines in broiler chickens, with special reference to Newcastle disease virus. Poult Sci. 99(6):2944–2954.
- Noack S, Chapman HD, Selzer PM. 2019. Anticoccidial drugs of the livestock industry. Parasitol Res. 118(7):2009–2026.
- NRC. 1994. US National Research Council. Nutrient requirements of poultry: 1994. ninth revised edition. National Academies Press. United States.
- Qaid MM, Al-Mufarrej SI, Azzam MM, Al-Garadi MA, Albaadani HH, Alhidary IA, Aljumaah RS. 2021. Growth performance, serum biochemical indices, duodenal histomorphology, and cecal microbiota of broiler chickens fed on diets supplemented with cinnamon bark powder at prestarter and starter phases. Animals (Basel). 11(1):94-112.
- Rakasivi KGJ, Chin KB. 2022. Antioxidant activity of Cinnamomum cassia extract and quality of raw chicken patties added with C. cassia powder and Pleurotus sajor-caju powder as functional ingredients during storage. Anim Biosci. 35(8):1279–1288.
- Rao PV, Gan SH. 2014. Cinnamon: a multifaceted medicinal plant. Evidence-Based Complementary Altern Med. 2014: 1-13.
- Ravis W, Parsons D, Wang S. 1988. Buffer and pH effects on propranolol binding by human albumin and α1-acid glycoprotein. J Pharm Pharmacol. 40(7):459–463.
- Safa Eltazi M. 2014. Effect of using cinnamon powder as natural feed additive on performance and carcass quality of broiler chickens. Int J Innov Agri Biol Res. 2(3):1–8.
- Saied A, Attia A, El-Kholy M, Reda F, Nagar A. 2022. Effect of cinnamon oil supplementation into broiler chicken diets on growth, carcass traits, haemato-biochemical parameters, immune function, antioxidant status and caecal microbial count. J Anim Feed Sci. 31(1):21–33.
- Sang-Oh P, Chae-Min R, Byung-Sung P, Jong H. 2013. The meat quality and growth performance in broiler chickens fed diet with cinnamon powder. J Environ Biol. 34(1):127-133.
- SAS. 2012. SAS Institute/OR 9.3 user's guide: mathematical programming examples. SAS Institute. Car. North Carolina. U.S. state.
- Song X, Li Y, Chen S, Jia R, Huang Y, Zou Y, Li L, Zhao X, Yin Z. 2020. Anticoccidial effect of herbal powder “Shi Ying Zi” in chickens infected with Eimeria tenella. Animals (Basel). 10(9):1484-1499.
- Syafiq R, Sapuan S, Zuhri M. 2021. Antimicrobial activity, physical, mechanical and barrier properties of sugar palm based nanocellulose/starch biocomposite films incorporated with cinnamon essential oil. J Mater Res aTechnol. 11:144–157.
- Symeon GK, Athanasiou A, Lykos N, Charismiadou MA, Goliomytis M, Demiris N, Ayoutanti A, Simitzis PE, Deligeorgis SG. 2014. The effects of dietary cinnamon (Cinnamomum zeylanicum) oil supplementation on broiler feeding behaviour, growth performance, carcass traits and meat quality characteristics. Annals Anim Sci. 14(4):883–895.
- Tanweer AJ, Saddique U, Bailey C, Khan R. 2014. Antiparasitic effect of wild rue (Peganum harmala L.) against experimentally induced coccidiosis in broiler chicks. Parasitol Res. 113(8):2951–2960.
- Thaxton J, Dozier W, Branton S, Morgan G, Miles D, Roush W, Lott B, Vizzier-Thaxton Y. 2006. Stocking density and physiological adaptive responses of broilers. Poult Sci. 85(5):819–824.
- Toghyani M, Toghyani M, Gheisari A, Ghalamkari G, Eghbalsaied S. 2011. Evaluation of cinnamon and garlic as antibiotic growth promoter substitutions on performance, immune responses, serum biochemical and haematological parameters in broiler chicks. Livest Sci. 138(1):167–173.
- Valavi M, Sarir H, FarhangFar H, Zarban A, Hosseini-Vashan SJ, Naeimipour Younosi H. 2016. Evaluation the effect of garlic and cinnamon powder on performance, antioxidant system, blood parameters of broilers under heat stress conditions. Res Anim Prod (Sci Res). 7(14):20-31.
- Wade B, Keyburn A. 2015. The true cost of necrotic enteritis. World Poult. 31(7):16–17.
- Wijewardhana U, Gunathilaka U, Navaratne S. 2019. Determination of total phenolic content, radical scavenging activity and total antioxidant capacity of cinnamon bark, black cumin seeds and garlic. Int Res J Adv Eng Sci. 4(2):55–57.
- Wondimu A, Mesfin E, Bayu Y. 2019. Prevalence of poultry coccidiosis and associated risk factors in intensive farming system of Gondar Town, Ethiopia. Vet Med Int. 2019: 1-7.
- Yang C, Kennes YM, Lepp D, Yin X, Wang Q, Yu H, Yang C, Gong J, Diarra MS. 2020. Effects of encapsulated cinnamaldehyde and citral on the performance and cecal microbiota of broilers vaccinated or not vaccinated against coccidiosis. Poult Sci. 99(2):936–948.
- Yang W-C, Yang C-Y, Liang Y-C, Yang C-W, Li W-Q, Chung C-Y, Yang M-T, Kuo T-F, Lin C-F, Liang C-L. 2019a. Anti-coccidial properties and mechanisms of an edible herb, Bidens pilosa, and its active compounds for coccidiosis. Sci Rep. 9(1):1–11.
- Yang Y-F, Zhao L-L, Shao Y-X, Liao X-D, Zhang L-Y, Lin L, Luo X-G. 2019b. Effects of dietary graded levels of cinnamon essential oil and its combination with bamboo leaf flavonoid on immune function, antioxidative ability and intestinal microbiota of broilers. J Integr Agric. 18(9):2123–2132.
- Zahraa H. 2008. Effects of commutative heat stress on immunoresponses in broiler chickens reared in closed system. Int J Poultry Sci. 7:964–968.
