ABSTRACT
This study was conducted to investigate the effect of Humulus scandens (HS) in diet on the production performance and intestinal barrier of rabbits. One hundred and sixty Laiwu black rabbits at 35 days of age with body weight of 692.5 ± 48.3 g were divided into four groups (five replicates per group and eight rabbits per replicate): fed on a basal diet free of HS (control), 4%, 8% or 12% HS powder supplement. The results showed that the dietary addition of HS decreased the ADFI, F/G, diarrhoea ratio and mortality ratio. Besides, the final body weight and ADG (P < .05) increased with the level of HS. Rabbits in 12% HS group had a higher sIgA and IgG concentration in serum and ileum than control, while lower TNFα, IFN-γ and IL-6 concentration (P < .05). Compared with the control, the HS supplementation groups for 12% level could decrease the mRNA expression of ZO-1, JAM3 and mucin1 (P < .05). In conclusion, dietary supplement of HS modulates immune responses and enhances intestinal barrier, meanwhile inhibits the synthesis of cytokine. Besides, our experiment offers positive evidence in improving rabbit health of HS as rabbit feed resource.
1. Introduction
The disease of digestive tract is the most important disease in rabbits, accounting for 70% (Carabaño et al. Citation2008). The mortality of the weaned rabbits up to 60% due to epizootic rabbit enteropathy (Fann et al. Citation2001; Carabaño et al. Citation2008). Rabbit diarrhoea is a big problem in present rabbit production, especially in the weaning stage (Martens and Van Herck Citation2000). Weaned rabbits had thinner intestinal walls, incomplete intestinal barrier, frequent diarrhoea or gastrointestinal flatulence (Fann et al. Citation2001). Antibiotics have been widely used to control mortality, increase breeding costs, affect product quality and reduce the economic benefits of many farms. For this reason, some herbs and plant extracts to substitute antibiotic have been found and studied by many researchers. Humulus scandens (HS) is widely used in China to treat dysentery and chronic colitis as one traditional Chinese medicine, and contains polysaccharides, flavonoids, volatile oil and other chemical components (Chen et al. Citation2012). Li et al. (Citation2008) had reported that HS was rich in flavonoids and there have been three kinds (vitexin, luteolin and cosmosiin) of flavonoids separated from HS. Flavonoids are a kind of common polyphenols in the plant world. They have biological activities such as anti-inflammatory, anti-oxidant and anti-cancer (Korkina and Afanas’ev Citation1997; Neuhouser Citation2005; Talhouk et al. Citation2007; Zhang et al. Citation2021; Nath et al. Citation2022). Li et al. (Citation2022) flavonoids could inhibit alcohol-induced hepatocyte injury, which might be attributed to alleviating oxidative stress and mitigating inflammatory response by activating Nrf2 and inhibiting NF-κB pathways. In addition, it is reported that flavonoids play a strong immune role by inhibiting the proliferation of immune cells and the production of pro-inflammatory cytokines (Rios Citation2010). HS is wild on the roadside in the field, which adapts to various environmental conditions and has high yield (Urziceanu et al. Citation2022). In addition, because of its strong vitality and harm to the growth of crops, it is often regarded as a malignant weed, so far it has not been systematically developed and utilized, resulting in serious waste of resources (Zhou and Feng Citation2020). However, there was little information available about HS use as feedstuff in rabbit production and special its effect on improving intestinal barriers. The present experiment was conducted to investigate the effect of HS on the performance and intestinal barrier of rabbits, to identify applicative effects HS as a feed resource in rabbit production.
2. Materials and methods
2.1. Production of HS powder
Harvest the HS from countryside in Shandong Province in the autumn mature stage, and naturally drying and pulverizing.
2.2. Animals, diet and feed chemical composition
One hundred and sixty Laiwu black rabbits (male–female rate of 1:1) at 35 days of age weight of 692.5 ± 48.3 g were divided into four groups (five replicates per group and eight rabbits per replicate): f fed on a basal diet with 0 (control), 4%, 8% or 12% HS powder, and the chemical of HS were listed in . The basal diets were formulated according to the values for growing from De Blas and Mateos (Citation2020) and the ingredients and composition of diet are listed in . Besides, the diet pelleted by the use of pressure and the diameter of the pellets was 4 mm. The experiment lasted for nine weeks which included one week adaptation period and eight weeks experimental period. During the rearing period, the rabbits were individually housed cage and had free access to feed and water.
Table 1. Chemical composition of Humulus scandens powder (air-dry basis, %).
Table 2. Composition and nutrient levels of basal diets (air-dry basis, %).
2.3. Sample collection and preparation
At the end of the trial (98 days), 40 rabbits (10 rabbits per group, male and female had half each, and the average body weight of the two rabbits equals to the average body weight of each replicate) were sacrificed by cervical dislocation. About 4 cm segment of the middle ileum was collected and divided into two segments. One intestinal segments were flushed gently with phosphate-buffered saline (PBS, pH 7.4) and then placed in 10% fresh, chilled formalin solution for histological measurements. The other intestinal segments were collected by cryopreservation tube, rapidly frozen in liquid nitrogen and stored at −80°C until analysis.
2.4. Determination of indicators and methods
2.4.1. Feed chemical analysis
The chemical composition of the SH and diets was analysed following the procedures of AOAC International (Citation2005). The dry matter was determined by oven drying at 105°C (procedure 934.01), and the ether extract was determined by extraction with petroleum ether in a Soxtec 1043 apparatus (FOSS Tecator AB, Hoganas, Sweden; procedure 920.39). The protein content was determined using a Kjeltec Auto 1030 Analyser (procedure 954.01). The ash content was determined according to procedure 920.153. Analysis of fibre components was performed according to Van Soest et al. (Citation1991). The calcium and phosphorus contents were determined by inductively coupled plasma atomic emission spectrometry (ICP-EMS; Optima 8000, Perkin Elmer, U.S.A.).
The content of polysaccharides in HS was detected by phenol-sulphuric acid method. Preparation of standard glucose solution: accurately weigh 20 mg of anhydrous glucose dried at 105°C to constant weight, put it in a 250 mL volumetric flask, fix the volume and shake well for use. Drawing the standard curve: accurately absorb 0, 0.2, 0.4, 0.6, 0.8, 1.0 and 1.2 mL of glucose standard solution, put them in a test tube with a stopper, add water to 2 mL respectively, and shake well, then add 1.0 mL of 5% phenol solution, quickly add concentrated H2SO4 5 mL, shake well and leave it at room temperature for 30 min until it is cooled. Then, use an ultraviolet spectrophotometer at 490 nm wavelength. Determination of polysaccharide content in the sample: the absorbance value is determined according to the above standard method, and the content is calculated according to the standard curve.
The content of flavonoids in HS was detected by colorimetry. Accurately weigh 1 g of raw materials, add 50% ethanol solution according to the ratio of material to liquid (1:20–1:40, g/ml), perform ultrasonic treatment for 2 h, centrifuge at 3000 r/min for 20 min and collect supernatant for determination. Taking rutin as standard, and mix 1.5 mL of extract with 1.5 mL of 2% AlCl3-methanol solution (containing 5% acetic acid), stand for 10 min, and measure the absorbance at 430 nm. With rutin as the standard sample, a standard curve was established in the range of 10–500 μg/mL.
2.4.2. Growth performance
Body weights of each replicate rabbits were measured before feeding at the beginning and end of the trial, and the ADG was calculated from 42 to 98 days of age. The ADFI was calculated according to total feed intake divided by the total number of experimental days in each replicate. The F/G was then calculated as the feed intake/weight gain. The number of rabbits with diarrhoea or dead was recorded daily throughout the study.
2.4.3. Slaughter performance
As described by Blasco and Ouhayoun (Citation1996), the slaughter procedure and carcass analysis were performed. Twelve hours before slaughter, rabbits were fasted and weighed, which was pre-slaughter body weight. After bleeding, the pelts, paws and full gastrointestinal tract were removed, and the commercial carcass was weighed (preserving the head, trachea, oesophagus, thoracic organs, liver, kidney and perirenal fat). The semi-clean carcass weight was commercial carcass weight removing the head at the first cervical vertebra, removing the trachea and oesophagus, retaining the liver, kidney and perirenal fat. The full-clean carcass weight is the semi-clean carcass weight removing the heart, liver, kidney and perirenal fat. At the same time, the thymus, spleen, heart, liver and kidney are weighed. At the same time, the commercial slaughter ratio, semi-clean slaughter ratio, full-clean slaughter ratio, thymus ratio, spleen ratio, heart ratio, liver ratio and kidney ratio were calculated by dividing the live weight before slaughter.
2.4.4. Morphological structure of small intestine
The ileum tissue samples were cut longitudinally at the mesenteric attachment and immediately fixed in 10% neutral formalin. The samples were then dehydrated in graded alcohols, cleared with xylene and embedded in paraffin. The serial microtome sections (5 µm thick) were stained with haematoxylin and eosin stain. In each slide, 10 well-oriented villi and crypts were analysed at a low magnification (40×) with a light microscope (using Image-Pro Plus 6.0 software, U.S.A.).
2.4.5. Immunoglobulins and cytokines levels
The ileum samples were disrupted and homogenized in PBS buffer (1 g samples: 9 ml PBS). After centrifuging with a speed of 5000 g for 10 min, the supernatant was isolated. The IgG, IgM and sIgA concentrations were measured with an immunoturbidimetry using Enzyme-Linked Immunosorbent Assay kits (CEA544Rb, CEA543Rb, SEA641Rb, respectively; Cloud-Clone Corp, Wuhan, China). The IgG, IgM and sIgA in supernatant could combine the IgG, IgM and sIgA antibody in 0.02 mol/L phosphate buffer substrate turnover was monitored spectrophotometrically at 450 nm with an automated reader (BioTek, Winooski, Vt.). The levels of IgA, TNFα, IFN-γ and IL-6 were determined using Enzyme-Linked Immunosorbent Assay kits (CSB-E06946Rb, CSB-E06998Rb, CSB-E06925Rb, CSB-E06903Rb, respectively; CUSABIO, Qingdao, China). After 1 h of incubation at 37°C, samples were removed and the plates were washed with a PBS buffer. After that, 100 μL horseradish peroxidase, and 100 μL of tetramethylbenzidine substrate was added to each well. The mixture was incubated in the dark for 30 min at room temperature. Optical density value at 450 nm was measured by a Bio-Rad ELISA reader (Bio-Rad, Richmond, CA).
2.4.6. RNA isolation and analysis
Total RNA Extraction Reagent Kit (RNAiso Plus, Takara, Code No. 9108/9109) was used to ileum RNA Isolation, and qRT-PCR by SYBR® Premix Ex Taq™ II (Tli RNaseH Plus, Takara, Code No. RR820A) was performed as described previously (Liu et al. Citation2017). The PCR primers used in this study () were designed using a Premier 5.0 Software and synthesized by Ruibo Biological Engineering Co., Ltd. (Qingdao, China). The relative mRNA expression levels were calculated using the arithmetic formula 2−△△Ct (Livak and Schmittgen Citation2001).
Table 3. Gene-specific primers used for the analysis of rabbit gene expression.
2.5. Statistical analysis
All of the data collected were subjected to one-way ANOVA analysis with the Statistical Analysis Systems statistical software package (Version 8e, SAS Institute, Cary, NC), and the primary effect of HS treatments on the responses among the various groups, and Duncan’s test was used for multiple comparisons. Besides, the data are shown as the means and root mean errors (RMSE), P < .05 was statistically significant.
3. Results
3.1. Growth performance
The growth performance of the experimental rabbits is presented in . Dietary HS supplemental level had significant effect on ADFI, ADG, F/G, diarrhoea ratio and mortality ratio (P < .05) of meat rabbits, with the increase of addition level, the feed intake, F/G, diarrhoea ratio and mortality ratio decreased, and the FBW and ADG increased.
Table 4. Effects of Humulus scandens supplementation on growth performance and feed intake in rabbits.
3.2. Slaughter performance
Dietary HS supplemental level had significant effect on pre-slaughter body weight, commercial carcass weight, semi-clean carcass weight, full-clean carcass weight and kidney weight (P < .05) of experimental rabbits, the pre-slaughter body weight, commercial carcass weight, semi-clean carcass weight, full-clean carcass weight in the higher group (8% and 12% group) were higher than those of the control group. Besides, the kidney weight of the experimental group (4%, 8% and 12% group) with HS was significantly higher than those of the control group. Furthermore, HS supplemental of maternal diets had no influence on the heart weight, lung weight, liver weight or the commercial slaughter ratio, semi-clean carcass ratio, full-clean carcass ratio, heart index, lung index, liver index (P > .05; ).
Table 5. Effects of Humulus scandens supplementation on carcass traits of rabbits.
3.3. Immune organ development
As shown in , dietary HS supplemental level had no significant effect on thymus weight, spleen weight or thymus ratio, spleen ratio (P > .05) of experimental rabbits.
Table 6. Effects of Humulus scandens supplementation on immune organ development of rabbits.
3.4. Morphological structure of small intestine
The HS supplementation in the diet did not alter the villus height, crypt depth, villus width, mucosal layer thickness and muscle layer thickness (P > .05) of the small intestine ().
Table 7. Effects of Humulus scandens supplementation on intestinal morphology of rabbits.
3.5. Immune active compounds
The HS supplementation in the diet could improve the titre of the IgG, IgA and sIgA (P < .05) in serum and intestinal (). However, the titre of the TNF-α, IFN-γ and IL-6 in serum and intestinal was decreased by HS supplementation level increased. Compared with the control, the HS supplementation in the diet did not alter the level of IgM (P > .05) in serum.
Table 8. Effects of Humulus scandens supplementation on immune active compounds of rabbits.
3.6. Gene expression of physical barrier
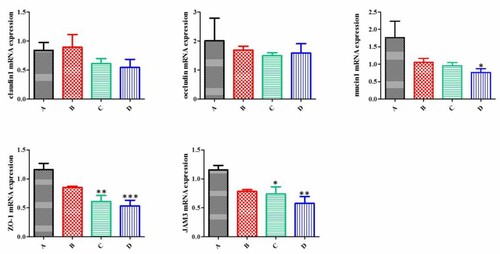
Agarose gel map for RNA quality was seen in . The HS supplementation in the diet did not alter the mRNA expression of claudin1 and occludin (P > .05) in the small intestine (P > .05, ). Compared with the control, the HS supplementation groups for 12% level could decrease the mRNA expression of ZO-1, JAM3 and mucin1 (P < .05).
Figure 1. Agarose gel map for RNA quality. A, B, C and D represent Humulus scandens were supplemented at 0, 4%, 8% and 12%, 1–6 represent different samples of this group.
Figure 2. Effect of Humulus scandens on mRNA expression of physical barrier gene in ileum of rabbits. Values are presented as the means ± std. error (n = 6). * indicates P < .05, ** indicates P < .001, *** indicates P < .0001. A, B, C and D represent Humulus scandens were supplemented at 0, 4%, 8% and 12%.
4. Discussion
As we all know, HS often grows wild in ditches, wastelands, ruins and forest edges, which adapts to various environmental conditions and can be harvested in large quantities in the autumn mature stage. HS has been used to treat inflammatory diseases in China. Luteolin is one of the flavonoids isolated from HS, it is a common flavonoid, which exists in many herbal medicines. Plants rich in luteolin have been used in traditional Chinese medicine to treat inflammatory disorders diseases (Ziyan et al. Citation2007; Lin et al. Citation2008). The main constituent of HS is fibre (). In our study showed that in rabbits of similar initial body weight (), the inclusion of HS in the diet decreased the ADFI and F/G in rabbits between 42 and 98 days of age. This result may be due to the difference in energy level in the four diets by different level with HS powder. Accordingly, the levels of fibre and fibre proportions in diet decreased when dietary HS levels increased (). The degree of lignification is the main factor responsible for a reduction in digestibility of raw feed and feedstuffs (Gidenne and Perez Citation1994). The cell wall lignification is lower in higher HS level (12%group) than that in control groups (). Thus, the lower fibre level should explain the higher energy and protein in diets containing HS and the subsequent reduction in ADFI. Carcass weight is closely related to live weight before slaughter, the results of slaughter ratio for rabbits showing no or few effects of fibre sources inclusion, which was agree with the previous studies (Dal Bosco et al. Citation2012; Margüenda et al. Citation2012). Guo et al. (Citation2008) reported that the mixture (50:50) of HS and alfalfa constitutes an alternative source of fibre for fattening rabbits and prevents diarrhoea effectively. In this study, we firstly investigate the addition effect of HS in rabbit production. After detecting the indicators of performance, intestinal morphology and intestinal barrier, the present study showed a positive effect of HS as rabbit feed resource.
The intestinal mucosal barrier is mainly composed of epithelial cells, tight junctions between adjacent enterocytes, and critical components of the mucosal immune system (Turner Citation2009). It acts as a selective barrier that allows the absorption and excretion of substances required for the homogenate state of the body and prevents the passage of various pathogens or their products. Under normal circumstances, epithelial cells allow only a small amount of intact antigens to enter the mucosa, where they interact with the mucosal immune system to reduce the inflammatory response. At the same time, intestinal pathogens can activate immune cells and initiate the inflammatory response required to eliminate infection (Chadwick et al. Citation2002). Under the condition of inflammatory bowel disease, the excessive penetration of antigen through the epithelial layer may lead to inappropriate immune stimulation and chronic gastrointestinal inflammation (Baumgart and Carding Citation2007). In addition, epithelial cells dilute, flush and bind harmful substances by secreting liquid and mucus and sIgA into the lumen, so as to play an important physiological defence role (Shang et al. Citation2008). sIgA is a major mucosal immune effector molecule, which provides an important first line of defence against pathogens (Woof and Kerr Citation2006). In line with the previous studies, rabbits after HS treatment increased the intestinal sIgA concentration. IgG is another important immunoglobulin, which can promote immune cells to phagocytize pathogens and neutralize bacterial toxins. In this study, the addition of HS to the diet increased the secretion of intestinal immunoglobulin in rabbits, which suggests HS treatment could increase the ability of immune cells to swallow pathogens and toxins.
Cytokines play an important role in the immune system and are also potential targets of immune regulation (Hill and Sarvetnick Citation2002). Some studies indicate that some herbs and plant extracts inhibit the synthesis of pro-inflammatory cytokines such as TNF-α and other cytokines related to inflammatory processes such as IFN-γ and IL6 (Pérez-Köhler et al. Citation2015). In our study, dietary addition of 12% HS decreased significantly the TNF-α, IFN-γ and IL6 concentration in ileum, which imply that the HS also had an inhibition in synthesis of cytokines, it is consistent with Aziz et al. (Citation2018), the luteolin in HS could regulate pro-inflammatory mediators by reducing the synthesis of a variety of pro-inflammatory cytokines (Aziz et al. Citation2018).
Tight junctions are assembled from at least three transmembrane proteins, including occludin, claudin and junction adhesion molecule (JAM), which are anchored to the cytoskeleton through peripheral membrane proteins, such as zonula occluden protein 1 (ZO1), ZO2 and ZO3 (Bazzoni et al. Citation2000). Mucin is a major glycoprotein expressed on the surface of breast epithelial cells and plays an important role in maintaining mucus protective layer and resisting inflammation. The main determinant of intestinal barrier function is intercellular tight junction (Martìn-Padura et al. Citation1998). These tight junctions proteins interact with other intracellular plaque ZO1, ZO2 and ZO3 proteins in turn anchor the transmembrane proteins to the actin cytoskeleton (Gonzalez-Mariscal et al. Citation2003). The association of tight junctions proteins with the perijunctional actin cytoskeleton ring is vital for maintaining the tight junctions structure and function (Gonzalez-Mariscal et al. Citation2003). RT–PCR analysis of the small intestine showed that the HS addition affects the gene expression of ZO-1, indicating that there may be improvement of the intestinal barrier mechanism.
5. Conclusion
Dietary supplement of Humulus scandens modulated immune responses via increasing the secretion of IgA and IgG and improved intestinal barrier might by regulating ZO1, JAM3 and mucin1 genes expression, meanwhile inhibit the synthesis of cytokine. These maybe cause low death rate of weaned rabbits, so our experiment offered a positive evidence of Humulus scandens as rabbit feed resource.
Ethics statement
The experimental procedures were approved by the Shandong Academy of Agricultural Sciences Animal Care and Use Committee (SAAS-2020-03) and were conducted in accordance with the Guidelines for Experimental Animals established by the Ministry of Science and Technology (Beijing, China).
Acknowledgement
The author thanks the staff of Shandong Zhengyu Rabbit Industry Co., Ltd. for their excellent support during this experiment.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- AOAC International. 2005. Official methods of analysis, 18th ed. Maryland: Association of Official Analytical Chemists.
- Aziz N, Kim MY, Cho JY. 2018. Anti-inflammatory effects of luteolin: a review of in vitro, in vivo, and in silico studies. J Ethnopharmacol. 225:342–352.
- Baumgart DC, Carding SR. 2007. Inflammatory bowel disease: cause and immunobiology. Lancet. 369:1627–1640.
- Bazzoni G, Martinez-Estrada OM, Orsenigo F, Cordenonsi M, Citi S, Dejana E. 2000. Interaction of junctional adhesion molecule with the tight junction components ZO-1, cingulin, and occludin. J Biol Chem. 275:20520–20526.
- Blasco A, Ouhayoun J. 1996. Harmonization of criteria and terminology in rabbit meat research. World Rabbit Sci. 4:93–99.
- Carabaño R, Badiola I, Chamorro S, García J, GarcíaRuiz AI, García-Rebollar P, Gómez-Conde MS, Gutiérrez I, Nicodemus N, Villamide MJ, et al. 2008. Review. New trends in rabbit feeding: influence of nutrition on intestinal health. Span J Agric Res. 6:15–25.
- Chadwick VS, Chen W, Shu D, Paulus B, Bethwaite P, Tie A, Wilson I. 2002. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology. 122:1778–1783.
- Chen Z, Meng S, Yang J, Nie H, Jiang H, Hu H, Meng F. 2012. Humulus scandens chemical constituents determination and pharmacological action research. Med Chem. 2(2):1–5.
- Dal Bosco A, Mourvaki E, Cardinali R, Servili M, Sebastiani B, Ruggeri S, Mattioli S, Taticchi A, Esposto S, Castellini C. 2012. Effect of dietary replacement with olive pomaces on the performance and meat quality of growing rabbits. Meat Sci. 92:783–788.
- De Blas C, Mateos GG. 2020. Feed formulation. In: Nutrition of the rabbit. Wallingford: CAB International p. 297–308.
- Fann MK, O'Rourke D, Aclam D. 2001. Normal bacterial flora of the rabbit gastrointestinal tract: a clinical approach. Semin Avian Exoti Pet. 10:45–47.
- Gidenne T, Perez JM. 1994. Dietary lignin in growing rabbits. I. Consequences on digestibility and rate of passage. Ann Zootech. 43:313–322.
- Gonzalez-Mariscal L, Betanzos A, Nava P, Jaramillo BE. 2003. Tight junction proteins. Prog Bio Mol Biol. 81:1–44.
- Guo W, Gu Z, Wei K, Liu Y, Wang Z. 2008. Effect of Humulus scandens for traditional fiber sources on digestion, diarrhea, and performance of growing rabbits. Agr Sci China. 8(4):497–501.
- Hill N, Sarvetnick N. 2002. Cytokines: promoters and dampeners of autoimmunity. Curr Opin Immunol. 14:791–797.
- Korkina LG, Afanas’ev IB. 1997. Antioxidant and chelating properties of flavonoids. Adv Pharmacol. 38:151–163.
- Li JJ, Wang XJ, Yi-Cheng FU. 2008. Study on chemical constituents of humulus scandens. Food Drug. 10(05):5–6
- Li QM, Yang XR, Zha XQ, Pan LH, Zang DD, Zhang FY, Luo JP. 2022. Protective effects of three flavonoids from Dendrobium huoshanense flowers on alcohol-induced hepatocyte injury via activating Nrf2 and inhibiting NF-κB pathways. Chem Biodivers. doi:10.1002/cbdv.202200471.
- Lin Y, Shi R, Wang X, Shen HM. 2008. Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr Cancer Drug Targets. 8:634–646.
- Liu GY, Wu ZY, Zhu YL, Liu L, Li FC. 2017. Effects of dietary vitamin B 6 on the skeletal muscle protein metabolism of growing rabbits. Anim Prod Sci. 57:2007–2015.
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C (T)) method. Methods. 25:402–408.
- Margüenda I, Nicodemus N, Vdddillo S, Sevilla L, García-Rebollar P, Villarroel M, Romero C, Carabaño R. 2012. Effect of dietary type and level of fiber on rabbit carcass yield and its microbiological characteristics. Livest Sci. 145:7–12.
- Martens L, Van Herck A. 2000. Performances of weaned rabbits raised in pens or in classical cages: First results. 7th World Rabbi congress; July 4–7; Valencia Spain Volume B. p. 435–440.
- Martìn-Padura I, Lostaglio S, Schneemann M, Williams L, Romano M, Fruscella P, Panzeri C, Stoppacciaro A, Ruco L, Villa A, et al. 1998. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol. 142:11–127.
- Nath R, Das C, Kityania S, Nath D, Das S, Choudhury MD, Patra JK, Talukdar AD. 2022. Natural flavonoids in the prevention and treatment of lung cancer: A pharmacological aspect. Comb Chem High T Scr. doi:10.2174/1386207325666220701121537.
- Neuhouser ML. 2005. Dietary flavonoids and cancer risk: evidence from human population studies. Nutr Cancer. 50:1–7.
- Pérez-Köhler B, García-Moreno F, Brune T, Pascual G, Bellón JM. 2015. Preclinical bioassay of a polypropylene mesh for hernia repair pretreated with antibacterial solutions of chlorhexidine and allicin: an in vivo study. PLoS One. 10:e0142768.
- Rios JL. 2010. Effects of triterpenes on the immune system. J Ethnopharmacol. 128:1–14.
- Shang L, Fukata M, Thirunarayanan N, Martin AP, Arnaboldi P, Maussang D, Berin C, Unkeless JC, Mayer L, Abreu MT, et al. 2008. Toll-like receptor signaling in small intestinal epithelium promotes B-cell recruitment and IgA production in lamina propria. Gastroenterology. 135:529–538.
- Talhouk RS, Karam C, Fostok S, El-Jouni W, Barbour EK. 2007. Anti-inflammatory bioactivities in plant extracts. J Med Food. 10:1–10.
- Turner JR. 2009. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 11(09):799–809.
- Urziceanu MM, Cîșlariu AG, Nagodă E, Nicolin AL, Măntoiu DS, Anastasiu P. 2022. Assessing the invasion risk of humulus scandens using ensemble species distribution modeling and habitat connectivity analysis. Plants. 11(7):857.
- Van Soest PJ, Robertson JB, Lewis BA. 1991. Methods for dietary fibre, neutral detergent fibre and non-starch polysaccharides in relation to animal nutrition. J Dairy Sci. 74:3583–3597.
- Woof JM, Kerr MA. 2006. The function of immunoglobulin A in immunity. J Pathol. 208:270–282.
- Zhang D, Tan LH, Feng YJ, Yao L, Yan XW, Cao WG. 2021. Evaluation of antioxidant, anti-inflammatory activity and identification of active compounds of Humulus scandens. S Afr J Bot. 141:126–132.
- Zhou G, Feng X. 2020. Study on feed processing and application of renewable plant Humulus scandens. IOP Conference Series: Materials Science and Engineering, 738.
- Ziyan L, Yongmei Z, Nan Z, Ning T, Baolin L. 2007. Evaluation of the anti-inflammatory activity of luteolin in experimental animal models. Planta Med. 73:221–226.
