ABSTRACT
This study addressed the different proportions of millet and sorghum silage biomass BRS 716 (BRS-716 silage) in the diet of crossbred heifers ¾ Holstein x ¼ Zebu on their nutrient intake, and digestibility, microbial protein synthesis, nitrogen balance, ingestive behaviour, and growth performance. Five experimental diets with 0, 25/75, 50/50, 75/25, and 100% of BRS-716 silage in compared to millet silage were evaluated. The roughage: concentrate ratio in the total dry matter (DM) of the diets was 75:25. The diets were evaluated in ten heifers with an initial body weight of 264.95 ± 19.4 kg (± SEM), following the experimental design in two 5 × 5 Latin squares, simultaneously. The total experimental period lasted 105 d, divided into five periods of 21 days, including a 17-d initial adaptation, and four for data collection, and samples. The increase of BRS-716 silage in the diet of crossbred dairy heifers did not change the dry matter intake (DMI), and indigestible neutral detergent fiber (iNDFI). For the purine derivatives, and microbial synthesis, there was no significant effect (P > 0.05). The average daily gain was 0.795 kg/d. The millet silage, and BRS-716 silage in the diet of crossbred Holstein × Zebu heifers used exclusively or combined in different proportions.
Highlights
The BRS-716 biomass sorghum silage has potential use in ruminant diet
The replacement of millet silage with BRS-716 biomass sorghum silage did not change dry matter intake
The replacement of millet silage with BRS-716 biomass sorghum silage maintains feed efficiency and daily weight gain of dairy heifers
Introduction
In Brazil, there is a growing need for forage alternatives with high productive potential and adapted to climatic conditions. Aiming at the sustainability of production systems with ruminant animals (Borges et al. Citation2019; Queiroz et al. Citation2021). In this sense, millet (Pennisetum glaucum (L.) R. Br.) is widely used in the world (Assis et al. Citation2017), and in Brazil, about five million hectares have been cultivated annually (Calegari et al. Citation2014; Carvalho et al. Citation2018). Due to the deep root system, capable of extracting nutrients and moisture from the soil, millet cultivation has stood out for its ability to grow under conditions of water stress (mean rainfall 400 mm). Furthermore, it is adapted to environments with high temperatures (up to 40°C), and presents an annual dry mass (DM) yield ranging from 10 to 20 t/ha (Assis et al. Citation2017), with good nutritional value (Carvalho et al. Citation2018).
In 2014, EMBRAPA Corn and Sorghum (Sorghum bicolor L. Moench) launched the biomass BRS 716 sorghum for the purpose of energy cogeneration through direct biomass burning, required by the thermoelectric, and sugar-cane industries. Some studies (Castro et al. Citation2015; Almeida et al. Citation2019) demonstrated the high productive potential of BRS 716 biomass sorghum, which presents high growth characteristics (up to six metres in height) and productivity of 50 t/ha DM, in two cycles. Furthermore, it is tolerant to pests, diseases, water deficit, and lodging. Due to these characteristics, this species is promising as a source of nutrients for ruminant animals in several regions of the world, including those with a semi-arid climate (Ramos et al. Citation2021; Queiroz et al. Citation2021).
Considering the important adaptability and dry mass yield of these two forage species, millet and biomass sorghum, to semi-arid conditions, it is of great relevance, the evaluation of their forage potential in the form of silage to feed ruminant animals. Moreover, forage plants with high growth can rapidly change cellular components, modifying nutrient intake, and animal performance (Monção et al. Citation2019; Monção et al. Citation2020; Queiroz et al. Citation2021).
Therefore, the objective of the study was to compare the effect of different proportions of millet BRS-716 silage in the diet of crossbred Holstein x Zebu heifers on their nutrient intake, and digestibility, microbial crude protein synthesis, nitrogen balance, ingestive behaviour, and growth performance.
Material and methods
This study was conducted in the Experimental Feedlot of the State University of Montes Claros (Unimontes), Janaúba (geographical coordinates: 15° 52’ 38 ‘South, 43 ° 20’ 05’ West), Minas Gerais, Brazil.
Animals, experimental design, and treatments
The study included ten crossbred heifers (264.95 ± 19.4 kg of body weight, BW; mean ± SD; averaging 14 mo old). The breed composition ranging from ¾ Holstein x ¼ Zebu. Heifers were allotted to 10 outdoor pens (3 m feedbunk length × 2 m width) having compacted soil surface. And equipped with concrete feedbunks and automatic waterers. All animals had the same origin and were selected from a herd of 30 heifers (Water ad libitum). Prior to the onset of the experiment, animals were vaccinated against clostridiosis (Vac Starvac, Basso Pancotte, Nova Alvorada, RS, Brazil), injected with A, D, and E vitamins supplement (ADE Injectable and Emulsifiable Pfizer, Zoetis, Morris County, NJ, USA). For deworming purposes also subcutaneously injected with 3.5% ivermectin (Ranger LA, Vallée S. A., São Paulo, SP, Brazil) with dosages adjusted according to BW.
The total experimental period lasted 105 d, divided into five periods of 21 days, including a 17-d initial adaptation, and four for data collection, and samples. Animals were assigned to an experimental design with two simultaneous 5 × 5 Latin squares, being five animals, five periods, and five treatments. Each heifer was considered as the experimental unit.
Dietary treatments were defined as follows: Treatment 1 - 100% of millet silage composing the roughage fraction of the diet (control); Treatment 2 - 75% of millet silage and 25% of BRS-716 silage; 3 - 50% of millet silage and 50% of BRS-716 silage; Treatment 4 - 25% of millet silage and 75% of BRS-716 silage; Treatment 5 - 100% BRS-716 silage composing the roughage fraction of the diet.
Crops, and feeding management
The sorghum used for silage production was Sorghum bicolour (L.) Moench cv. BRS 716 biomass and the millet used for silage production was Pennisetum glaucum (L) R. Br. ADR 500, cultivated at the Unimontes Experimental Farm. Sorghum was planted in an eutrophic clayey red-yellow latosol with the following chemical characteristics: pH in CaCl2, 6.0; P (Mehlich), 20.5 mg/dm3; K (Mehlich), 90 mg/dm3; Na (Mehlich), 0.1 cmolc/dm3; Ca 2+, 3.5 cmolc/dm3; Mg 2+, 1.2 cmolc/dm3; Al 3+, 0.0 cmolc/dm3; H + Al (0.5 mol/L of calcium acetate), 1.4 cmolc/dm3; cation exchange capacity of 6.7 cmolc/dm3; base saturation (V) of 72%. The soil was meshed and levelled mechanically using harrows attached to the tractor (Massey Ferguson 275 tractor; Massey Ferguson ® AGCO, Duluth, Georgia, USA). During planting 300 kg/ha of 04-30-10 (N-P-K) phosphate was used. Atrazine herbicide was used to control invasive plants. Millet (seeding rate of 10 kg/ha) and BRS-716 biomass sorghum (seeding rate of 7 kg/ha) were managed and harvested (direct-harvested) 122 and 164 days after planting (Queiroz et al. Citation2021), respectively. The dry matter yield of millet was 10.90 t/ha and that of biomass sorghum, 36.0 t/ha. For millet silage and BRS-716 silage, surface type silos were used. The cost of the silage produced was $/kg 0.03 for millet and $/kg 0.01 for BRS-716.
The diets were formulated according to the NRC (Citation2001) for heifers with an average of 265 kg of body weight (BW), average daily gain of 0.900 kg/d, and the roughage: concentrate ratio in the five experimental diets was approximately 75:25 on the basis of dry matter. The diets were supplied twice a day, at 07:00 h and at 14:00 h, in a complete diet system (Total mixed ration-TMR), homogenized in the trough ad libitum. The leftovers were collected and weighed daily, in the morning, before the first feeding, to adjust consumption and the quantity supplied was calculated based on the leftovers, which represented 5% of the total dry matter supplied.
Urea was used to correct the crude protein (CP) contents of the roughage fraction of the diets, using a single concentrate in the five experimental diets. To ensure the maintenance of the roughage: concentrate ratio in the total DM of the diets and that they were kept isoproteic, the DM contents, and CP of the roughages were analyzed weekly.
Feed analyses
On the 18th, 19th and 20th day of each experimental period, samples of the feed supplied, leftovers (refusals) and faeces were collected in the morning and stored in a freezer, −20°C. Afterwards, the samples were thawed, dried in a forced-air oven at 55◦C for 72 h, and ground with a Wiley mill (MA340, Marconi, Piracicaba, Brazil) to pass a 1-mm screen. The DM of 1-mm ground samples was determined with an oven at 105°C for 24 h (method 934.01; AOAC Citation1990), and organic matter (OM) was determined by difference after heating at 600°C in muffle for 2 h (Method 942.05; AOAC Citation1990). Nitrogen was determined according to AOAC (Citation1990) (method 972.43) using a micro Kjeldahl apparatus (TE-036/1 model, Tecnal, Piracicaba, SP, Brazil). The CP content was calculated by multiplying N content by 6.25. Ether extract (EE) was measured using a Soxhlet apparatus (TE-044 extractor, Tecnal, Piracicaba, SP, Brazil) based on extraction with petroleum ether for 6 h (method number 920.39; AOAC Citation1990). Lignin (cellulose solubilization with 72% w/w sulfuric acid) and NDFap were determined according to Van Soest et al. (Citation1991). For NDFap procedure (TE-149 fiber analyzer, Tecnal Laboratory Equipment Inc., Piracicaba, Brazil; INCT-CA F-002/1), a heat-stable α-amylase (A3306, Sigma Chemical. Co., St. Louis, MO, USA) was used without sodium sulfite nor ash- and protein-corrections. Acid detergent fiber (ADF) without ash and protein corrections was obtained as described in Goering and Van Soest (Citation1970). The indigestible neutral detergent fiber (iNDF) (INCT-CA F-008/1) and non-fibrous carbohydrates, following the recommendations described in Detmann et al. (Citation2012). The TDN was calculated according to Weiss et al. (Citation1992). The ingredients and chemical composition of experimental diets are in and .
Table 1. Proportion of ingredients and chemical composition of experimental diets.
Table 2. Chemical composition of ingredients (g/kg dry matter) used in the formulation of experimental diets.
Ruminal kinetics
After pre-drying, the silage samples were ground in mills equipped with sieves with 2 mm sieves and placed in non-woven fabric bags in the amount of approximately 3.0 g of dry matter (DM)/bag, in order to maintain a ratio close to 20 mg DM/cm2 of bag surface area. The incubation periods corresponded to times of 0, 3, 6, 12, 24, 48, 72, 96, 120, and 144 hours, with the bags being placed at different times to be removed all at the same time from the rumen. Two cannulated crossbred steers were used, with an average body weight of 580 ± 60 kg and mean age 8 years. The animals were adapted for 14 days to the diet containing 4 kg of concentrate (25% CP and 65% TDN), divided into two meals, morning and afternoon, in addition to the provision of roughage based on sorghum silage (50% millet silage and 50% BRS-716 silage). All the proportions of the silages used during the experiment were evaluated (100% of millet silage; 75% of millet silage and 25% of BRS-716 silage; 50% of millet silage and 50% of BRS-716 silage; 25% millet silage and 75% BRS-716 silage and 100% of BRS-716 silage).
After the incubation period, the nylon bags were washed in running water until it was clean, then drying. The DM determination was made in an oven regulated at 55°C, for 72 hours. In situ dry matter degradability data were obtained from the difference observed between the weights performed before and after ruminal incubation and expressed as a percentage.
As it is a first-order asymptotic growth model, which was reparametrized by subdividing the asymptote value into two fractions, ‘a’ and ‘B’, the DM degradation rates were calculated using the equation proposed by Ørskov and McDonald (Citation1979): Dt = a + B (1 - e-ct), where: Dt = fraction degraded over time ‘t’ (%), ‘a’ = soluble fraction (%); ‘B’ = potentially degradable insoluble fraction (%); ‘c’ = rate of degradation of fraction ‘B’ (/h); and ‘t’ = time (h).
The degradation of NDF was interpreted using the model of Mertens and Loften (Citation1980): Rt = B*e-ct + I, where: Rt = fraction degraded in time ‘t’ (%); I = undegradable fraction; and ‘B’, ‘c’ and ‘t’ as defined above. Fractions were standardized according to the proposition by Waldo et al. (Citation1972), according to equations: BP = B/(B + I) * 100; IP = I/(B + I) * 100; I = 100 - (a + b), where: BP = standardized potentially degradable fraction (%); IP = standardized non-degradable fraction (%); B and I = as defined above.
The nonlinear parameters ‘a’, ‘b’ and ‘c’ were estimated using least squares iterative procedures. The effective degradability (ED) of DM in the rumen were calculated using the model: ED = a + (B x c / c + k), where: k corresponds to the rate of passage of particles in the rumen, of according to the AFRC (Citation1993). For the ED of NDF, the model was used: ED = BP*c/(c + k), where BP is the standardized potentially degradable fraction (%).
Intake and digestibility of nutrients
Feed intake was monitored from d 1–105. Feed delivery was adjusted daily and fed to appetite allowing ad libitum intake and orts below 5% of daily intake. The feedbunk was cleaned and orts weighed daily before morning feeding. Feed offered and orts were sampled weekly and frozen at −20°C for further DM determination. The DMI was calculated daily per pen by subtracting orts from offered feed (on a DM basis). To estimate daily metabolizable energy intake (MEI), the ME concentration was multiplied by the DMI. The faecal dry matter production was estimated using indigestible neutral detergent fiber (iNDF) as an internal indicator. Samples of feed, leftovers and faeces were ground in a knife mill with a 2 mm diameter sieve and ruminally incubated in two cannulated crossbred adult cattle (480 ± 30 kg and 8 years mean age) for 288 hours, following the methodology (INCT-CA F-009/1) presented by Detmann et al. (Citation2012). The digestibility coefficient of all nutrients was calculated using the following equation: [quantity ingested - quantity excreted in the faeces]/quantity ingested. Based on the digestibility coefficients, the value of total digestible nutrients was calculated.
Nitrogen balance and microbial synthesis
Spot urine samples were obtained on the 18th day of each experimental period, approximately four hours after feeding in the morning, during spontaneous urination. 10 mL aliquots of this sample were filtered and immediately diluted in 40 mL of 0.036 N H2SO4 for further analysis of creatinine. These aliquots were stored in plastic flasks, identified and frozen for further analysis and quantification of urea, total nitrogen, creatinine, uric acid and allantoin.
Blood samples were collected on the first and last day of each experimental period, via puncture of the jugular vein, using 5 mL test eppendorf tubes (400 mm) (Vacutainer TM) with EDTA (anticoagulant). Immediately, centrifugation was carried out at 5000 rpm for 15 minutes and, subsequently, plasma samples were taken, which were packed in eppendorf and stored at −15°C for further analysis of urea.
The concentrations of urea, creatinine and uric acid in the urine and urea in the plasma were estimated using commercial kits (Bioclin, Belo Horizonte, Minas Geras, Brazil). The conversion of urea values into urea nitrogen was performed by multiplying the values obtained by the factor 0.4667.
The urinary contents of allantoin and uric acid were estimated by colorimetric methods, as specified by Chen and Gomes (Citation1992), and the total nitrogen content estimated by the Kjeldhal method (Detmann et al. Citation2012). The balance of nitrogen compounds (Nitrogen balance; g/day) was calculated as: N retained (g) = {N ingested (g) – N faecal (g) – N urine (g)}, where: Nitrogen balance = nitrogen retained in the animal's organism; N ingested = nitrogen ingested by the animal; N faecal = nitrogen excreted in faeces and N urine = nitrogen excreted in urine. The excretion of creatinine (mg/kg BW) was used to estimate urinary volume for each animal according to the equation described by Chizzotti et al. (Citation2008): EC = {32.27–0.01093 x BW}, where: EC = daily excretion of creatinine (mg/kg BW). Since, in growing animals, the percentage of muscle tissue varies according to body weight and, consequently, the excretion of creatinine (mg/kg of BW) can be altered. The total daily urinary volume was estimated by dividing the daily urinary excretions of creatinine by the observed values of creatinine concentration in the urine.
The excretion of total purines was estimated by the sum of the amounts of allantoin and uric acid excreted in the urine and the amount of absorbed purines (mmol/day), by the excretion of total purines (mmol/day), by means of equation proposed by Verbic et al. (Citation1990):AP = {(total purines − 0.385 × BW 0.75)/0.85}, where: AP = absorbed purines (mmol/day); 0.85 = recovery of purines absorbed as purine derivatives in the urine; and 0.385 = endogenous excretion of purine derivatives in the urine (mmol) per unit of metabolic size (0.75 BW).
To estimate microbial protein production (MCP), purine bases (mmol/day) were used as a microbial indicator, whose quantification was performed according to the technique of Chen and Gomes (Citation1992): MCP (g/day) = {(70 × AP) / (0.85 × 0.116 × 1000)}, assuming the value of 70 for the nitrogen content in the purines (mg/mmol); 0.83 for intestinal digestibility of microbial purines and 0.116 for the N PURINE: N TOTAL ratio in bacteria.
The microbial crude protein synthesis efficiency was calculated as follows: microbial crude protein synthesis efficiency = {(0.629 × AP) × 6.25)/TDN intake}, where: AP = absorbed purines (mmol/day); TDNI – total digestible nutrients intake; 0.629 represents the absorbed purine without considering the contribution of the endogenous fraction.
Determination of ingestive behaviour
Ingestive behaviour followed the method described in Monção et al. (Citation2020). Visual observations for each pen (n = 1) were recorded every 5 min during the 24-h cycle on d 19 and 20 of each experimental period. Different groups of two observers each were assigned every 5-h interval. Each observer was responsible for recording the ingestive behaviour of animals in 5 pens (5 animals). Pen observations were conducted sequentially, always following the same order per observer. Eating, ruminating, and total chewing times (min/d) were calculated by the number of observations multiplied by 5 min. Total chewing time was the sum of eating and ruminating times. Ingestive variables were also expressed as min/kg DMI. The rumination efficiency was calculated according to the methodology of Burger et al. (Citation2000).
Growth performance, and biometric measurements
At the beginning and at the end of each experimental period, body weight was measured after a 16-hour fast of solid feed using a mechanical scale (Valfran, Votuporanga, São Paulo, Brazil). Moreover, measurements were made of the thoracic perimeter (heart girth), withers and croup height (hip height), and body length. The measurements were made according to the methodology of Hoffman (Citation1997), with the animals in a forced station, that is, front and rear members perpendicular on a flat floor, forming a rectangular parallelogram. Feed efficiency was calculated by dividing weight gain (kg/day) with DM intake (kg/day).
Multivariate analysis
A principal component analysis (PCA) was applied to better understand the nature of the relationship between the studied variables and the independent variables (Treatments). For this analysis, 49 characteristics studied were considered. Based on the correlation matrix between the characteristics, data were subjected to PCA, in which the variables were standardized to mean equal to zero and variance equal to one. A correlation matrix was used instead of a covariance matrix (Johnson and Wichern Citation2002). Kaiser's method (Citation1960) was used to select which principal components best simplified the variability present in the data set and to compose the other analyses and interpretations.
Statistical analyses
Data were evaluated by analysis of variance using the MIXED procedure. Data normality (Shapiro–Wilk test at 5% probability) was verified by the UNIVARIATE procedure. The statistical model used for analyses was Yk(ijl) = μ + Pi + Aj + Ql + Tk(ijl) +PI + Ql + ek(ijl), where Yk(ijl) is the observation concerning the treatment ‘k’, within period I, animal j and Latin square (Q) l; μ is a constant associated with all observations; Pi is the effect of period i, with i = 1, 2, 3, 4 and 5; Aj is the animal effect j, with j = 1, 2, 3, 4, and 5; Ql is the Latin square effect l; Tk(ijl) is the treatment effect k, with k = 1, 2, 3, 4, and 5; PI is the initial body weight as a covariable and ek (ijl) is the experimental error associated with all observations (Yk (ijl)), which is independent and by hypothesis has a normal distribution with mean zero and variance δ2. The treatments (Tk(ijl)) were considered to be fixed effects; animals (Aj), experimental period (Pi), initial body weight and the error term (ek(ijl)) were random effects.
The ruminal degradability of DM and NDF was conducted in a randomized complete block design in subdivided plots, with five treatments (plots), 10 incubation times (subplots) and five replications. The variation of the animals’ body weight was the blocking factor. Ruminal fermentation variables were analyzed as repeated measures using the PROC MIXED, according to the following model: Yijklm = μ +Ai +Pj +Bk +αkl + ωijkl +Tm + T × Ami + ϵijklm,
where ωijkl ≈ N (0, α²ω) and ϵijklm ≈ MVN (0, R), and Yijklm = observation on animal l, given treatment i, at period j, in block k, in time m; μ, Ai, Pj, Bk, and αkl were previously defined; ωijkl = the residual error associated with cows within experimental period; Tm = the fixed effect of the sampling time (m = 1–10); T × Ami = the fixed interaction effect between the time and treatment; ϵijklm = random residual error; α²ω = the estimated variance associated with experimental units (cows within period); MVN = multivariate normal; and R = the variance-covariance matrix of residuals due to repeated measurements. Variance – covariance matrices were evaluated [UN, UN1, CS, CSH, AR(1), ARH(1), TOEP, TOEPH, FA(1), and ANTE(1)] and chosen by the Bayesian method. The covariance matrix that best fit the data according to the corrected Bayesian information criterion (BIC) was variance components (UN). When determined to be significant by the F test, the means of the treatments were compared by decomposing the sum of squares into orthogonal linear contrasts and quadratic effects at 5% probability, with subsequent adjustments to the regression equations. Outliers were identified and deleted if the absolute values of Studentized residuals exceeded ±3. The mean values were considered to be different when p < 0.05. For exploratory data analysis by principal component analysis (PCA), the PAST® 4.5 software was used (Hammer et al. Citation2001).
Results
Ruminal kinetics
The increase in the proportion of BRS-716 silage linearly reduced the readily soluble fraction (fraction ‘a’), potential degradability and effective degradability of DM at different passage rates 2, 5, and 8%/h (P < 0.01). For each percentage unit of inclusion of the BRS-716 silage, there was a reduction of 0.10, 0.11, 0.06, 0.07, and 0.08 percentage points (pp), respectively. The indigistible fraction of DM linear increased as proportion BRS-716 silage increased ().
Table 3. Ruminal kinetics of dry matter and fibrous fraction of different proportions of millet silage and BRS-716 silage.
For the standardized degradable potential insoluble fraction (Fraction Bp), degradation rate of fraction Bp ‘c’, standardized indigestible fraction (Ip) and effective degradability (k = 2, 5, and 8%/h) of NDF, the means were adjusted to fit the quadratic regression model. The maximum point of fraction Bp was estimated to be at an inclusion of 30% BRS-716 silage. The minimum point for the degradation rate of the fraction Bp ‘c’, and the indigestible fraction of NDF was estimated to be at a proportion of 30 and 32.5% inclusion of BRS-716 silage, respectively.
Intake and digestibility of nutrients
Diets with different proportions of BRS-716 silage and millet silage for crossbred dairy heifers did non-affected the dry matter intake (DMI; P = 0.99), crude protein intake (P = 0.99), NDF intake (P = 0.68), ether extract intake (P = 0.53), non-fibrous carbohydrates intake (P = 0.99), total digestible nutrients intake (P = 0.95), indigestible neutral detergent fiber intake (iNDFI; P = 0.63), and metabolizable energy intake (P = 0.88) (). The means observed for DMI and iNDFI were 7.85 and 1.18 kg/day, respectively.
Table 4. Nutrient intake and digestibility in crossbred Holstein x Zebu heifers fed diets containing different proportions of millet silage and BRS-716 silage.
The DMI and nutrients when expressed as a percentage of BW, was non-affected due to experimental diets, mean of 3.12% BW for DMI. There was a quadratic effect on the digestibility of the ether extract, with an increase in the proportion of BRS-716 silage in the diet. As for the other variables related to apparent digestibility, no differences were found between the diets (P > 0.05).
Nitrogen balance and microbial synthesis
The different proportions of BRS-716 silage and millet silage in the diet of crossbred dairy heifers did non-affected the daily nitrogen intake (N; mean of 132.42 g; P = 0.32; ).
Table 5. Nitrogen balance and microbial synthesis in crossbred Holstein x Zebu heifers fed diets containing different proportions of millet silage and BRS-716 silage.
For the retained nitrogen (Nitrogen balance; P = 0.42), expressed in grams and percentage of the nitrogen ingested, plasma urea nitrogen (P = 0.21) and urinary nitrogen (P = 0.82), there was no differences between experimental diets, with the means of 104.84 g/day, 75.18%, 17.66, and 0.86 mg/dL, respectively. Regarding the excretion of purine derivatives (P = 0.85) and microbial synthesis (P = 0.84), there was no significant effect due to diets with different proportions of BRS-716 silage and millet silage.
Ingestive behaviour
The feeding behaviour of crossbred Holstein x Zebu heifers was not influenced with the different proportions of BRS-716 silage and millet silage in the diet (). The means of feeding, rumination and idle time were 369.1, 495.5 and 575.4 minutes/day, respectively.
Table 6. Feeding behaviour in crossbred Holstein x Zebu heifers fed diets containing different proportions of millet silage and BRS-716 silage.
Body weight and biometric measurements
The final body weight (P = 0.89), weight gain (P = 0.08), feeding efficiency (P = 0.50) and the body measurements were non-affected (P > 0.05) by the replacement millet silage with BRS332 716 silage in the diets (). The final body weight and average daily weight gain of heifers were 278.46 and 0.795 kg/day, respectively.
Table 7. Body performance and biometric measurements in crossbred Holstein x Zebu heifers fed diets containing different proportions of millet silage and BRS-716 silage.
Multivariate analysis
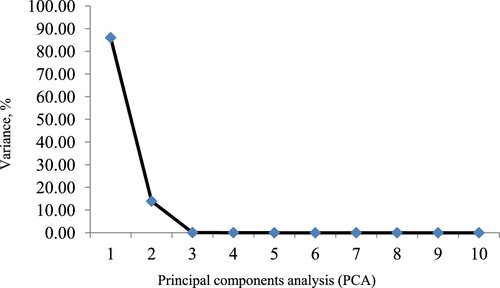
The highest eigenvalues were verified in PCA 1 and 2 (). These PCA explained 86.03%, and 13.91% of the total variation in results, respectively. Within PCA 1, the most important dependent variables were, in that order, nitrogen in the urine, crude protein digestibility, and dry matter digestibility. In PCA 2, the dry matter intake, %BW, was appointed as the most important dependent variable in the data variation. These main components explained 99.95% of the variation in the overall results.
Discussion
The DM content of millet silage (24.07%) and BRS-716 silage (29.75%) influenced the DM content of the experimental diets, with an increase as the BRS-716 silage inclusion. This increase in DM content in the diets compensated for the decrease in DM degradability with BRS-716 silage, favouring the maintenance of DMI, and nutrient intake.
The higher value of the soluble fraction ‘fraction a’ of DM in the diet with 100% millet silage can be explained by the greater proportion of soluble carbohydrates in this silage due to the presence of grains (Ramos et al. Citation2021). The higher proportion of fibrous components (NDF, ADF and iNDF) in the BRS-716 silage compared with the millet silage justify the results observed in the ruminal degradability of the silages. However, according to Van Soest (Citation1994), levels of ADF above 40% can impair the DMI due to reduced digestibility. However, in experimental diets with different proportions of BRS-716 silage and millet silage, the levels of ADF did not exceed this limit, maintaining the DMI and digestibility of DM by dairy heifers in the growing phase. Additionally, the NDF intake, which has a negative correlation with the DMI due to the effect of rumen filling, was similar between diets.
Among the factors that affect animal performance, the DMI is one of the main factors when compared to environmental and sanitary factors (NRC Citation2001). The similarity in the DMI and nutrients and in the digestibility of diets with different proportions of BRS-716 silage and millet silage demonstrates the forage potential of BRS-716 biomass sorghum as a roughage basis in the diet of dairy heifers. The amount of DM ingested observed in this study was of 7.86 kg/day or 3.12% of BW, having a direct relationship with the increase in body weight of the animal. The greater digestibility of the ether extract with the inclusion of BRS-716 silage may reflect the higher content of this nutrient in its chemical composition. Rigueira et al. (Citation2021) evaluated the performance of crossbred heifers fed different banana crop wastes verified a DMI of 9.8 kg/d in the sorghum silage diet, obtaining a daily average weight gain of 1 kg/d.
The similarity in the TDN intake and DMI, despite the greater concentration of fibrous components in the BRS-716 silage in comparison the millet silage, demonstrates that the BRS 716 biomass sorghum has compatible fiber quality to provide adequate animal performance. Corroborating with Ramos et al. (Citation2021), who studied the effect of replacing forage sorghum silage with BRS-716 biomass sorghum silage in diets for crossbred lactating cows. The authors concluded that although the BRS-716 silage has a higher content of components fibrous (NDF; 725.6 vs. 664.0 g/kg), its fiber is of satisfactory quality for adequate consumption. Furthermore, the authors observed that the replacement of forage sorghum silage with BRS 716 silage reduced DMI and dry matter digestibility, but did not alter the mean of milk yield (13.42 kg/day) and feed efficiency.
Even with the linear reduction of faecal nitrogen excretion, the nitrogen balance remained positive and similar between diets, as well as animal performance. The decrease in nitrogen excretion may result in less environmental impact and greater economic return on the production system by decreasing the use of nitrogen inputs. The nitrogen balance quantifies protein retention or loss by the animal, and is related to feed intake, when the nitrogen balance is positive it means that probably the protein requirements have been met (Gonçalves et al. Citation2014). Furthermore, despite the higher proportion of fibrous components and less energy availability with BRS-716 silage, the TDN intake and DMI were similar, justifying once again the similarity in growth performance results of crossbred Holstein x Zebu heifers.
The similarity for allantoin and uric acid excretions is due to the diets being isoproteic. According to Braga et al. (Citation2012), the total excretion of purine derivatives in the urine results from protein catabolism in ruminants and are important variables for estimating the amount of synthesized microbial protein. Microbial protein synthesis was similar between diets, due to similarities in nitrogen and energy intake between experimental diets (Van Soest Citation1994; Rigueira et al. Citation2021). The mean microbial efficiency verified in the diets was 130.5 g MCP/kg TDN, corroborating the NRC (Citation2001) which proposes the value of 130 g MCP/kg TDN intake. Microbial efficiency can allow an increase in the availability of microbial protein to be absorbed in the intestine, thus meeting the requirements of growing animals (Rigueira et al. Citation2021).
The variations in the NDF, ADF and iNDF contents between experimental diets with different proportions of millet silage and BRS-716 silage were not sufficient to affected the ingestive behaviour of heifers, which presented similar nutrients intake and digestibility values. It should be noted that the fibrous fraction is the component of the diet of main relevance under the activities of ingestive behaviour, especially for diets with a high participation of roughage feeds. In the growing phase of the heifers, provide a balanced diet to ensure gain enough weight to body development and early coverage is essential. In this study, the average daily weight gain of 0.80 kg/day is within the desirable range, demonstrating the potential use of millet silage and BRS-716 silage for this animal category.
Conclusion
Millet silage and BRS-716 silage in the diet of crossbred Holstein x Zebu heifers, used exclusively or combined in different proportions non-affected parameter dry matter intake and nutrients, digestibility, nitrogen balance, microbial synthesis, feeding behaviour, and growth performance.
Compliance with ethical standards
This study was conducted in the Experimental Feedlot of the State University of Montes Claros. Experimental protocol (no 215/2020) and animal-use procedures were approved and followed guidelines recommended by the Animal Care Committee of the same institution. The manuscript does not contain clinical studies or patient data.
Acknowledgments
The authors thank the Foundation for Research Support of the State of Minas Gerais (FAPEMIG, Brazil), Unimontes Janaúba (Experimental Farm, Brazil), the National Council for Scientific and Technological Development (CNPq, Brazil), EMBRAPA Corn and Sorghum, and Instituto Nacional de Ciência e Tecnologia (INCT – Ciência Animal, Brazil). The authors would like to thank the undergraduate students for their contribution towards the accomplishment of the research.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials.
Additional information
Funding
References
- Agricultural and food research council - AFRC. 1993. Energy and protein requirements of ruminants. Wallingford: CAB International, 159 p.
- Almeida LGF, Parrella RAC, Simeone MLF, Ribeiro PCO, Santos AS, Costa ASV, Guimarães AG, Schaffert RE. 2019. Composition and growth of sorghum biomass genotypes for ethanol production. Biomass Bioenergy. 122:343–348.
- Aoac international (association of official analytical chemists international). 1990. Official methods of analysis of the AOAC international, 15th ed. Arlington, VA: AOAC International.
- Assis RL, Freitas RS, Mason SC. 2018. Pearl millet production practices in Brazil: a review. Exp Agric. 54:699–718.
- Borges LDA, Rocha Júnior VR, Monção FP, Soares C, Ruas JRM, Silva FV, Rigueira JPS, Costa NM, Oliveira LLS, Rabelo WO. 2019. Nutritional and productive parameters of Holstein/Zebu cows fed diets containing cactus pear. Asian-Australas J Anim Sci. 18:1–12.
- Braga JMDS, Valadares RF, Pellizzoni SG, Valadares Filho SDC, Prates LL, Costa e Silva LF. 2012. Estimation of endogenous contribution and urinary excretion of purine derivatives from the total digestible nutrient intake in Nellore heifers. Rev Bras Zootec. 41:1899–1906.
- Burger PJ, Pereira JC, Queiroz AC, Silva JFC, Valadares Filho SC, Cecon PR, Casali ADP. 2000. Ingestive behavior in Dutch calves fed diets containing different concentrate levels. Rev Bras Zootec. 29:236–242.
- Calegari AG, Costa A, Lanillo RF, Casão Junior R, Santos DR. 2014. Conservation agriculture in Brazil. In: R. A. Jat, K. L. Sahrawat, A. H. Kassam, editor. In conservation agriculture: global prospects and challenges. Oxfordshire: CAB International; 54–88.
- Carvalho GGP, Freitas PMD, Santos EM, Araújo GGL, Oliveira JS, Pires AJV, Maranhão CMA, Rodrigues TCGC, Freitas Júnior JE, Rufino LMA, et al. 2018. Effect of pearl millet silage ammoniated with urea on lamb production and metabolic performance. Grass Forage Sci. 1:1–9.
- Casali AO, Detmann E, Valadares Filho SC, Pereira JC, Henriques LT, Freitas SG, Paulino MF. 2008. Influência do tempo de incubação e do tamanho de partículas sobre os teores de compostos indigestíveis em alimentos e fezes bovinas obtidos por procedimentos in situ. Rev Bras Zootec. 37:335–342.
- Castro FMR, Bruzi AT, Nunes JAR, Parrella RAC, Lombardi JMR, Albuquerque CJB, Lopes M. 2015. Agronomic and energetic potential of biomass sorghum genotypes. Am J Plant Sci. 06:1862–1873.
- Chen XB, Gomes MJ. 1992. Estimation of microbial protein supply to sheep and cattle basedon urinary excretion of purine derivatives – an overview of technical details (Occasional Publication). Aberdeen: Rowett Research Institute, International Feed Resources Unit.
- Chizzotti ML, Valadares Filho SC, Valadares RFD, Chizzotti FHM, Tedeschi LO. 2008. Determination of creatinine excretion and evaluation of spot urine sampling in Holstein cattle. Livest Sci. 113:218–225.
- Detmann E, Souza MA, Valadares Filho SC, Queiroz AC, Berchielli TT, Saliba EOS, Cabral LS, Pina DS, Ladeira MM, Azevedo JAG. 2012. Methods for food analysis = Métodos para análise de alimentos. Suprema, Visconde do Rio Branco, MG, Brasil (in Portuguese).
- Goering HK, Van Soest PJ. 1970. Forage fiber analysis (apparatus, reagents, procedures and some applications). ARS-USDA, Washington, DC. Agric. Handbook No. 379 [accessed 2020 Nov 5]. https://naldc.nal.usda.gov/download/CAT87209099/PDF
- Goncalves GS, Pedreira MS, Pereira MLA, Santos DO, Souza DD, Porto Junior AF. 2014. Nitrogen metabolism and microbial production of dairy cows fed sugarcane and nitrogen compounds. Revista Brasileira de Saúde e Produção Animal. 15:48–61.
- Hammer O, Harper DAT, Ryan PD. 2001. Past: Paleontological statistics software Packaged for education and data analysis. Version 1.94b. Palaentologia Electronica, 4:1-9. Disponível em: < http://folk.uio.no/ohammer/past/>. Acesso em: 2009.
- Hoffman PC. 1997. Optimum body size of Holstein replacement heifers. J Anim Sci. 75:836–845.
- Johnson RA, Wichern WD. 2002. Applied multivariate statistical analysis. New Jersey: Prentice Hall.
- Kaiser HF. 1960. The application of electronic computers to factor analysis. Educ. Psychol. Meas. XX:141–151. https://doi.org/10.1177/001316446002000116
- Mertens DR, Loften JR. 1980. The effect of starch on forage fiber digestion kinetics in vitro. J Dairy Sci. 63:1437–1446.
- Monção FP, Costa MAMS, Rigueira JPS,Moura MMA, Rocha VR, Gomes VM, Leal DB, Maranhão CMA, Albuquerque CJB, Chamone JMA. 2019. Yield and nutritional value of BRS Capiaçu grass at different regrowth ages. Semina Ciências Agrárias. 40:2045–2055. https://doi.org/10.5433/1679-0359.2019v40n5p2045
- Monção FP, Santana PF, Rocha Júnior VR, Ruas JRM, Rigueira JPS, Borges LDA, Menezes GCC, Sousa TES, Costa MD, Oliveira LLS, Queiroz FE. 2020. Nutritional efficiency of feed-restricted F1 Holstein/Zebu cows in early lactation. Trop Anim Health Prod. 52:141–149.
- Neter J, Wasserman W, Kutner MH. 1985. Applied linear statistical models. Regression, analysis of variance, and experimental designs, 2nd ed. Athens: Richard D. Irwin, Inc.1127 p.
- NRC. 2001. Nutrient requirements of dairy cattle, 7th rev. ed. Washington, DC: Natl. Acad. Press.
- Ørskov ER, McDonald I. 1979. The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. J Agric Sci. 92:499–503.
- Queiroz FE, Rocha Júnior VR, Monção FP, Rigueira JPS, Parrella RAC, Rufino LDA, Santos AS, Cordeiro MWS. 2021. Effect of row spacing and maturity at harvest on the fermentative profile, aerobic stability, and nutritional characteristics of biomass sorghum (BRS 716) silage in the semiarid region of Brazil. Rev Bras Zootec. 50:e20200254.
- Ramos JCP, Rocha Júnior VR, Monção FP, da Costa Parrela RA, Caxito AM, Cordeiro MWS, da Hora FF, de Assis Pires DA. 2021. Effect of replacing forage sorghum silage with biomass sorghum silage in diets for F1 Holstein × Zebu lactating cows. Trop. Anim. Health Prod. 53:1–12. https://doi.org/10.1007/s11250-020-02503-3
- Rigueira JPS, Jesus NG, Rocha Júnior VR, Monção FP, Costa NM, David GSS, Silva FV, Carvalho CCS. 2021. Effects of different banana crop wastes on nutrient intake and digestibility, microbial protein synthesis, feeding behavior, and animal performance of ¾ Holstein × Zebu heifers in a semiarid rangeland. Trop Anim Health Prod. 53:1–13.
- Van Soest PJ, Robertson JB, Lewis BA. 1991. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci. 74:3583–3597.
- Van Soest PJ. 1994. Nutritional ecology of the ruminant, 2nded. Ithaca, NY: Cornell University Press, 476 p.
- Verbic J, Chen XB, Macleod NA. 1990. Excretion of purine derivatives by ruminants. Effect of microbial nucleic acid infusion on purine derivative excretion by steers. J Agric Sci. 114:243–248.
- Waldo DR, Smith LW, Cox LE. 1972. Model of cellulose disappearance from the rumen. J Dairy Sci. 55:125–129.
- Weiss WP, Conrad HR, St. Pierre NR. 1992. A theoretically-based model for predicting total digestible nutrient values of forages and concentrates. Anim Feed Sci Technol. 39:95–110.