ABSTRACT
This study aimed to investigate the effects of different concentrations of in ovo injection of 25-hydroxycholecalciferol (25(OH)D3) on early growth, immune and tibia development in Daheng broilers. The results showed that 25(OH)D3 can significantly improve the hatchability of broilers and improve body weight (p < 0.05). The low concentration of 25(OH)D3 (8 μg/mL) not only significantly increased tibial fresh weight, tibial length, tibial diameter, thymus and liver weight and immune organ index (p < 0.05), but also significantly increased the content of bone metabolism-related factors OC and CaBP in serum and the expression levels of OC in the liver (p < 0.05). However, a high concentration of 25(OH)D3 group (16 μg/mL) significantly increased bursa of Fabricius weight and immune organ index (p < 0.05), the content of immune factor IL-2, IFN-γ and TLR15 in serum and the expression level of IFN-γ and TLR15 in the spleen (p < 0.05). Furthermore, the content of ash, calcium and phosphorus in the tibia was negatively correlated with the expression level of OC and positively correlated with the expression level of ALP (p > 0.05). Our data suggested that 25(OH)D3 has positive effects on the early growth, immune, bone development of broiler during the early stage, which is a supplement to improve disease resistance and skeletal development of broilers.
Highlights
25(OH)D3 can improve the hatchability of broilers and body weight.
25(OH)D3 have a beneficial effect on the immune system and tibia development of broilers at different time point.
High concentration of 25(OH)D3 has the better effect on increasing the content of calcium and phosphorus in tibia.
Introduction
It is well known that, unlike mammals, embryonic development in poultry takes place separately from the matrix, mainly in vitro, and that all the nutrients needed for embryonic development are provided by the breeding egg (Li et al. Citation2016). However, the supply of nutrients, especially energy is limited, and over time, the number of carbohydrates in the egg will gradually decrease, which will undoubtedly have an impact on the growth and development of the embryo. Meanwhile, a lack of nutrients in the breeding eggs can also interfere with the chicken’s early immune system response, which in turn affects the chicken’s ability to resist disease (Yi et al. Citation2005; Abbasi et al. Citation2017). Therefore, early nutritional supplementation is essential for the growth and development of broilers before and after hatching. Fortunately, in ovo injection technology has emerged to provide poultry embryos with specific nutrients, such as vitamins, amino acids and carbohydrates and so on, which thus can improve the growth performance, body weight, bone and immune systems of poultry (Kornasio et al. Citation2011; Nowaczewski et al. Citation2012; Elnesr et al. Citation2019; Taha et al. Citation2019; Zhu et al. Citation2019). Therefore, the in ovo injection technology is widely used in the poultry industry.
25-hydroxycholecalciferol (25(OH)D3) is not only one of the active metabolites of vitamin D3 but also the storable and stable form of vitamin D3 in birds’ circulation (Klasing Citation1998; Holick and Chen Citation2008). As early as 1995, Ward (Citation1995) indicated that the addition of 25(OH)D3 in commercial broiler diets was generally considered safe. A large number of studies have reported the effects of dietary 25(OH)D3 supplementation on the egg production and egg quality of laying hens (Akbari Moghaddam Kakhki et al. Citation2019; Chen et al. Citation2020a). Moreover, 25(OH)D3 was found to be more effective than D3 in improving production performance, breast meat production and reducing inflammation (Morris et al. Citation2014; Vignale et al. Citation2015; Fatemi Citation2016). The reason may be due to its long half-life, which can enhance the absorption of calcium in the gut or increased calcium storage in muscle tissue (Morris et al. Citation2014; Burild et al. Citation2016). Meanwhile, other studies have shown that diets supplemented with 25(OH)D3 not only produced a better cellular immune response in broilers (Gómez-Verduzco et al. Citation2013) but also improves vitamin D status and stimulates breast muscle growth in broilers by increasing skeletal muscle satellite cells proliferation (Avila et al. Citation2022). Similarly, egg injection of 25(OH)D3 reduced embryonic mortality and improved the performance of broilers in the early postnatal period (Fatemi et al. Citation2020). In Swiatkiewicz et al. (Citation2006), they found that dietary supplementation with 25(OH)D3 decreased tibia dysplasia. In contrast, however, another study has shown that 25(OH)D3 has no effect on bone development and mineralization in broilers (Bello et al. Citation2014).
Therefore, given the above advantages of dietary 25(OH)D3 supplementation on the growth and development of poultry, based on previous studies, we injected 25(OH)D3 at the early embryo stage to investigate the effects of different concentrations of 25(OH)D3 on immunity, bone development, and calcium and phosphorus content in broilers. The findings provide a theoretical foundation for the application of 25(OH)D3 in the early development of poultry.
Materials and methods
Experimental design and egg incubation
A total of 410 incubating eggs of Daheng broiler without shell abnormalities or malformations were used. Daheng broiler is a specialized meat-type breed by Sichuan Daheng Poultry breeding Company (Chengdu, China) through native chickens from Sichuan and Guangdong provinces of China. It is a breed with stable production performance and good meat quality (Zhou et al. Citation2017; Li et al. Citation2020). All eggs were incubated in a microcomputer automatic incubator-KFP (Dezhou Keyu Incubation Equipment Co. Ltd., Shandong, China) with a temperature of 37.8 ± 0.1℃ and relative humidity of 60% in the Animal Genetics and Breeding Laboratory of Sichuan Agricultural University. Every egg in the incubator was turned every 1.5 h until the 19th day of incubation during incubation. On day 16 of incubation, the dead embryos or unfertilized eggs were removed after candling for eggs. On day 17.5 of incubation, the remaining 400 embryonated eggs with similar weight were randomly divided into four groups, the different groups were treated as follows: (1) the non-injected group was not injected with any substance (KB); (2) the control group was only injected with sterilized water (NC); (3) the treatment group was injected with 8 μg/mL 25(OH)D3 (D1); (4) the treatment group was injected with 16 μg/mL 25(OH)D3 (D2). These concentrations of 25(OH)D3 were determined according to the preliminary experiment in our laboratory and previous literature.
In ovo injection
In ovo injection was performed on a sterile table on day 17.5 of incubation. Briefly, the blunt end of the labeled egg was first wiped and disinfected with 75% ethanol and then gently poked a small hole with a diameter of about 0.1 cm with tweezers. After that, a 1 mL disposable syringe and an 18-gauge needle were quickly used to inject 0.1 mL of warm injection (about 30℃) of different concentrations into the amniotic cavity through the small hole (the depth of the injection was about 2.49 cm). Finally, the pinhole was sealed with solid paraffin wax and transferred to the hatching tray at 37.2℃ under 70% humidity for further incubation until the hatching.
Feeding management
After hatching, a total of 200 healthy male 1-day-old chicks were divided into 4 treatment groups, 5 replications, and 10 chicks in each replication. All chicks were housed in a battery brooder and raised under a standard commercial broiler-rearing environment at the poultry breeding farm of Sichuan Agricultural University. Namely, the temperature of the brood house was maintained at 32–34℃ for the first week, and then the temperature reduced by 1–2℃/week until it reached 21℃. The relative humidity was set at 50% throughout the study. Meanwhile, the basal diet of all chicks was composed of corn-soybean meal-type during the feeding period () and the chicks had free access to water and commercial diet under the 23 h lighting. The trial lasted for 28 days.
Table 1. Composition and nutritional level of basal diet from 1 to 28 days of age.
Sample collection
Blood samples were taken from the wing vein (12 chickens/treatment/time point) on days 1 (d1), day 14 (d14) and day (d28) post-hatch. After the blood was collected, the serum samples were obtained by centrifugation at 3000 rpm for 6 min at 4℃ and then frozen at −20℃ for further analysis. Subsequently, the chicks were euthanized, the thymus, bursa of Fabricius, spleen and liver were collected. About 2–3 g of each of the tissue samples mentioned above were placed into RNAase-free EP tubes as well as frozen in liquid nitrogen, and then transferred to a −80℃ refrigerator for subsequent analysis. In addition, the right tibia of each group of chicks was separated from the femur at the knee joint and ankle joint. After removing the muscle and tendons on the surface of the tibia, the tibia was weighted, and the length and middle diameter of the tibia was measured with a Vernier caliper.
Determination of production performance
After 21 days of incubation, the number of hatching squabs was recorded. Moreover, the body weight (BW) was recorded for each treatment group on d1, d7, d14, d21 and d28. After that, the hatchability of fertilized eggs was calculated. The formulae are as follows: the hatchability of fertilized eggs (%) = 100% × number of hatching squabs/the number of fertilized eggs.
Immune organ index
On days 1, 14 and 28, thymus, bursa of Fabricius and liver (with the attached tissue removed) weight of each chick measured respectively (12 chicks/treatment/time point), and then the immune organ index was calculated. The formula is as follows: the immune organ index (%) = organ weight/live weight before slaughter × 100%.
Serum biochemical indexes
Blood samples were collected from 12 chicks in each treatment group on days 1, 14 and 28 respectively, and were placed at room temperature for 6 h, followed by centrifuged at 3000 r/min for 15 min. The precipitated serum was absorbed into the new 1.5 mL centrifuge tube, and the content of interleukin-2 (IL-2), interferon-γ (INF-γ), osteosclerotic gene (OC), alkaline phosphatase (ALP), Toll-like receptor 15 (TLR15) and calcium-binding protein (CaBP) in serum was detected by using ELISA kits purchased from Jiangsu Baolai Biotechnology Co., Ltd (Nanjing, Jiangsu, China) according to the instruction of the manufacturer. Absorbance was measured at 450 nm by a Varioskan Lux multimode microplate reader (Thermo Scientific, Wilmington, DE, USA).
The tibia indicators
For tibia strength, the right tibia of different days of age was measured by WD-1 electronic universal testing machine (Changchun nonmetallic material testing machine factory, Changchun, China). Three different points of the tibia were taken for testing. In brief, the tibia was placed under the probe of the tester, the distance between the two fulcrum points was 30 mm, and the upper compression head was uniformly loaded onto the surface of the tibia at a speed of 10 mm/min to record the strength of the tibia. Bone strength was expressed in Newtonian (N). After the determination, the tibia was placed in a marked sealed plastic bag and frozen at 4℃ for subsequent analysis of bone ash content, calcium and phosphorus.
For the content of tibia ash, calcium and phosphorus, the defatted tibia bones were dried at 105°C in an oven for 24 h, soaked in 99.5% purity ether for 96 h, and dried at 65°C for 4 h to a constant weight. The crushed tibia was through a 40-mesh sieve and then put in a muffle furnace at 600°C for 24 h, and the residues were weighed and expressed in %. In addition, the content of Ca and P in ash was determined on an inductively coupled plasma emission spectrometer (Agilent, Victoria, Australia).
RNA extraction and real-time quantitative PCR
Total RNA was isolated from the spleen and liver of chicks using RNAiso Plus (Takara Biotechnology Co. Ltd, Dalian, China) according to the manufacturer’s protocol. The purity and quality of RNA were determined by Nanodrop 2000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA) at 260 and 280 nm. RNA with the OD260/OD280 values between 1.8 and 2.0 was reverse transcribed into cDNA using the PrimeScriptTM RT reagent Kit (Takara Biotechnology Co. Ltd, Dalian, China) following the manufacturer’s instructions. About 25 μL cDNA was stored at −20℃ for further real-time PCR. The mRNA expression levels of INF-γ, OC, ALP and TLR15 in the spleen or liver were determined with SYBR® Premix Ex TaqTM II (Takara Biotechnology Co. Ltd, Dalian, China) on CFX96-TouchTM Real-time PCR System (Bio-Rad Laboratories, CA, USA). The reaction system contained: 5.0 μL of SYBR Premix Ex Taq (2×), 0.5 μL of forward and reverse primer respectively (10 μM), 1.0 μL of cDNA and 3.0 μL of sterile, double-distilled water. Protocols were set as follows: initial denaturation at 98℃ for 4 min, 40 cycles of denaturation at 98℃ for 15 s, annealing for 20 s at X℃ (X is determined by the annealing temperature of different primers in ) and extension for 10 s at 72℃. The primers for the above genes were designed using Primer Primer 5 () and synthesized by Sangon Biotech (Shanghai, China). All samples were run in triplicate. The glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the housekeeping gene to calculate relative mRNA levels using the 2-△△Ct method (Livak and Schmittgen Citation2001).
Table 2. The primers sequences of Real-time PCR.
Statistical analysis
The experimental data were collected by Excel 2019, one-way ANOVA was performed by SPSS software (version 22 for windows, SPSS Inc, Chicago, USA) with ANOVA program, and the means of different treatment groups were compared using Duncan’s multiple comparisons. Pearson’s correlation analysis test was used to analyse the relationship between the expression level of bone calcium and phosphorus metabolism genes in the liver and the contents of enzymes and hormones in serum. Meanwhile, the overall data are expressed as the mean ± SEM, and differences were considered statistically significant at p < 0.05.
Results
Hatchability and weight
The effects of in ovo injection of 25(OH)D3 on hatchability are given in . Compared with the KB and NC group, the hatchability in the 25(OH)D3 group was significantly increased (p < 0.05). The hatchability in the 25(OH)D3 group was about 7.9% or 10.1% higher than that of the KB and NC groups. Therefore, we suggested that in ovo injection of a certain amount of vitamin could improve the hatchability.
Table 3. Effect of in ovo injection of 25(OH)D3 on hatchability.
Higher chick BW was observed in ovo injection of 25(OH)D3 groups in comparison with the KB and NC groups at each time point (p < 0.05, ). The BW of the 25(OH)D3 group (8 μg/mL) reached the maximum at different days of age, which was significantly higher than that of the high concentration group (16 μg/mL), especially at days 28, its BW was 589.275 g, which was 28.6% and 32.7% higher than that of the KB and NC group, respectively. Moreover, the interaction between time and treatment type also had a positive effect on mean body weight (p < 0.001).
Table 4. Effect of in ovo injection of 25(OH)D3 on live body weight of broiler from 1 to 28 days of age.
Immune organ index
According to , compared with the KB and NC groups, the immune organ weight of chicks at different days of age was significantly increased with the injection with 25(OH)D3 (p < 0.05). The weight of thymus and liver was reduced by increasing concentration of 25(OH)D3, but increased the weight of bursa of Fabricius (p < 0.05). Interestingly, the thymus immune organ index decreased significantly with the increase of 25(OH)D3 concentration on days 1 and 14, but increased significantly on day 28 (p < 0.05). However, the change in liver immune organ index was opposite to that of the thymus index, that is, it increased significantly on days 1 and 14, but decreased significantly on day 28 (p < 0.05). Different with thymus and liver immune organ index, the bursa of Fabricius index was increased significantly with the increase of 25(OH)D3 concentration at different time points (p < 0.05). Additionally, the interaction between time and treatment type also had a significantly positive effect on both immune organ weight and index (p < 0.001).
Table 5. Effect of in ovo injection of 25(OH)D3 on immune organs weight and immune organ index.
Tibial index and its calcium and phosphorus content
As illustrated in , the weight, strength, length and diameter of tibia were significantly improved in the 25(OH)D3 injection group compared with the KB and NC group (p < 0.05). Among them, the weight, length and diameter of tibia decreased significantly with the increase of 25(OH)D3 concentration (p < 0.05), on the contrary, the tibial strength increased with the increase of 25(OH)D3 concentration at 14 and 28 days. Meanwhile, with the increase of 25(OH)D3 concentration, the contents of ash, calcium and phosphorus in tibia also significantly increased (p < 0.05). Curiously, the contents of tibia ash, calcium and phosphorus were lower in the low-concentration 25(OH)D3 group than that in the KB and NC groups (p < 0.05). In addition, we found that all tibial indexes increased significantly with the increase of time (p < 0.05) and reached the maximum value on 28 days. Besides, the interaction between time and treatment type had no significant effect on weight and length of tibia (p = 0.364 and p = 0.438, respectively), but had a significant effect on strength, diameter, ash, calcium and phosphorus of tibia (p < 0.01).
Table 6. Effect of in ovo injection of 25(OH)D3 on the tibia.
Analysis of immune and bone metabolism-related factors and hormones in serum
Overall, compared with the KB and NC groups, the contents of IL-2, IFN-γ and TLR15 in serum of broilers in 25(OH)D3 injection group were significantly improved at each time point (, p < 0.05). Meanwhile, the contents of IL-2, IFN-γ and TLR15 in the high concentration group (16 μg/mL) were significantly higher than those in the low concentration group (8 μg/mL) (p < 0.05), but there was no significant difference between KB and NC group (p > 0.05). Together, these results suggest that a high concentration of 25(OH)D3 (16 μg/mL) increased the levels of immunomodulatory factors IL-2, IFN-γ and TLR15 in serum.
Figure 1. Effect of in ovo injection of 25(OH)D3 on serum IL-2, IFN-γ, TLR15, CaBP, OC and ALP contents. (1) A: serum IL-2 content; B: serum IFN-γ content; C: serum TLR15 content; D: serum CaBP content; E: serum OC content; F: serum ALP content; (2) KB: blank group; NC: control group; D1: injection of 8 μg/mL 25(OH)D3 group; D2: injection of 16 μg/ml 25(OH)D3 group; (3) * and ** indicate significant and highly significant differences, respectively (p < 0.05, p < 0.01).
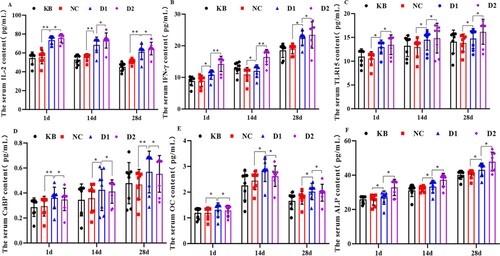
For the contents of hormones and enzymes related to bone metabolism in serum, the results showed that the level of CaBP, OC and ALP were significantly improved in the 25(OH)D3 group compared with the KB and NC group (p < 0.05, ). Among them, the contents of CaBP and OC in the low-concentration group (8 μg/mL) were significantly higher than those in the high-concentration group (16 μg/mL) (p < 0.05). On the contrary, the content of ALP in the low-concentration group was significantly lower than that in the high-concentration group (p < 0.05, ). Furthermore, there was no significant difference between the KB and NC groups (p > 0.05). These results suggest that in ovo injection different concentrations of 25(OH)D3 can improve the content of hormones and enzymes related to bone metabolism in serum of broilers, to promote bone development.
Expression of immune and bone metabolism-related genes in the spleen and liver
We also detected the expression of immune-related genes IFN-γ and TLR15 in the spleen. The results showed that 25(OH)D3 injection significantly improved the expression level of IFN-γ and TLR15 in the spleen compared with KB and NC groups (p < 0.05) (A and B). Meanwhile, with the increase of 25(OH)D3 concentration, the expression of IFN-γ and TLR15 in the spleen also increased, and the difference between high concentration and low concentration was significant (p < 0.05). However, there was no significant difference in the expression of the two genes between the KB and NC groups (p > 0.05).
Figure 2. Effect of in ovo injection of 25(OH)D3 on the expression of IFN-γ, TLR15 in spleen and OC, ALP in the liver (1) A: The expression of IFN-γ in the spleen; B: The expression of TLR15 in the spleen; C: The expression of OC in the liver; D: The expression of ALP in the liver; (2) KB: blank group; NC: control group; D1: injection of 8 μg/mL 25(OH)D3 group; D2: injection of 16 μg/mL 25(OH)D3 group; (3) * and ** indicate significant and highly significant differences, respectively (p < 0.05, p < 0.01).
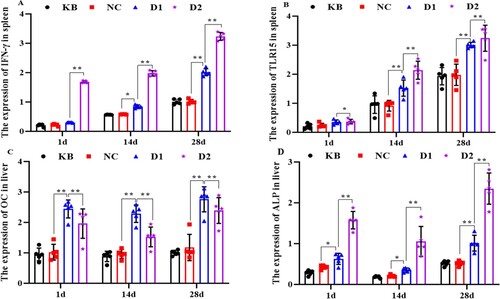
The expression of genes OC and ALP related to calcium and phosphorus metabolism in the liver is shown in (C and D). The expression level of OC significantly decreased with the increase of 25(OH)D3 concentration, while the expression level of ALP was opposite to the expression level of OC, that is, it significantly increased with the increase of 25(OH)D3 concentration, and the expression of two genes was significantly different between the high concentration group (16 μg/mL) and the low concentration group (8 μg/mL, p < 0.01). However, there was no significant difference in the expression of the two genes between the KB and NC groups (p > 0.05). Therefore, these results suggest that the injection of low or high concentrations of 25(OH)D3 can effectively increase the expression levels of OC and ALP in the liver of broilers, thus regulating the bone calcium and phosphorus metabolism.
Correlation analysis
To further verify the previous results, we analysed the correlation between the expression levels of immune and bone metabolism-related genes in tissues and serum hormone levels and tibia indices. The results showed that the expression level of IFN-γ and TLR15 in the spleen was negatively correlated with the content of IL-2 in serum (p > 0.05), but both had a significant positive correlation with the content of IFN-γ and TLR15 in serum (p < 0.01) (). Furthermore, the expression levels of OC and ALP in the liver were significantly positively correlated with the content of ALP and CaBP in serum (p < 0.05 and p < 0.01, respectively). Interestingly, tibia ash content, calcium and phosphorus content were negatively correlated with the expression level of OC but positively correlated with the expression level of ALP (p > 0.05). Also, the expression level of OC and ALP was positively correlated with tibial strength and tibial diameter (p < 0.01 and p < 0.05, respectively). In addition, there is a very significant positive correlation between the tibia index including contents of ash, calcium, phosphorus, tibia weight, tibia strength, length and diameter and the contents of OC, ALP and CaBP in serum (p < 0.01) ().
Table 7. Effect of in ovo injection of 25(OH)D3 on the correlation between the expression of IFN-γ and TLR15 in spleen and the serum IL-2, IFN-γ and TLR15 contents.
Table 8. Effect of in ovo injection of 25(OH)D3 on the correlation of calcium and phosphorus metabolism indexes in bone.
Discussion
Our results show that in ovo injection of 25(OH)D3 alone improved hatchability percentages of fertile eggs and body weight compared to the KB and NC groups. This result is consistent with that of Wang et al. (Citation2021), who found that dietary supplementation of 25(OH)D3 and VD3 eutectic also can significantly increase the ADG of meat duck from 1 to 35 days. Also, some studies have reported that dietary supplementation of 25(OH)D3 can increase growth performance and daily gain of broilers, and improve the hatchability and immunity of hens (Yarger et al. Citation1995; Bello et al. Citation2014; Saunders-Blades and Korver Citation2015; Vazquez et al. Citation2018). However, another study states that compared with the control group, adding 25(OH)D3 into the diets did not affect the weight gain, growth performance and feed efficiency during the breeding period, but reduced chicken feed intake during the grower period (Chou and Chuang Citation2009; Hutton et al. Citation2014). The reason for the inconsistent results may contribute to differences in 25(OH)D3 concentration and growth stages. In conclusion, the injection of 25(OH)D3 has a certain protective effect on the early development of chicken embryos.
The weight of immune organs and the index of immune organs are important indexes to evaluate the immune performance of poultry. The relative size of organ index can indicate the strength of its function to some extent (Qi and Zhu Citation2015). When the weight or index of immune organs is reduced, it means immune suppression; otherwise, it means immune enhancement. In the present study, the injection of 25(OH)D3 greatly improved the characteristics of the immune index such as weight and index of early immune organs in broilers. As the largest immune organ, the spleen plays an important role in cellular and humoral immunity in poultry (Zhu et al. Citation2019). In adult poultry, the spleen provides immune defense in two main ways, one is blood filtration, which involves phagocytosis of damaged cells and antigens; the other is the production, maturation and storage of lymphocytes responsible for humoral (antibody-mediated) and cellular immune response (Smith and Hunt Citation2004). Cytokines such as IL-2, IL-4 and IFN-γ and so on play an important role in poultry immunity, so their mRNA expression level is often used as an indicator of spleen immune status (Parkin and Cohen Citation2001; Alkhalifa Citation2015). In this study, a high-concentration injection group of 25(OH)D3 (16 μg/mL) could increase the level of IL-2 and IFN-γ in serum and the expression levels of IFN-γ in the spleen. This shows that 25(OH)D3 has a critical effect on the resistance to inflammatory disease in poultry. Our results are consistent with previous reports that indicated that 25(OH)D3 culture significantly increased the level of IL-2, IL-6, IL-10 and IFN-γ in mouse bone marrow-derived dendritic cells (Yang et al. Citation2012). Moreover, Li et al. (Citation2017) reported that the level of 25(OH)D3 in hypothyroidism patients is significantly correlated with the level of IL-2, IFN-γ and IL-4, suggesting that 25(OH)D3 may affect the immune function of the body by affecting the secretion of cytokines. Furthermore, toll-like receptors (TLRs) are innate immune receptors that are responsible for recognizing the conserved components of pathogens and play a key role in the early detection of infection (Medzhitov Citation2001), and TLR15 is one of them. In this study, the expression level of the TLR15 gene was the highest in the high concentration 25(OH)D3 group and was significantly higher than that in other treatment groups. Therefore, based on this result, we think that the high concentration of 25(OH)D3 could increase the innate immunity of poultry, to improve disease resistance of poultry.
For poultry, the tibia is an important organ for growth and production, parameters related to tibia development including bone strength, bone morphology, calcium and phosphorus content and density are critical to our understanding of leg abnormalities. The development of the tibia, especially the epiphysis, has been extensively studied in broilers (Williams et al. Citation2000; Applegate and Lilburn Citation2002), turkeys (Burs et al. Citation2008), geese (Charuta et al. Citation2014) and duck (Zhang et al. Citation2019). However, to our knowledge, there are few studies on the effects of vitamin injection into embryonic eggs on tibia development and its calcium and phosphorus content. Previous studies have shown that dietary supplementation of 25(OH)D3 can improve the bone health of laying hens, thus helping to prolong the laying period (Silva Citation2017). Furthermore, Sakkas et al. (Citation2019) found that femur and tibia mineralization could be improved when diets were supplemented with a high concentration of 25(OH)D3 (3000 IU/kg). Wang et al. (Citation2020) also found that dietary 25(OH)D3 led to better connectivity of tibial bone trabeculae and more complete tibial structure, which increased tibial strength. In addition, Chen et al. (Citation2020b) also showed that the dietary addition of 25(OH)D3 can promote bone growth and increase bone volume of laying hens, which has a positive effect on bone quality. Similar to the results observed in this study, supplementation with 25(OH)D3 increased tibial breaking strength, body weight and plasma concentration of 25(OH)D3 on d 21 in broilers (Levya-Jimenez et al. Citation2019). In addition, in our study, compared with the KB, NC and low concentration groups (8μg/mL), the contents of ash, calcium and phosphorus in tibia were significantly increased with the injection of high concentration of 25(OH)D3 (16μg/mL) group, which is consistent with the results of Zhang et al. (Citation2017, Citation2019), that he also found that dietary supplementation of 25(OH)D3 could significantly increase tibia weight, ash, calcium and phosphorus content of tibia in broilers. Therefore, 25(OH)D3 supplementation can promote the increase of calcium, phosphorus and ash content in tibia at an early stage of broilers, which lays a solid foundation for the development of the tibia at a later stage.
ALP is one of the biochemical indicators reflecting bone metabolism (Van Straalen et al. Citation1991). When osteoblasts are active, bone metabolism is vigorous and bone ALP secretion is increased. Moreover, since 50% of serum ALP comes from bone, serum ALP levels will also increase. Meanwhile, Osteocalcin (OC) is a marker of osteoblasts, which can also reflect the body's bone metabolism to some extent (Farrugia et al. Citation1989). Previous studies have shown that genetic polymorphisms in the OC gene are associated with serum OC levels and bone fractures (McGuigan et al. Citation2010). Our studies suggested injecting 25(OH)D3 increased the concentration of serum ALP, OC and CaBP and the expression level of liver ALP and OC, indicating that 25(OH)D3 can promote skeletal health.
Conclusion
In conclusion, under the conditions of this experiment, 8 μg/mL injection of 25(OH)D3 in an egg could significantly improve early body weight, thymus, liver, tibia strength, tibia length and tibia diameter, while 16 μg/mL injection of 25(OH)D3 in eggs can significantly increase tibia calcium and phosphorus contents. As a result, 8 μg/mL 25(OH)D3 supplementation is optimal for early bone development and broiler health. Although injection of 25(OH)D3 opens up a new field for early broilers, the molecular mechanism of the effect of in ovo injection of 25(OH)D3 on early immunity and tibia development of broilers needs further investigation. In addition, more studies are needed to determine the safety and efficacy of in ovo injection of 25(OH)D3.
Ethic approval
This study follows the guideline for animal use and management at Sichuan Agricultural University. All experimental protocols involving animals were approved by the Animal Welfare Committee in the College of Animal Science and Technology of Sichuan Agricultural University. Permit number is 2018-14.
Acknowledgment
We thank the Daheng poultry Breeding Company and the Farm Animal Genetic Resources Exploration and Innovation Key Laboratory of Sichuan Province of Sichuan Agricultural University for providing care for our laboratory animals and maintaining the research instruments.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Abbasi T, Shakeri M, Zaghari M, Kohram H. 2017. Growth performance parameters, bone calcification and immune response of in ovo injection of 25-hydroxycholecalciferol and vitamin K3 in male ross 308 broilers. Theriogenology. 90:260–265.
- Akbari Moghaddam Kakhki R, Heuthorst T, Mills A, Neijat M, Kiarie E. 2019. Interactive effects of calcium and top-dressed 25-hydroxy vitamin D3 on egg production, eggshell quality, and bones attributes in aged lohmann LSL-lite layers1. Poult Sci. 98(3):1254–1262.
- Alkhalifa H. 2015. Immunological techniques in avian studies. World Poultry Sci J. 72:573–584.
- Applegate TJ, Lilburn MS. 2002. Growth of the femur and tibia of a commercial broiler line. Poult Sci. 81:1289–1294.
- Avila LP, Leiva SF, Abascal-Ponciano GA, Flees JJ, Sweeney KM, Wilson JL, Meloche KJ, Turner BJ, Litta G, Waguespack-Levy AM, et al. 2022. Effect of combined maternal and post-hatch dietary 25-hydroxycholecalciferol supplementation on broiler chicken pectoralis major muscle growth characteristics and satellite cell mitotic activity. J Anim Sci. 100(8):skac192.
- Bello A, Bricka RM, Gerard PD, Peebles ED. 2014. Peebles effects of commercial in ovo injection of 25-hydroxycholecalciferol on broiler bone development and mineralization on days 0 and 21 posthatch. Poult Sci. 93:1053–1058.
- Burild A, Lauridsen C, Faqir N, Sommer HM, Jakobsen J. 2016. Vitamin D3 and 25-hydroxyvitamin D3 in pork and their relationship to vitamin D status in pigs. J Nutr Sci. 5:e3.
- Burs M, Zdybel A, Faruga A, Laskowski J. 2008. Effect of housing conditions on the mechanical strength of the femur and tibia in turkeys. Med Weter. 64:202–206.
- Charuta A, Tatara MR, Grużewska A, Pierzchala M, Kalinowski L, Trusewicz M, Łuszczewska-Sierakowska I. 2014. Morphological and densitometric research of the tibial bone in the postnatal development in domestic geeses. Anim Sci Pap Rep. 32(3):251–260.
- Chen C, Turner B, Applegate TJ, Litta G, Kim WK. 2020a. Role of long-term supplementation of 25-hydroxyvitamin D (3) on egg production and egg quality of laying hen. Poult Sci. 99(12):6899–6906.
- Chen C, Turner B, Applegate TJ, Litta G, Kim WK. 2020b. Role of long-term supplementation of 25-hydroxyvitamin D3 on laying hen bone 3-dimensional structural development. Poult Sci. 99(11):5771–5782.
- Chou S, Chuang T. 2009. Effects of supplemental hydroxycholecalciferol on growth performance, small intestinal morphology, and immune response of broiler chickens. Poult Sci. 88:2333–2341.
- Elnesr SS, Elwan HAM, Xu QQ, Xie QQ, Xie C, Dong XY, Zou XT. 2019. Effects of in ovo injection of sulfur-containing amino acids on heat shock protein 70, corticosterone hormone, antioxidant indices, and lipid profile of newly hatched broiler chicks exposed to heat stress during incubation. Poult Sci. 98:2290–2298.
- Farrugia W, Fortune CL, Heath J, Caple IW, Wark JD. 1989. Osteocalcin as an index of osteoblast function during and after ovine pregnancy. Endocrinology. 125:2785–2790.
- Fatemi SA. 2016. University of Alberta; Edmonton, Canada: 2016. Effects of Dietary 25-hydroxycholecalciferol and Vitamin D3 on Performance, Meat Yield, Bone Characteristics, Innate Immune Response and Gene Expression of Ross 308 Broilers Grown on Reused or Fresh Litter. M.Sc. Diss.
- Fatemi SA, Elliott KEC, Bello A, Durojaye O, Zhang H, Turner B, Peebles ED. 2020. The effects of in ovo injected vitamin D3 sources on the eggshell temperature and early posthatch performance of ross 708 broilers. Poult Sci. 99:1357–1362.
- Gómez-Verduzco G, Morales-López R, Avila-Gozàlez E. 2013. Use of 25 hydroxycholecalciferol in diets of broiler chickens: effects on growth performance, immunity and bone calcification. Poult Sci. 50:60–64.
- Holick MF, Chen TC. 2008. Vitamin D deficiency: a world-wide problem with health consequences. Am J Clin Nutr. 87:1080s–1086S.
- Hutton KC, Vaughn MA, Litta G, Turner BJ, Starkey JD. 2014. Effect of vitamin D status improvement with 25-hydroxycholecalciferol on skeletal muscle growth characteristics and satellite cell activity in broiler chickens. J Anim Sci. 92:3291–3299.
- Klasing KC. 1998. Comparative avian nutrition. Cambridge, UK: University Press.
- Kornasio R, Halevy O, Kedar O, Uni Z. 2011. Effect of in ovo feeding and its interaction with timing of first feed on glycogen reserves, muscle growth, and body weight. Poult Sci. 90:1467–1477.
- Levya-Jimenez H, Gardner K, AL-Jumaa Y, Padgett JC, Bailey CA. 2019. Partial replacement of dietary cholecalciferol with 25-hydroxycholecalciferol on broiler chickens subjected to a coccidiosis vaccine challenge. J Appl Poult Res. 28:743–754.
- Li J, Yang C, Peng H, Yin H, Wang Y, Hu Y, Yu C, Jiang X, Du H, Li Q, Liu Y. 2020. Effects of slaughter age on muscle characteristics and meat quality traits of Da-heng meat type birds. Animals (Basel). 10(1):69.
- Li S, Zhi L, Liu Y, Shen J, Liu L, Yao J, Yang X. 2016. Effect of in ovo feeding of folic acid on the folate metabolism, immune function and epigenetic modification of immune effector molecules of broiler. Br J Nutr. 115:411–421.
- Li Y, Xu L, Zhou YH, Ouyang XY, Cao T. 2017. Combination of periodontal combination of periodontal, orthodontic and endodontic therapy in upper anterior teeth with hopeless prognosis and long-time follow-up: a case report. J Peking Univ (Health Sci). 49(4):740–744.
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (T) (-delta dlta C) method. Methods. 25:402–408.
- McGuigan F, Kumar J, Ivaska KK, Obrant K, Gerdhem P, Akesson K. 2010. Osteocalcin gene polymorphisms influence concentration of serum osteocalcin and enhance fracture identification. J Bone Miner Res. 25:1392–1399.
- Medzhitov R. 2001. Toll-like receptors and innate immunity. Nat Revs Immunol. 1(2):135–145.
- Morris A, Shanmugasundaram R, Lilburn MS, Selvaraj RK. 2014. 25 hydroxycholecalciferol supplementation improves growth performance and decreases inflammation during an experimental lipopolysaccharide injection. Poult Sci. 93:1951–1956.
- Nowaczewski S, Kontecka H, Krystianiak S. 2012. Effect of in ovo injection of vitamin C during incubation on hatchability of chickens and ducks. Folia Biol (Krakow). 60:93–97.
- Parkin J, Cohen B. 2001. An overview of the immune system. Lancet. 357:1777–1789.
- Qi Q, Zhu D. 2015. Research progress of vitamin D effect on the immune system. China Modern Doctor. 53(22):157–160. (In Chinese).
- Sakkas P, Smith S, Hill TR, Kyriazakis I. 2019. A reassessment of the vitamin D requirements of modern broiler genotypes. Poult Sci. 98:330–340.
- Saunders-Blades JL, Korver DR. 2015. Effect of hen age and maternal vitamin D source on performance, hatchability, bone mineral density, and progeny in vitro early innate immune function. Poult Sci. 94:1233–1246.
- Silva FA. 2017. Effects of dietary 25-hydroxycholecalciferol on growth, production performance, eggshell quality and bone traits of brown egg layer housed under commercial conditions. Edmonton: University of Alberta.
- Smith KG, Hunt JL. 2004. On the use of spleen mass as a measure of avian immune system strength. Oecologia. 138:28–31.
- Swiatkiewicz S, Koreleski J, Kopowski J. 2006. Effect of phytase and 25-hydroxycholecalciferol on performance and bone quality in broiler chickens. Med Weter. 62(1):81–84.
- Taha AE, Abdallah OA, Attia KM, Abd El-Karim RE, Abd El-Hack ME, El-Edel MA, Saadeldin IM, Hussein EOS, Swelum AA. 2019. Does in ovo injection of two chicken strains with royal jelly impact hatchability, post-hatch growth performance and haematological and immunological parameters in hatched chicks? Animals (Basel). 9:486.
- Van Straalen JP, Sanders E, Prummel MF, Sanders GT. 1991. Bone-alkaline phosphatase as indicator of bone formation. Clin Chim Acta. 201(1-2):27–33.
- Vazquez JR, Gómez GV, López CC, Cortés AC, Díaz AC, Fernández SRT, Rosales EM, Avila AG. 2018. Effects of 25-hydroxycholecalciferol with two D3 vitamin levels on production and immunity parameters in broiler chickens. J Anim Physiol Anim Nutr. 102(1):e493–e497.
- Vignale K, Greene ES, Caldas JV, England J, Boonsinchai N, Sodsee P, Pollock ED, Dridi S, Coon CN. 2015. 25-Hydroxycholecalciferol enhances male broiler breast meat yield through the mTOR pathway. J Nutr. 145:855–863.
- Wang H, Liu Y, Zhang K, Ding X, Bai S, Wang J, Peng H, Xuan Y, Su Z, Zeng Q. 2021. Effects of 25-hydroxyvitamin D3 and vitamin D3 eutectic on growth performance, immune function and tibia development of meat ducks. Chinses J Anim Nutr. 33(3):1469–1481. (Chinese).
- Wang J, Qiu L, Gong H, Celi P, Yan L, Ding X, Bai S, Zeng Q, Mao X, Xu S, et al. 2020. Effect of dietary 25-hydroxycholecalciferol supplementation and high stocking density on performance, egg quality, and tibia quality in laying hens. Poult Sci. 99(5):2608–2615.
- Ward NE. 1995. Research examines use of 25-OH vitamin D3 in broiler diets. Feedstuffs. 67:53–62.
- Williams B, Solomon S, Waddington D, Thorp B, Farquharson C. 2000. Skeletal development in the meat-type chicken. Br Poult Sci. 41:141–149.
- Yang HF, Zhang ZH, Xiang LB, Tang KL, Luo F, Liu CY, Zhou JB, Li JQ, Xu JZ. 2012. 25(OH)D(3) affects the maturation and function of mouse bone marrow-derived dendritic cells stimulated by mycobacterium bovis BCG. PLos One. 7(11):e48062.
- Yarger JG, Saunder CA, McNaughton JL, Quarles CL, Holis BW, Gray RW. 1995. Comparison of dietary 25-hydroxycholecalciferol and cholecalciferol in broiler chickens. Poult Sci. 74:1159–1167.
- Yi G, Allee G, Knight C, Dibner J. 2005. Impact of glutamine and oasis hatchling supplement on growth performance, small intestinal morphology and immune response of broilers vaccinated and challenged with eimeria maxima. Poult Sci. 84:283–293.
- Zhang JL, Zhang N, Yang X, Chen GH, Wang ZX, Qu HX, Han JC, Chen JC. 2017. Effects of 25-hydroxyvitamin D3 on growth performance, bone mineralization and intestinal vitamin D receptor gene expression in broilers. China Feed. 24:24–29. (In Chinese).
- Zhang LH, He TF, Li M, Hu J, Piao X. 2019. Effects of dietary calcium and phosphorus levels and supplementation of 25-hydroxycholecalciferol on performance and bone properties of broiler starters. Arch Anim Nutr. 73(6):445–456.
- Zhou YG, Xiong Y, Yang CW, Jiang XS, Ran JS, Jin J, Wang Y, Lan D, Ren P, Hu YD, Liu YP. 2017. Experimental verification of CAPN1 and CAST gene polymorphisms in different generation of Da-heng broilers. BioMed Res Int. 2017:7968450.
- Zhu YF, Li SZ, Sun QZ, Yang XJ. 2019. Effect of in ovo feeding of vitamin C on antioxidation and immune function of broiler chickens. Animal. 13(9):1927–1933.
