ABSTRACT
The effects of cold and heat shocks on serum electrolyte and biochemical changes in Panagasianodon gigas. Cold shock was conducted as follows: 25–22°C, 25–19°C and 25–13°C; and heat shock: 25–28°C, 25–31°C and 25–37°C. The lowest Na+ was found in the group exposed to 25–13°C and 25–37°C, while the highest Na+ was observed in the 25–31°C group. The K+ significantly increased with decreases in water temperature. The Cl- was significantly decreased and increased when decreasing and increasing water temperature, respectively. The serum CO2 level was affected by cold shock but was not affected by heat shock. The highest and lowest Na+/K+ ratio was found in the group exposed to 25°C–31°C and 25°C–13°C, respectively. Moreover, the Na+/Cl- ratio showed a similar trend to that of the Na+/K+ ratio. Furthermore, the highest (Na+ + K+)/Cl- ratio was found in 25–31°C group. The highest glucose was found in all the cold shock groups. Albumin, globulin, total protein, triglyceride and cholesterol levels were significantly decreased after exposure to cold and heat shocks. These results demonstrated that 25–28°C is the appropriate temperature range for this species whilst other ranges influenced biochemical and physiological indices.
Introduction
Nowadays, extreme global weather events have been occurring more often than in past decades. Air temperatures in 2020 were only the second warmest on record, behind 2016, with at 0.98°C above the twentieth-century average. Also, every month of 2020, with the exception of December, was one of the top four warmest on record (Liberto Citation2021). Moreover, the ocean has been heating up at a rate of 0.06°C per decade over the past century (Stevens Citation2020). Extreme weather events in Thailand have included both heat and cold shocks and have occurred in the northern part of Thailand. Data collected from 1951 to 2018 revealed that the coldest month on record was 2 January 1974 at −1.4oC in Sakon Nakhon province and the hottest as 44.6oC on 28 April 2017, at Mae Hong Son province (Meteorological Department Citation2017). Besides, Thailand is located in a tropical area between latitude 5° 37′ N to 20° 27′ N and longitude 97° 22′ E to 105° 37′ E. March to May is the hot season of the year, especially in April. The effects of cold and heat shocks have affected fishery resources, especially aquatic animals. According to Dalvi et al. (Citation2017) found that some biochemical indices of Horabagrus brachysoma after acclimatized at different temperatures for 30 days were significantly increased with increasing water temperatures. For heat shock experiment (30°C, 300 min), oxygen consumption and plasma cortisol concentrations of Carassius auratus were significantly increased when compared to the control group (17°C) (Wang et al. Citation2019). Besides, Phrompanya et al. (Citation2021) revealed that water temperature changes (heat and cold shocks) evoked oxidative stress (malondialdehyde), histological injury, neutral and acidic mucous cells on the gills of Oreochromis niloticus. In addition, Sutthi et al. (Citation2022) exhibited that some biochemical indices, cortisol level and electrolyte changes in Chao Phraya catfish, Pangasius sanitwongsei were affected by cold shock. According to reports, subtropical and tropical regions are more susceptible to climate change or extreme weather events (Knutson et al. Citation2010; Clusella-Trullas et al. Citation2011). For example, 16 years ago in Phayao province (located in the northern part of Thailand), a rapid decrease in air temperature, or cold shock, happened from 25 to 27 January 2016, the coldest period on record. The air temperature dropped from 25°C to 8°C within 9 h (Meteorological Department Citation2016). Which resulted in huge economic damage, especially with regard to fishery resources in Phayao Lake (Kwan Phayao). Many kinds of fish died and one variety effected was Pangasianodon gigas. No report was done on the effects of heat or cold shocks on the responses of biochemical and physiological indices in P. gigas. This species is economically valuable in Southeast Asia because it has a high nutritional content and is rich in protein, carbohydrates and omega-6 fatty acids (Mengumphan and Saengkrachang Citation2008) and is listed in IUCN red list as critically endangered (CR) status (Hogan Citation2015). This species is the largest freshwater fish in the world, up to 3 m of a total length and up to 300 kg of body weight was found. P. gigas is one endemic fish in the Mekong river basin but in the present time, this species is extremely scarce in natural waterways and has almost become extinct in the wild (Sukumasavin Citation2006). However, P. gigas is now being cultured and sold in the fresh market in Thailand, more expensive than Nile tilapia (Oreochromis niloticus) as 2–3 times. Besides, studies done on the effects of cold and heat shocks on O. niloticus revealed that serum biochemistry, hematology and cortisol levels were changed significantly (Panase et al. Citation2018; Panase et al. Citation2019); this was also found to be true for Albula vulves when subjected to cold shocks (7°C and 14°C below ambient temperature), the physiological and behavioural were changed (Szekeres et al. Citation2014). Normally, the biochemical and physiological response studies of aquatic animals are focused on glucose, albumin, globulin, total protein, triglyceride and cholesterol because they are primarily associated with pathways of energy product or cellular respiration (Schreck Citation2000) and serum electrolytes are indicators of the performance of homeostatic mechanisms in the body (Pollock et al. Citation2014; Takvam et al. Citation2021). Especially, Na+, K+, and Cl− are performed significantly in osmoregulation and homeostasis in aquatic animals (Davis Citation2004; Tavares-Dias et al. Citation2008)
Although Pangasianodon gigas is important to commercial and conservative sectors, there is limited information available on their biochemical and physiological responses when they are exposed to a rapid decrease and increase in water temperatures (cold and heat shocks). Hence, this experiment was conducted to improve our understanding of the effects of rapid decreases and increases in water temperatures on their biochemical and physiological functions. The serum biochemistry (glucose, albumin, globulin, total protein, triglyceride and cholesterol), serum electrolyte (Na+, Cl-, K+ and CO2), mathematical curve fitting to the polynomial equation of each parameter were investigated. Moreover, it was hoped that suitable temperatures for culturing this species might be discovered through the course of this experiment, based on biochemical and physiological indices; this could also help to prevent economic losses when this fish faces extreme weather events.
Materials and methods
Collection and acclimation of Pangasianodon gigas
Healthy fish (55.4 ± 8.9 g mean body weight) were purchased from a commercial fish farm in Phrae province, Thailand. After being transferred to the laboratory, the experimental fish were acclimatized in the plastic tanks for 1 week under a natural photoperiod, in a continuous aeration and water recirculation system weekly. During the acclimatization, the observed water quality parameters were: the temperature at 25.1 ± 1.59°C, dissolved oxygen (DO) concentration 7.30 ± 1.5 mg/l, pH 7.2 ± 0.43 and Conductivity 0.3 ms/cm3 (multi-probes, HORIBA, U50 series). The experimental fish were fed twice daily (08.00; 17.00) with commercial fish feed at 3% body weight per day (28% crude protein).
Experimental design
A completely randomized design (CRD) was used in this experiment, 7 water temperature levels were assigned (13°C, 19°C, 22°C, 25°C, 28°C, 31°C, and 37°C). For cold shock experimentation, water temperatures were rapidly decreased from 25°C to 13°C by chiller Hailea, HS-90A. For heat shock experimentation, water temperatures were rapidly increased from 25°C to 37°C using a combination of heater (MIN JIANG, MJ-HC500) and hot water. In both cold and heat studies, the water temperatures were changed an average of 3°C per 1 h. All of the different levels were conducted using an automatic program setting. Throughout the process, water tanks were oxygenated using the aerator. During the process, the water quality in each tank was monitored using multi-probes, HORIBA, U50 series, Japan.
Serum biochemistry study
Nine fish (three fish/replicate) from each degree level were randomized to collect blood samples. Serum preparation, 0.8–1.0 ml individual blood sample was obtained from the caudal vein using non-heparinized syringes, the blood samples were transferred in micro-centrifuge tubes and allowed to clot for 4 h at room temperature (25°C), then spun down at 5000 rpm for 15 min at 25°C, supernatants were equally divided into 2 parts and placed in sterile serum tubes at −20°C until used (not for more than 3 days). The frozen serum samples were transferred to the Chiang Mai Veterinary Laboratory Centre limited Partnership, Chiang Mai, Thailand for serum analysis. The first part of the serum sample used for biochemical indices, such as glucose, albumin, globulin, protein, triglyceride and cholesterol, were investigated blood serum analyzer (P400 and PC400, HORIBA, Japan).
Serum electrolyte study
Other parts of serum sample used for the serum electrolytes study such as Na+, Cl-, K+ and CO2 were determined using the principle of bottom-read reflectance spectrophotometry, with an IDEXX dry-slide, which used a catalyst one chemistry analyzer (IDEXX laboratories, Inc., Westbrook, Maine, USA). In this study, monovalent ratios {Na+/K+, Na+/Cl-, (Na+ + K+)/Cl-} were also evaluated.
Statistical analysis
Before the statistical analysis, the normality and homogeneity of variance were evaluated. Data analysis involved a one-way analysis of variance (ANOVA) followed by a Tukey’s test at a significance level of 95% (p < 0.05). SPSS software version 17.0 for windows (SPSS Inc., Chicago, USA) was used in this analysis. All data were presented as mean ± SD. After clustered column chart of each parameter was generated, a mathematical curve fitting to find out the suitable polynomial equation was determined by Excel program. In this study, the R2 nearest to 1 is used as the best suitable equation.
Results
Serum electrolyte study
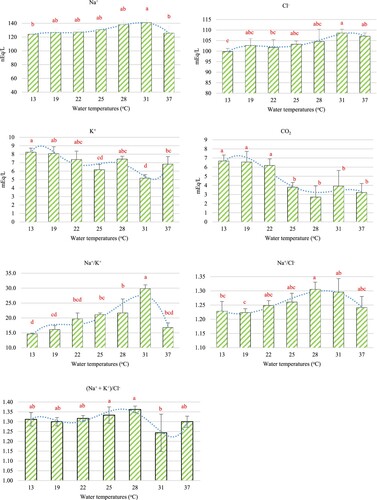
The four serum parameters were affected by rapid increases and decreases in water temperatures. Na+ was slightly decreased with rapid decreases in temperature and was continuously at its lowest level at 13°C. For heat shock, Na+ slightly increased with increasing water temperatures, to be at the highest level of 31°C; it then rapidly decreased until reaching its lowest level at 37°C ((a)). Cl- was shown to have slightly decreased when the water temperature dropped and to have slightly increased with increasing water temperatures. The highest and lowest levels were found at 31°C and 13°C, respectively ((b)). K+ fluctuated after the fish were subjected to heat shock, whilst slightly increasing when they were exposed to cold shock; it reached its highest level at 13°C, but the lowest K+ level was found at 31°C ((c)). The dominant result of serum CO2 levels was that they increased with decreasing water temperatures, whereas experimental fish exposed to heat shock were not changed when compared with the 25°C exposure group. Moreover, the CO2 levels in fish exposed to cold shock were shown to be higher than those undergoing heat shock exposure ((d)). The Na+/K+ ratio was shown to be significantly different (P < 0.05), the ratio significantly increased with increasing water temperatures but rapidly decreased at 37°C ((e)). Na+/Cl- ratio showed a similar trend to the Na+/K+ ratio, it significantly increased with increasing water temperatures up to 31°C but at 37°C rapidly decreased; the highest and lowest ratio were found at 28°C and 19°C, respectively ((e)). Besides, the sum of the Na+ and K+ to Cl- ratio {(Na+ + K+)/ Cl- } was significantly different, it fluctuated after the fish were exposed to heat shock while in the cold shock group, the ratio changed little when compared with the heat shock groups ((e)).
Serum biochemistry study
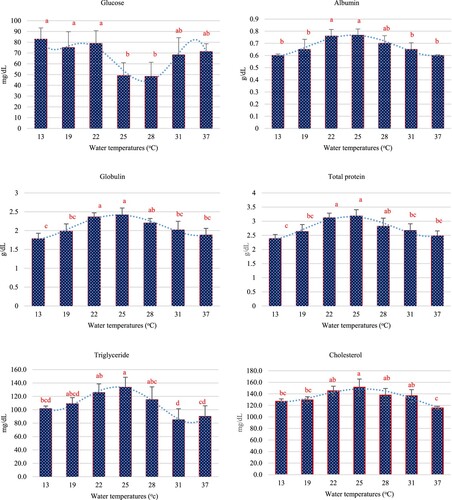
As the water temperature rapidly decreased and increased from between 25°C to 13°C in 4 h and between 25°C and 37°C in 4 h, respectively, the serum biochemistry showed significant difference (p < 0.05). Both with heat and cold shocks, serum glucose was shown to have significantly increased when compared with the 25°C group, except when at 28°C. Higher glucose levels were found in the cold shock groups than in the heat shock groups ((a)). Albumin, globulin and total protein showed the same trend, these parameters significantly decreased with increasing and decreasing water temperature from 25°C ((b) and (c)). Triglyceride was affected by heat and cold shocks, it slightly decreased with rapidly decreasing water temperatures but showed rapid decreases in the heat shock experiments. The lowest and highest triglyceride levels were found at 31°C and 25°C, respectively ((d)). Besides, serum cholesterol was also affected by heat and cold shocks: it decreased after rapid lowing and raising of water temperatures, the lowest level was found at 37°C, while the highest level was found at 25°C ((e)).
Mathematical curve fitting
Biochemical indices
After the clustered column chart of each parameter was generated by Excel program. The polynomial equation that was found to most match these results is considered from the R2. The polynomial equations for all biochemical indices are shown in . When the equations were taken up to 4 or 5 degrees causing the R2 nearest to 1. The R2 of biochemical indices ranged between 0.89 and 0.99, which is the highest R2 was triglyceride (0.99) and follow by globulin (0.98), albumin (0.96), glucose (0.95), total protein (0.94) and cholesterol (0.89), respectively.
Table 1. Polynomial equations and the coefficient of determination (R2) of serum biochemical indices of Pangasianodon gigas after subjected to different water temperatures.
Serum electrolytes
After the clustered column chart of each parameter was generated by Excel program. The polynomial equation that was found to most match these results is considered from the R2. The polynomial equations for all biochemical indices are shown in . When the equations were taken up to 3, 4 or 5 degrees this caused the R2 nearest to 1. The R2 of serum electrolytes ranged between 0.82 and 0.99, of which the highest R2 was Na+ (0.99) and followed by Cl- (0.98), Na+/Cl- (0.95), CO2 (0.93), (Na++K+)/Cl- (0.90), Na+/K+ (0.85) and K+ (0.82), respectively ().
Table 2. Polynomial equations and the coefficient of determination (R2) of serum electrolyte indices of Pangasianodon gigas after subjected to different water temperatures.
Discussion
In our study, serum indices were found to be more focused than plasma because serum contains proteins, electrolytes, antibodies, antigens and hormones. This indicates that serum is an important source of electrolytes. Vijayasamundeeswari et al. (Citation2017) reported that serum electrolytes are more stable than plasma electrolytes when the serum and plasma were kept at room temperature and 2–4°C for 48 h. Besides this, serum electrolytes were used for a comparative study of Notopterus notopterus from three aquatic bodies (Kulkarni Citation2015). In this study, serum electrolytes were evaluated, Na+, K+, Cl- and CO2 levels are indicators of the operation of a variety of homeostatic mechanisms in the body (Kelley and Kelley Citation2000; Pollock et al. Citation2014; Takvam et al. Citation2021). Moreover, sodium, potassium and chloride play an important role in osmoregulation and homeostasis. Normally, K+ should be at a higher level in the intracellular fluid when compared to the extracellular fluid. In fish, sodium and potassium are the predominant electrolytes. The function of electrolytes is to regulate the acid-basic balance and thus help in maintaining the ionic sufficiency of tissue functions (Davis Citation2004; Tavares-Dias et al. Citation2008). The results from this experiment showed a significant change in serum electrolytes and biochemistries when the experimental fish were subjected to cold and heat shocks. According to our results, K+ levels were shown to be much lower than Na+ serum levels, which accords with various other reported findings (Koeypudsa and Jongjareanjai Citation2011; Ghahremanzadeh et al. Citation2014; Geng et al. Citation2016). This may be attributed to the fact that K+ levels afford a relatively low contribution to osmolality when compared with those of Na+ and Cl- electrolytes, but K+ has been shown to have a significantly important role in the nervous system (sodium-potassium pump), secretory system (kidney), heartbeat and digestive system (Ham et al. Citation2003; Pillans et al. Citation2006). In this study, hypernatremia was found in the group exposed from 25°C to 31°C, whilst the levels found in the other groups tended to be normal, something that may be attributed to an impaired osmoregulation ability, because water temperature changes can affect osmoregulation (Fiess et al. Citation2007). Moreover, hyponatremia was observed in the groups exposed from 25°C to 15°C, 25°C to 15°C, respectively. This was due to NaCl being replaced by an over uptake across the gills to counteract Na+ diffusive losses (Tsuzuki et al. Citation2001). In addition, the highest level of Na+ in the 25°C–31°C group and the lowest levels in the groups at 25°C–31°C and 25°C–13°C may be an indication of gill and kidney injuries, which may have influenced the fish’s osmoregulatory abilities (Grabriel et al. Citation2019). Hyperkalemia was found in cold shock groups when compared with control group (25°C), but a fluctuating curve was found in heat shock groups. This result revealed that Na+ and K+ have opposing transmission rates because the two electrolytes go across and through cell membranes (Therien and Blostein Citation2000; Castillo et al. Citation2015), this happens when they are activated by stimulants that lead to action potential occurring, especially with regards to repolarized stage K+, released from the cell body into the extracellular fluid, while Na+ is still located in cell body; this results in more K+ in the extracellular fluid than in the intracellular fluid. According to Gabriel et al. (Citation2019), levels of Na+ and K+ in Heterobranchus bidorsalis are shown to move in different directions from one another after cypermethrin-treated stress. When fish are exposed to environmental change, physiological responses such as hormonal, metabolic, and behavioural are significantly changed (Mazeaud et al. Citation1977; Barton Citation2002). Na+/K+, Na+/Cl- and (Na++K+)/Cl- ratio investigations were mostly revealed an effect on salinity, whilst these ratios, as a consequence of rapid water temperature changes, have not been reported. This is the first report revealing that changes are caused by heat and cold shock experiments. It has shown that Na+/K+ and Na+/Cl- ratios trend to increase and decrease with increasing and decreasing water temperatures, respectively. This may have been due to abnormalities that primarily reflect aldosterone deficiency, with diminished renal and gill preservation of sodium (Na+) and excretion of potassium (K+) ions and a lack of the extracellular fluid (Feldman and Nelson Citation1996; Dibartola Citation2000). However, not only serum electrolytes but also serum biochemical indices were evaluated.
In the current study, biochemical indices such as glucose, albumin, globulin, total protein, triglyceride and cholesterol were significantly different when the fish were exposed to cold shock and heat shocks. Glucose level was affected by the cold and heat shocks, serum glucose is more sensitive to temperature changes than serum protein and cholesterol, glucose molecules have a major role to play in the bioenergetics of animals, which is transferred to ATP synthesis (Lucas Citation1996). Besides, glucose levels are closely related to cortisol levels which, within these parameters, are the most common stress indicator. As in the study by Panase et al. (Citation2018), serum glucose and cortisol levels were significantly increased as water temperature levels decreased, while a 15°C water temperature caused significantly increased levels of serum cortisol in the fish. This result was supported by the report of Inoue et al. (Citation2008), where plasma cortisol and glucose levels of Brycon amazonicus were shown to have been significantly increased after the fish were subjected to cold shock. In this experiment, the water temperature dropped from 28°C to 18°C for 1 h and then returned to the original temperature. According to our results, glucose levels were affected by heat and cold shocks, and were shown to be significantly higher when the water temperature dropped to 22°C and changed consistently until the temperature dropped to 13°C. When water temperatures were increased to 31°C and 37°C, glucose levels were shown to have slightly increased, this was due mainly to the physiological responses, cortisol hormone effects on chromaffin cells that result in the occurrence of glycogenolysis, gluconeogenesis and modulates cardiovascular and respiratory function (Reid et al. Citation1992, Citation1998). Surprisingly, all serum biochemical indices except for glucose showed a similar trend, their levels were significantly decreased with decreasing and increasing water temperatures when compared to the control group (25°C). According to Panase et al. (Citation2018) the total serum protein exhibited by Nile tilapia (Orechromis niloticus) was slightly decreased with decreases in water temperature from 25°C to 13°C (3oC/h, decreasing rate). The reason for this was that serum protein, albumin and globulin were reduced, this may have been due to it being transformed into amino acid then becoming an ATP through the cellular respiration process for maintenance of body temperature (Alberts et al. Citation2002). Similarly, serum cholesterol was serum protein, and this may have been caused by its use as precursor of cortisol hormone to cope with stress response. Although the Panase et al. (Citation2018) study did not determine the effects of heat and cold shocks on cortisol levels, it did reveal that cold shock resulted in increases in cortisol and decreases in cholesterol levels in O. niloticus. Moreover, serum triglyceride was affected by water temperatures which, according to Islam et al., (Citation2022), explains why triglyceride significantly decreases with increasing water temperatures.
Conclusion
Based on our finding, 25°C −28°C is the appropriate temperature range for culturing P.gigas, because the biochemical and physiological indices were not shown to be significantly different. The results will be used to find appropriate practices for the prevention of detrimental effects on fish by finding ways to help them adapt to extreme weather events that may occur, especially with regards to rapidly decreasing water temperatures. One recommendation we can make, based on our results, is that those doing fish farming in the cold season and putting water in fish ponds should try to prevent the water temperature from dropping too rapidly.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. 2002. Molecular biology of the cell, 4th ed. New York: Garland Science. https://www.ncbi.nlm.nih.gov/books/NBK21054/.
- Barton BA. 2002. Stress in fishes: a diversity of responses with particular reference to changes in circulating corticosteroids. Integr Comp Biol. 42:517–525.
- Castillo J, Rui H, Basilio D, Das A, Roux B, Latorre R, Bezanilla F, Holmgren M. 2015. Mechanism of potassium ion uptake by the Na+/K+-ATPase. Nat Commun. 6:7622. doi:10.1038/ncomms8622.
- Clusella-Trullas S, Blackburn TM, Chown SL. 2011. Climatic predictors of temperature performance curve parameters in ectotherms imply complex responses to climate change. Am Nat. 177(6):738–751. PMID: 21597251.
- Dalvi RS, Das T, Debnath D, Yengkokpam S, Baruah K, Tiwari LR, Pal AK. 2017. Metabolic and cellular stress responses of catfish, Horabagrus brachysoma (Günther) acclimated to increasing temperatures. J Therm Biol. 65:32–40.
- Davis K. 2004. Temperature affects physiological stress responses to acute confinement in sunshine bass (Morone chrysops×Morone saxatilis). Comp Biochem Physiol A Mol Integr Physiol. 139:433–440.
- Dibartola SP. 2000. Disorders of sodium and water: Hyponatremia and hypernatremia. In: DiBartola SP, editor. Fluid therapy in small animal practice. 2nd ed. Philadelphia (PA): WB Saunders Company; p. 45–72.
- Feldman EC, Nelson RW.1996. Hypoadrenocorticism (Addison's disease). In: Canine and feline endocrinology and reproduction. 2nd ed. Philadelphia (PA): WB Saunders Company; p. 266–306.
- Fiess JC, Kunkel-Patterson M, Mathias L, Riley LG, Yancey PH, Hirano T, Grau EG. 2007. Effects of environmental salinity and temperature on osmoregulatory ability, organic osmolytes and plasma hormone profiles in the Mozambique tilapia (Oreochromis mossambicus). Comp Biochem Physiol A Mol Integr Physiol. 146:252–264. doi:10.1016/j.cbpa.2006.10.027.
- Geng C, Tian Y, Shang Y, Wang L, Jiang Y, Chang Y. 2016. Effect of acute salinity stress on ion homeostasis, Na+/K+–ATPase and histological structure in sea cucumber Apostichopus japonicas. SpringerPlus. 5, 1977. doi: 10.1186/s40064-016-3620-4
- Ghahremanzadeh Z, Namin JI, Bani A, Hallajian A. 2014. Cytological comparison of gill chloride cells and blood serum ion concentrations in kutum (Rutilus frisii kutum) spawners from brackish (Caspian Sea) and fresh water (Khoshkrood River) environments. Arch Pol Fish. 22:189–196.
- Grabriel UU, Edori OS, Egobueze EC. 2019. Plasma enzymes and electrolytes in heterobranchus bidorsalis treated with cypermethrin. Biochem Anal Biochem. 08:380.
- Ham EHV, Anholt RDV, Kruitwagen G, Imsland AK, Foss A, Sveinsbo BO, FitzGerald R, Parpoura AC, Stefansson SO, Bonga SEW. 2003. Environment affects stress in exercised turbot. Comp Biochem Physiol A Mol Integr Physiol. 136:525–538.
- Hogan Z. 2015. Pangasianodon gigas. The IUCN Red List of Threatened Species 2011: e.T15944A5324699. https://doi.org/10.2305/IUCN.UK.2011-1.RLTS.T15944A5324699.en.
- Inoue LA, Moraes G, Iwana GK, Afonso LOB. 2008. Physiological stress responses in the warm-water fish matrinxã (Brycon amazonicus) subjected to a sudden cold shock. Acta Amazonica. 38:603–609.
- Islam JM, Kunzmann A, Slater MJ. 2022. Responses of aquaculture fish to climate change-induced extreme temperatures: A review. J World Aquacult Soc. 53:314–366.
- Kelley GA, Kelley KS. 2000. Progressive resistance exercise and resting blood pressure: a meta-analysis of randomized controlled trials. Hypertension (Dallas, TX: 1979). 35: 838–843. doi: 10.1161/01.hyp.35.3.838.
- Knutson TR, McBride JL, Chan J, Emanuel K, Holland G, Landsea C, Held I, Kossin JP, Srivastava AK, Sugi M. 2010. Tropical cyclones and climate change. Nat Geosci. 3:157–163.
- Koeypudsa W, Jongjareanjai M. 2011. Impact of water temperature and sodium chloride (NaCl) on stress indicators of hybrid catfish (Clarias gariepinus Burchell x C. macrocephalus Gunther). Songklanakarin J Sci Technol. 33:369–378.
- Kulkarni RS. 2015. Comparative studies on blood electrolytes of the fresh water fish, Notopterus notopterus from three aquatic bodies. Int Lett Nat Sci. 40:1–5.
- Liberto TD. 2021. Eighth-warmest December on record helps 2020 end as second-hottest year on record. Department.
- Lucas A. 1996. Physical concepts of bioenergetics. In: Lucas A, editor. Bioenergetics of aquatic animals. English Edition. London: Taylor & Francis.
- Mazeaud MM, Mazeaud F, Donaldson EM. 1977. Primary and secondary effects of stress in fish: some new data with a general review. Trans Am Fish Soc. 106:201–212. Features, NOAA, Climate government, USA.
- Mengumphan K, Saengkrachang J. 2008. Production of generation 2 Mekong giant catfish (Pangasainodon gigas) cultured with Spirulina sp. Maejo International J SciTechnol. 2:559–567.
- Meteorological Department. 2016. Monthly weather summary in Thailand January 2016. Climatological Center. Meteorological Development Bureau. Meteorological Department, Thailand. p. 13.
- Meteorological Department. 2017. The lowest and highest temperature statistics in winter in Thailand during the period of 68 years 1951–2018. Climatological Center. Meteorological Development Bureau. Meteorological Department, Thailand. (in Thai). p.10.
- Panase P, Saenphet S, Saenphet K. 2018. Biochemical and physiological responses of Nile tilapia Oreochromis niloticus Lin subjected to cold shock of water temperature. Aqua Rep. 11:17–23.
- Panase P, Saenphet S, Saenphet K, Pathike P, Thainum R. 2019. Biochemical and physiological responses of Nile tilapia (Oreochromis niloticus Linn.) subjected to rapid increases of water temperature. Comp Clin Path. 28:493–499.
- Phrompanya P, Panase P, Saenphet S, Saenphet K. 2021. Histopathology and oxidative stress responses of Nile tilapia Oreochromis niloticus exposed to temperature shocks. Fish Sci. 87:491–502. doi:10.1007/s12562-021-01511-y.
- Pillans RD, Anderson WG, Good JP, Hyodo S, Takei Y, Hazon N, Franklin CE. 2006. Plasma and erythrocyte solute properties of juvenile bull sharks, Carcharhinus leucas, acutely exposed to increasing environmental salinity. J Exp Mar Biol Ecol. 331:145–157. doi:10.1016/j.jembe.2005.10.013.
- Pollock JS, Ryan MJ, Samson WK, Brooks David P. 2014. Water and electrolyte homeostasis brings balance to physiology. Am J Physiol-Regul Integr Comp Physiol. 307:R481–R483.
- Reid SD, Moon TW, Perry SF. 1992. Rainbow trout hepatocyte beta-adrenoceptors, catecholamine responsiveness, and effects of cortisol. Am J Physiol. 262:794–799.
- Reid SG, Bernier NJ, Perry SF. 1998. The adrenergic stress response in fish: control of catecholamine storage and release. Comp Biochem Phys C. 120:1–27.
- Schreck CB. 2000. Accumulation and long-term effects of stress in fish. In: Moberg G.P., Mench J.A., editors. The biology of animal stress. Wallingford: CABI Publishing; p. 147–158.
- Stevens A. 2020. A warm pool in the Indo-Pacific Ocean has almost doubled in size, changing global rainfall patterns. Department: Images &. Video, NOAA, Climate government, USA.
- Sukumasavin, N. 2006. Status of the Mekong giant catfish, Pangasianodon gigas Chevey, 1930 stock enhancement program in Thailand. In: Primavera, JH, Quinitio, ET, Eguia, MRR, editors. Proceedings of the regional technical consultation on stock enhancement for threatened species of international concern; Jul 13–15; Iloilo: Aquaculture department, Southeast Asian Fisheries Development Center. p. 85–90.
- Sutthi N, Panase A, Phinrub W, Srisuttha P, Panase P. 2022. Cold shock and its effect on biochemical indices, cortisol and electrolyte changes in Chao Phraya catfish, Pangasius Sanitwongsei Smith, 1931. Comp Clin Path. 31:757–764.
- Szekeres P, Brownscombe JW, Cull F, Danylchuk AJ, Shultz AD, Suski CD, Murchie KJ, Cooke SJ. 2014. Physiological and behavioural consequences of cold shock on bonefish (Albula vulpes) in The Bahamas. J Exp Mar Biol Ecol. 459:1–7.
- Takvam M, Wood CM, Kryvi H, Nilsen TO. 2021. Ion transporters and osmoregulation in the kidney of teleost fishes as a function of salinity. Front Physiol. 12:664588.
- Tavares-Dias M, Moraes FR, Imoto ME. 2008. Hematological parameters in two neotropical freshwater teleost, Leporinus macrocephalus (Anostomidae) and Prochilodus lineatus (Prochilodontidae). Biosci J. 24:96–101.
- Therien AG, Blostein R. 2000. Mechanisms of sodium pump regulation. Am J Physiol-Cell Physiol. 279:C541–C566. doi:10.1152/ajpcell.2000.279.3.C541.
- Tsuzuki MY, Ogawa K, Strussmann CA, Maita M, Takashima F. 2001. Physiological responses during stress and subsequent recovery at different salinities in adult pejerrey Odontesthes bonariensis. Aquaculture. 200:349–362.
- Vijayasamundeeswari CK, Ananthi N, Sudha R. 2017. Comparison of electrolyte levels in serum and plasma. Int J Clin Biochem Res. 4:115–118.
- Wang Y, Han G, Pham CV, Koyanagi K, Song Y, Sudo R, Lauwereyns J, Cockrem JF, Furuse M, Chowdhury VS. 2019. An acute increase in water temperature can increase free amino acid concentrations in the blood, brain, liver, and muscle in goldfish (Carassius auratus). Fish Physiol Biochem. 45:1343–1354.