ABSTRACT
A species of catfish belonging to the Anguilla genus called Anguilla marmorata has long been exploited and grown in Vietnam. Marbled eels are regarded as a specialty seafood species with several therapeutic and nutritive benefits. This study used High-Performance Liquid Chromatography (HPLC) to identify various biochemical and nutritional ingredients in A. marmorata collected in its natural habitat. There was 16 different amino acids analysed in the muscle, skin, and mucus of marbled eels at the rates of 14.01%, 5.48%, and 9.96%, respectively. Twenty-four fatty acids were found in muscle and skin, while only nine fatty acids were detected in the mucus with proportions of 11.93, 25.91, and 0.37 g/100 g, respectively. Six different vitamins (A, B3, B5, D3, C, E) are found in the muscle of the eel; five types of vitamins (A, B3, B5, D3, E) were detected in the skin; and three vitamins (B3, B5, C) are found in mucus. Using HPLC analysis has shown remarkable effectiveness in micronutrient analysis in nutritional research. The results will increase our knowledge of the nutritive benefits of the A. marmorata and point the way toward the creation of functional foods and cosmetics with natural ingredients that may be used on people.
Introduction
The animal’s shell serves an important purpose as its main line of defence against its surroundings. The aquatic environment renders fish particularly susceptible to outside forces, hence this function is crucial for them. Fish skin may easily blend in with its environment, making it a useful signal for communication within or across species. Being able to travel on or between hard platforms or dig into soft bottoms while causing the least amount of damage to the fish's body shells makes the outer layers of the skin very crucial (Fishelson Citation1996). The skin mucosa of fish consists of part cellular and part humoral. The skin’s mucus is made up of extracellular molecules, while the cellular component is the mucous membrane and its supporting connective tissue (Salinas et al. Citation2011). Proteins, carbohydrates, lipids, and metabolites are some of the components found in the skin mucosa of fish (Zaccone et al. Citation2001). The characterization of numerous significant proteins and enzymes in fish mucus, including protease, antibacterial peptide (AMP), lectin, lysozyme, immunoglobulin, complement protein, C-reactive protein (CRP), transferrin, Alkaline phosphatase (ALP), and many other antibacterial proteins and peptides, is crucial for understanding fish natural immunity (Swain et al. Citation2007). For centuries, the life history of freshwater eels (Anguilla) has been a mystery to biologists. The freshwater eel is a typical giant fish; they spend most of their life in freshwater rivers and estuaries before finally migrating to their spawning grounds in the ocean for reproduction (Tesch Citation2003). In total, there are 16 species and three subspecies of freshwater eels have been identified in the world (Arai Citation2016), which can be classified into two groups based on body colour: speckled skin and uniformly coloured skin, known as ‘marble eel’ and ‘plain eels’ (Smith Citation1999). Marble eels are distributed mainly in the tropics and subtropics, while plains eels are distributed in tropical, subtropical, and temperate regions (Tesch Citation2003).
Previous research on eels (Anguilla marmorata) has revealed that they have a high nutritional value, including protein, amino acids, minerals, fatty acids, and several vitamins (Seo et al. Citation2013; Widyasari et al. Citation2014; Nafsiyah et al. Citation2018; Lee et al. Citation2020; Kieu and Nguyen Citation2020a). It is beneficial for human health such as tonic yang, blood circulation, bone and joint tonic, beautiful skin, enhanced vision, and nervous system functions. The nutritional makeup of fish flesh gathered under natural living conditions in Thua Thien Hue, Vietnam, is being studied as a follow-up to earlier research on genetic diversity and species identification (Kieu et al. Citation2020; Kieu and Nguyen Citation2020b). Even though a lot of research has been done on fish skin (Pickering Citation1974; Bullock Citation1980; Whitear Citation1986), and mucus (Kelly and Salinas Citation2017; Dash et al. Citation2018; Reverter et al. Citation2018; Tagami and Kuwahara Citation2020) very little of it has focused on eels, a group of fish that inhabits that stratum and is highly diverse (Casimir and Fricke Citation1971; Leonard and Summers Citation1976). Therefore, in this study, we use the HPLC technique to determine the composition of several important nutrients used for human survival in the muscle, skin, and mucus of marble eels in their natural habitat, thereby providing a database for assessing nutritional value and potential exploitation of products from eel for different purposes such as research and production of functional foods, cosmetics to build a strategy to exploit and develop sustainably eel resources in the future.
Materials and methods
Materials
Marbled eels at yellow eel stage in size of 500–900 g collected in word wild, in Thua Thien Hue, Vietnam packed in ice-insulated polystyrene boxes and shipped to the laboratory of the Faculty of Fisheries, University of Agriculture and Forestry, Hue University within 24 h after purchasing. Muscle, skin, and mucus samples were taken from the dorsal of marbled eels. In which, the mucus is carefully scraped from the dorsal side of the body with a sterile spatula.
The mucus samples were collected aseptically from fish and thoroughly mixed with an equal amount of sterile physiological saline (0.85% NaCl) and centrifuged at 5000 rpm for 15 min to remove insoluble particles dissolved and supernatants and kept at 4°C until use (Sridhar et al. Citation2021). Meanwhile, the muscle and skin samples were cut into 1–2 cm dice before being pureed, and stored at −20°C until analysed amino acid, fatty acid, and vitamin compositions were within one month (Tasleem et al. Citation2020).
Methods
Analysis of fatty acids, amino acids, and vitamins in the oil of eel was determined by the method of high-performance liquid chromatography (UHPLC).
Amino acid analysis of proteins in eels
The amino acid composition of the protein in the muscle, skin, and mucus of the eel was analysed according to TCVN 8764:2012 (ISO 13903:2005) (Ministry of Science and Technology of Vietnam Citation2012) modified as described by Tagami and Kuwahara (Citation2020). Proteins in 10 mg of sample were hydrolyzed in 200 µL 6 M HCl at 110°C for 24 h under low-pressure conditions. The nitrogenous bulk co-extracts were precipitated with sulfosalicylic acid and removed by filtration through a 0.45 µm filter. The filtrate was adjusted to pH = 2.20. Amino acids were separated by ion exchange chromatography and determined by reaction with ninhydrin using a sing spectrophotometric detector at 570 nm (440 nm for proline) on a Shimadzu (models 10A, 20A) instrument. The amino acid content was determined by the retention time and peak spectrum after comparison on the spectrum bank.
The composition of fatty acids in the muscle, skin, and mucus of marble eels
Analysis of fatty acid composition in muscle, skin, and mucus of eel by UHPLC according to the description of TCVN 9969: 2013 (ISO 15885:2002) (Ministry of Science and Technology of Vietnam Citation2013a, Citation2013b) modified according to the description of Lee et al. (Citation2020). To determine the fatty acid composition, lipids in the samples were extracted according to the method of Folch et al. (Citation1957). The sample is saponified and converted to a methyl ester by reaction with BF3 – MeOH (boron trifluoride-methanol) according to AOCS Ce 2–66 (AOCS Citation1989). The composition of fatty acid methyl esters (FAME) was analysed on a gas chromatograph with flame ionization detector GC-FID (FID-Flame Ioniation Detetor), mobile phase: Helium gas, Highly polar column (HP- Silica), 100 m × 0.25 mm × 0.2 µm on Shimadzu equipment system (GC 2010, GC 2010 Plus) at Case laboratory company, Ho Chi Minh city, Vietnam. Fatty acids were determined by comparing retention times and mass spectra with analytical standards.
The composition of vitamins content in eels
The contents of vitamin A, vitamin C, Vitamin D3, Vitamin E, and vitamin K1 were analysed by the HPLC-UV method. Five grams of sample used for each analytical parameter, after being extracted according to instructions, were filtered through 0.45 µm filter and injected into HPLC-UV liquid chromatography equipment, UV wavelength = 265 nm, is current programme; analytical column C18 ((250*4.6) mm; 5 µm) on Shimadzu equipment system (model 10A, 20A) at Case laboratory company, Ho Chi Minh City, Vietnam. Which, vitamin A content in the sample was determined by measuring b-carotene, 13-cis-retinol, and all-trans-retinol isomers (all-trans-retinol). Retinol is saponified with either ethanol or methanolic potassium hydroxide and extracted with a suitable solvent according to TCVN 8972-1:2011 (Ministry of Science and Technology of Vietnam Citation2011a) and Jamaluddin et al (Citation2018b). The content of vitamin C in the sample was extracted by mobile phase solution with KH2PO4 concentration of 0.05M (pH = 2.5) according to TCVN 8977:2011 (EN 14130:2003) (Ministry of Science and Technology of Vietnam Citation2011c); Samples for analysis of vitamin D3 content were saponified with potassium hydroxide solution in alcohol and extracted with a suitable solvent as described in TCVN 8973:2011 (Ministry of Science and Technology of Vietnam Citation2011b). The determination of vitamin D3 in a suitable sample extract was carried out by semi-preparative HPLC using normal phase and then further analysed by reverse phase HPLC, Vitamin D2 was used as internal standard; Samples for analysis of vitamin E content were saponified with KOH solution with concentration ρ(KOH) = 50 g/100 ml of sample. α-, β-, γ- and δ-tocopherol in the sample solution were determined by HPLC detector UV according to the instructions of TCVN 8973:2011 (Ministry of Science and Technology of Vietnam 2011) with adjustment. The content of vitamins B3 and B5 in the sample was determined by HPLC-LC/MS-MS method according to the guidance of TCVN 13263-2:2020 (Ministry of Science and Technology of Vietnam Citation2020); and (Kahoun et al. Citation2022). Samples were extracted with 0.1 M HCl solution. Filter the sample through a 0.45 µm filter into a 2 mL vial. Inject into liquid chromatography coupled to mass spectrometry (LC/MS/MS). Mobile phase: Channel A: H2O (0.1%HCOOH) and channel B: MeOH (0.1%HCOOH), gradient programme. Analytical column C18 ((150*4.6) mm; 3.5 µm) on GC Varian 3800 system at Case laboratory company, Ho Chi Minh City, Vietnam. The identification of all vitamins in the sample is based on the retention time and quantified by the external standard method using the peak area or peak height obtained.
Results
Amino acids content
The findings of our study, which analysed 16 essential amino acids found in the muscle, skin and mucus of a marbled eel (A. marmorata) captured in its natural habitat in Thua Thien Hue, Vietnam, and . This result reveals that the total amount of amino acids found in marbled eel skin was the highest accounting for 15.48% (w/w), the white amino acid content in muscle (14.01%) and mucus (9.96%). The composition of each essential amino acid (w/w) concentration varies from 0.17% to 3.04%, in which Tyrosine had the smallest percentage (0.17% (w/w)) and Glycine had the greatest (3.04%). The composition of essential amino acids in muscle was ranging from 0.32% (Methionine) to 2.34% (Glutamic acid); and that in mucus was ranked from 0.26% (Histidine) to 1.37% (Glutamic acid). The sample of eel protein-rich material combination may be seen in the amino acid composition.
Figure 1. UHPLC chromatography of the amino acid essential ingredients in muscle (a), skin (b), and mucus (c) of marbled eel.
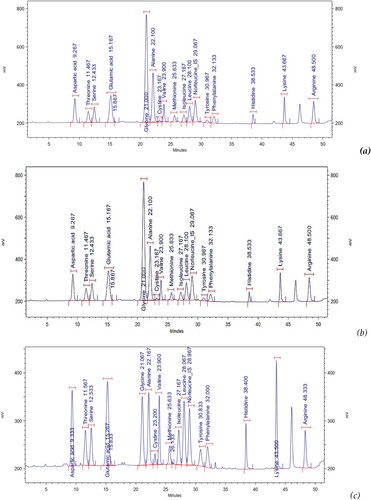
Table 1. Ingredients of essential amino acids in marbled eel (% w/w).
Fatty acid composition
Total fatty acids present in marbled eel (A. marmorata) skin accounted for 25.91 g/100 g sample, 2.17 times higher than the total fatty acid content in muscle (11.93 g/100 g) and up to 70 times higher than the fatty acid content in the mucus (only 0.37 g) (). The analytical findings for each fatty acid composition, and reveal that up to 24 of the entire 37 fatty acids were found in the muscle and skin of marbled eel, meanwhile, only nine types of fatty acids are found in the mucus of marbled eels collected in the natural habitat in Thua Thien Hue, Vietnam. The composition of fatty acids in the flesh of the eel fish includes C12:0 (Lauric acid), C14: 0 (Myristic acid), C14:1 (Myristoleic acid), C15:0 (Pentadecanoic acid), C16:0 (Palmitic acid), C16:1 (Palmitoleic acid), C17:0 (Heptadecanoic acid), C17:1 (Cis-10- Heptadecanoic acid), C18:0 (Stearic acid), C18:1n9 T (Elaidic acid), C18:1n9C (Oleic acid), C18:2n6 T (Linolelaidic acid), C18:2n6C (Linoleic acid), C18:3n3 (α-Linolenic acid), C18:3n6 (γ-Linolenic acid), C20:0 (Arachidic acid), C20:1 (Eicosenoic acid), C20:2 (cis-11,14-Eicosadienoic acid), C20:3n6 (cis-8,11,14-Eicosatrienoic acid), C20:5n3 (Eicosapentaenoic acid), C22:0 (Behenic acid), C22:1n9 (Erucic acid), C22:6n3 (Docosahexaenoic acid), and C24:0 (Lignoceric acid) with rates ranging from 0.01 to 5.34 g/100 g sample. The composition of fatty acids in the skin of marbled eel ranges from 0.01 to 13.35 g/100 g. Compared with the fatty acid composition of meat, amino acids such as C18:1n9T (Elaidic acid), C18:1n9C (Oleic acid), C22:1n9 (Erucic acid) were not detected in the skin but were replaced by the presence of the following amino acids: C20:3n3 (cis-11,14,17-Eicosatrienoic acid), C21:0 (Heneicosanoic acid), and C24:1 (Nervonic acid). Meanwhile, out of nine types of fatty acids found in mucus, only seven fatty acids are found in skin and muscle, including C16:0 (Palmitic acid), C16:1 (Palmitoleic acid), C17: 0 (Heptadecanoic acid), C17:1 (Cis-10-Heptadecanoic acid), C18:0 (Stearic acid), C18:2n6C (Linoleic acid), C20:1 (Eicosanoid acid). Of the remaining two fatty acids, C18:1n9C (Oleic acid) was found in muscle and mucus with the largest concentrations of 5.34 and 0.1 g/100 g of the sample, respectively. C20:4n6 (Arachidonic acid) is a fatty acid found only in the mucus of eels and has the second largest concentration of 0.09 g/100 g of sample ( and ).
Figure 2. UHPLC chromatography of fatty acids ingredients in muscle (a), skin (b), and mucus (c) of marbled eel.
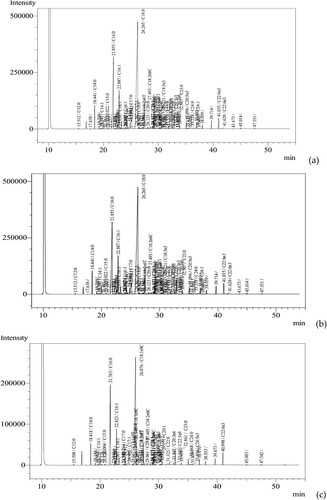
Table 2. Ingredients of fatty acids in marbled eel (g/100 g).
Ingredients of vitamins
The results of the analysis of the composition of seven different vitamins in the muscle, skin, and mucus of marbled eels naturally distributed at the yellow eel stage are presented in , showing that most of the vitamins surveyed, including A, D3, E, C, B3, and B5 are present in the study sample, except vitamin K1. Vitamins A, vitamin D3, and vitamin E were found only in the muscle and skin and were not detected in the mucus of the eel. Which, the vitamin A content in muscle was 8890 IU/kg, 3.34 times higher than the content in the skin (2.663 IU/kg). Vitamin E content was detected with a higher value in the muscle of 23.9 mg/kg which was 1.65 times higher than 14.5 mg/kg in the skin. The content of vitamin D3 detected in the skin was 350 µg/kg which was 1.25 times higher than the 279 µg/kg in muscle. Vitamins B3 and B5 were present in all samples studied. The content of vitamin B3 in muscle (17.7 mg/kg) and skin (15.7 mg/kg) was more than double that of mucus (7.29 mg/kg). However, the vitamin B5 content in the mucus showed a superior value with 104 mg/kg 33.55 times higher than the content in muscle (3.10 mg/kg) and 13.87 times higher than the content in the skin. Vitamin C in the mucus was three times higher than its content in muscle (12.6 and 4.55 mg/kg respectively), and vitamin C was not found in the skin of the eel.
Table 3. Composition of vitamins in marbled eel.
Discussion
Some previous studies have determined the composition of amino acids, fatty acids, and some vitamins in the flesh of eels (Nafsiyah et al. Citation2018; Kieu and Nguyen Citation2020a; Gómez-Limia et al. Citation2021; Citation2022; Jamaluddin et al., Citation2018a,Citationb; Sari and Jamaluddin Citation2019). In this study, the composition of amino acids, fatty acids, and seven different vitamins in the muscle, skin, and mucus of wild eel collected from the wild in Thua Thien Hue, Vietnam was determined and compared based on the HPLC technique. There were 16 amino acids present in the muscle, skin, and mucus of the eel ( and ), of which 12 amino acids are similar to examining the presence of amino acids in the meat and skin of eels by Gómez-Limia et al. (Citation2021; Citation2022), demonstrated that the skin and flesh of the marbled eel contain 13 amino acids in varying amounts depending on age and living conditions, including Threonine, Valine, Isoleucine, Leucine, Lysine, Glutamic acid, Tyrosine, Methionine, Glycine, Arginine, Alanine, and Proline. Four amino acids including, Aspartic acid, Histidine, Phenylalanine, and Serine were not found in the study of Gómez-Limia et al. (Citation2021; Citation2022) but were found in the skin, muscle, and mucus of the eel in Thua Thien Hue, while Hydroxyproline was not detected in our results (Gómez-Limia et al. Citation2021; Citation2022; ). Furthermore, a relatively high percentage of total protein in the mucus eel (9.69%) is an interesting result. According to several previous reports, many types of bioactive molecules participate in many biological roles and interactions such as glycoprotein lectins, mucins, antioxidants, and antimicrobial peptides (Dash et al. Citation2018; Reverter et al. Citation2018; Tagami and Kuwahara Citation2020) have been identified and are abundant in the mucus of fish, including Anguilla eels such as Japanese eels and European eels, but not reported in marbled eels (A. marmorata) (Kelly and Salinas Citation2017; Dash et al. Citation2018; Reverter et al. Citation2018; Tagami and Kuwahara Citation2020). This result opens up the potential for studies on active protein composition related to life activities and the discovery of new values of the marble eels.
In some previous studies, the total lipid composition analysed in the flesh (including muscle and skin) of wild eels ranged from 17.18% to 19.22% (for natural eels) (Kieu and Nguyen Citation2020a) and from cultured models was 21.35% (for cultivated eels) (Nafsiyah et al. Citation2018). In this study, the total lipid content in muscle and skin obtained was 25.91% and 11.93% respectively, lower than the results of Kieu and Nguyen Citation2020a, which was 26.3% ± 2.14% and 20.2% ± 2.02% respectively (for marbled eels weighing 500–1000 g). In this study, for the first time, we determined that the total lipid content in the mucus of A. marmorata was 0.37%; the composition of 24/36 fatty acids found in muscle and skin; and 9/36 fatty acids present in mucus of A. marmorata collected from Thua Thien Hue, Vietnam ( and ). Our findings demonstrate that the skin of A. marmorata species collected naturally in Thua Thien Hue, Vietnam has a higher fatty acid content than the fatty acids reported by Lee et al. Citation2020 with the predominant fatty acid composition of various eel species, including A. japonica, A. australis, and A. anguilla, was found to be C18: 1n 9 (24.4%–33%), C16: 0 (17.7%–22%), C18: 2n − 6 (0.6%–6.1%), C20: 5n − 3 (1.5%–5.1%), C14: 0 (2.4%–5.1%), C18: 0 (3.4%–4.5%) and C20: 4n − 6 (1.0%–2.9%), respectively.
In addition to essential fatty acids found in the muscle, skin, and slime of the eel, such as Linoleic acid (present in both muscle, skin, and mucus with the proportion of 0.54; 1.12% and 0.02%, respectively) and α-Linolenic acid (with a ratio of 0.02% and 0.63% in muscle and skin respectively), a relatively high proportion of other fatty acids that play an important role and are widely used in the production of cosmetic products to help protect skin defence in humans involves the function of retaining moisture and protecting skin structures against environmental damage. Among them, palmitic acid (C16:0) was found in both muscles (2.27%), skin (5.59%), and mucus (0.07%) with a relatively high proportion of total fat detected, 19.03%, 21.58%, and 18.92% respectively. Stearic acid (C18:0) has the highest percentage in the skin (13.35% or 51.14% of the total fat in the skin), but it accounts for only 4.86% of the total fat in the muscle and 8.11% of the total fat in the mucus (). Manufacturers frequently favour stearic acids because they are primarily used in formulations for skin and hair care cosmetics. Stearic acid thickens the emulsion base, gives the skin a cool sensation, acts as a mild surfactant, and has a good cleansing effect. It is also a skin-friendly wax, so the likelihood of irritation is almost nonexistent. Stearic acids and palmitic acid are frequently included in conjunction together in commercial cosmetic skin care products (Yu et al. Citation1995). Oleic acid (C18:1n9C) was also detected in relatively large concentrations in the muscle and mucus of marbled eels (the highest concentration in muscle was 5.34%, accounting for 44.76% of total muscle fat; and 0.1% accounted for 27.03% of the total fat in the mucus) (). Oleic acid is an omega very good for heart health, controlling sugar levels in the body; and has effective antioxidant and fat metabolism effects (Ernesto Citation2016; Preedy and Watson Citation2020). In skin care, Oleic acid is used to soothe the skin, helping to reduce the signs of dryness and sensitivity (Choulis Citation2011; Preedy and Watson Citation2020). It may impede the progression of adrenal dystrophy, enhance memory (Choulis Citation2011), and may also inhibit lung cancer cells (Cassiano et al. Citation2016). In addition, an important derivative of Oleic acid has also been found in mucus (not detected in muscle and skin) is C20:4n6 (Arachidonic acid) with 0.09% content, accounting for the second highest proportion out of nine fatty acids detected in mucus (24.32%) (). In addition to being involved in signalling as a lipid second messenger involved in the regulation of signalling enzymes, such as PLC-γ, PLC-δ, and PKC-α, -β, and -γ isoforms, arachidonic acid is a key inflammatory intermediate and can also act as a vasodilator (Baynes and Marek Citation2005). As a result, there is a high level of certain fatty acids in the marbled eels harvested in their natural habitat in Thua Thien Hue, Vietnam, which has the potential to be used in the manufacture of very safe cosmetic skin care and medical products for people.
Analysis of the content of some vitamins in the marbled eel A. marmorata has been performed by several previous studies. The study of Jamaluddin et al., Citation2018b determined the vitamin A content in marbled eels was 3243.34–3766.67 IU/100 g and the highest value was found in the silver eel. Meanwhile, Nafsiyah et al. Citation2018, determined vitamin A content in marbled eels was 1839 μg/100 g; and 2068.55 mg/100 g for culture and 3316.38 mg/100 g for wild were determined by Wijayanti and Setiyorini (Citation2018). The vitamin A content in our study was much lower than in previous studies (8890 IU/kg for muscle; 2663 IU/kg for skin; and not found in mucus) (). According to the research of Atsuko et al. (Citation1984), values of vitamin D3 in eel body oils were very low (16–43 IU/g) and showed no appreciable change despite differences in the farming conditions. This was lower than the values of vitamin D3 in skipjack and tuna liver oils were 57,760 and 16,200 IU/g, respectively. In the muscle and skin of marbled eels in Thua Thien Hue, Vietnam, the vitamin D3 content was 279 and 350 µg/kg, respectively (). According to Wijayanti and Setiyorini (Citation2018), the vitamin E content of the wild eel was 0.21% while the cultured eel was 0.224%. This value corresponds to the concentration found in muscle 23.9 mg/kg (0.239%) and is higher than the value in the skin (14.5 mg/kg or 0.145%) in . In the research of Jamaluddin et al. (Citation2020), vitamin B3 of the yellow eel is 2.97 × 10−6 g/100 g; the silver eel is 1.17 × 10−5 g/100 g; and the levels of vitamins B1, B5, B6, and B9 are not detected. This is consistent with our study on the content of vitamin B3 found in the muscle, skin, and mucus of marbled eels as 17.7, 15.7, and 7.29 mg/kg, respectively () lower than the value found in the study by Jamaluddin et al. (Citation2020) at the yellow eel stage (2.97 × 10−6 g/100 g) and corresponding to the silver eel stage ((1.17 × 1.17 × 100 g) · 10−5g/100 g).
In this study, we found significant levels of vitamin B5 in the muscle and slime of marbled eels with concentrations of 3.10 and 104 mg/kg, respectively, whereas Jamaluddin et al. (Citation2020) reported no detectable B5 in the muscle of marbled eels. Similarly, Vitamin C in the muscle and mucus of marbled eels was determined to be 4.55 and 12.6 mg/kg respectively (). However, Jamaluddin et al. (Citation2018a), previously reported not detected there was the content of vitamin C in A. marmorata from the Poso lake and estuary of Palu rive. Vitamin E and vitamin K1 were evaluated for the first time in this study, the results showed that the vitamin E content detected with a higher value in the muscle of 23.9 mg/kg was 1.65 times higher than 14.5 mg/kg in the skin. Vitamin K1 was not detected in eel samples with Method Detection Limit = 200 based on the HPLC-UV technique ().
Moreover, there are significant differences (p < 0.05) in the composition and content of different vitamins in different parts of the marbled eels, indicating their nutritional value and potential applications are related to different purposes. A vitamin is an organic molecule (or a set of chemically related molecules, i.e. vitamers) that is an essential micronutrient that an organism needs in small amounts to process. Its metabolism works normally. The term vitamins exclude three other groups of essential nutrients: minerals, essential fatty acids, and essential amino acids (Maton et al. Citation1993). The major health organizations list thirteen types: vitamin A (as all-trans-retinol, all-trans-retinyl-esters, as well as all-trans-beta-carotene and other provitamin A carotenoids), vitamin B1 (thiamine), vitamin B2 (riboflavin), vitamin B3 (niacin), vitamin B5 (pantothenic acid), vitamin B6 (pyridoxine), vitamin B7 (biotin), vitamin B9 (folic acid or folate), vitamin B12 (cobalamin), vitamin C (ascorbic acid), vitamin D (calciferol), vitamin E (tocopherol and tocotrienols) and vitamin K (phylloquinone and menaquinone) (World Health Organization Citation2005). Six of them were detected in marbled eels (), in which, Vitamin A acts as a regulator of cell and tissue growth and differentiation. Vitamin D provides hormone-like function, regulating mineral metabolism for bones and other organs. B complex vitamins act as enzyme cofactors (coenzymes) or precursors. Vitamins C and E act as antioxidants (Bender Citation2003). In addition, vitamins are also a source of research for the production of cosmetic and pharmaceutical products, in which, therapeutic vitamins (vitamins A and D), and their analogues, and antioxidant vitamins (vitamins C, E, and coenzyme Q) play an increasing role in skin care. Their benefits range from skin conditions such as acne and psoriasis to protect against environmental damage (Stanley and Claude Citation2001). Studies on the discovery and commercial production of vitamins have been carried out and have greatly contributed to the completion of human nutritional needs (Stanley and Claude Citation2001; Blaner Citation2020).
Conclusion
In this study, by applying the HPLC analysis technique, we analysed the composition of amino acids, fatty acids, and seven different vitamins in the muscle, skin, and mucus of the marbled eel (A. marmorata). The use of the HPLC technique allows us to analyse the trace elements in the nutritional composition of fish. Moreover, the results obtained from this study have confirmed that eel is a valuable source of nutrients, including protein and vitamins, that can be used to promote human health. At the same time, it has opened up new potentials in nutritional research, application, and exploitation of the value of marbled eel in pharmaceuticals and natural cosmetics for humans.
Ethics approval and consent to participate
The uses of all animals and samples in the study applied international, national, and regional and institutional guidelines for animal care and rules in Vietnam.
Data availability statement
All data and materials are available in the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- AOCS. 1989. Official and recommended methods of the American Oil chemists’ society. 4th ed. Champaign: American Oil Chemists’ Society.
- Arai T. 2016. Taxonomy and distribution. In: Arai T, editor. Biology and ecology of anguillid eels. CRC Press; p. 1–20. https://bit.ly/3lHk3lU.
- Atsuko T, Toshio O, Mari A, Harumi Y, Sumiko T, Yumiko M, Tadashi K. 1984. High-performance liquid chromatographic determination of vitamin D3 in fish liver oils and eel body oils. J Nutr Sci Vitaminol. 30(5):421–430. doi:10.3177/jnsv.30.421.
- Baynes JW, Marek HD. 2005. Medical biochemistry. 2nd ed. Philadelphia: Elsevier Mosby; p. 555.
- Bender DA. 2003. Nutritional biochemistry of the vitamins. Cambridge, UK: Cambridge University Press.
- Blaner WS. 2020. Vitamin A. In: BP Marriott, DF Birt, VA Stallings, AA Yates, editors. Present knowledge in nutrition. 11th ed. London: Academic Press (Elsevier); p. 73–92. ISBN 978-0-323-66162-1.
- Bullock AM. 1980. A comparative study of the epidermal morphology of fishes of the order Anacanthidea, with references to their ecology and distribution. Oceanogr Mar Biol Ann Rev. 18:251–315.
- Casimir MJ, Fricke HW. 1971. Zur Funktion, Morphologie und Histochemie der Schwanzdnise bei Riihrenaalen (Pisces, Apodes, Heterocongridae). Mar Biol. 9:339–346. doi:10.1007/BF00372828.
- Cassiano FG-d-A, Silva AR, Burth P, Castro-Faria MV, Castro-Faria-Neto HC. 2016. Chapter 23 – Oleic acid and lung injury. In: Ronald Ross Watson, Fabien De Meester, editors. Handbook of lipids in human function. AOCS Press; p. 605–634. doi:10.1016/B978-1-63067-036-8.00023-8.
- Choulis NH. 2011. Chapter 49 – Miscellaneous drugs, materials, medical devices, and techniques. In: J.K. Aronson, editor. Side effects of drugs annual, 33. Elsevier; p. 1009–1029. doi:10.1016/B978-0-444-53741-6.00049-0.
- Dash S, Das SK, Samal J, Thatoi HN. 2018. Epidermal mucus, a major determinant in fish health: a review. Iran J Vet Res. 19:72–81.
- Ernesto MH. 2016. 4 – Specialty oils: functional and nutraceutical properties. In: Thomas A.B. Sanders, editor. Woodhead publishing series in food science, technology and nutrition, functional dietary lipids. Woodhead Publishing; p. 69–101. doi:10.1016/B978-1-78242-247-1.00004-1.
- Fishelson L. 1996. Skin morphology and cytology in marine eels adapted to different lifestyles. Anat Rec. 246:15–29. doi:10.1002/(SICI)1097-0185(199609)246:1<15::AID-AR3>3.0.CO;2-E.
- Folch J, Lees M, Stanley GS. 1957. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 226:497–509. doi:10.1016/S0021-9258(18)64849-5.
- Gómez-Limia L, Carballo J, Rodríguez-González M, Martínez S. 2022. Proximate composition and amino acid profile of European eel skin: influence of body weight. Eur Food Res Technol. doi:10.1007/s00217-022-03978-0.
- Gómez-Limia L, Cobas N, Martínez S. 2021. Proximate composition, fatty acid profile and total amino acid contents in samples of the European eel (Anguilla Anguilla) of different weights. Int J Gastron Food Sci. 25:100364. doi:10.1016/j.ijgfs.2021.100364.
- Jamaluddin J, Damayanti AT, Widodo A. 2018a. Vitamin C Ikan Sidat (Anguilla marmorata) Asal Danau Poso Dan Muara Sungai Palu. Jurnal Gizi dan Kesehatan. 2(1). DOI: 10.22487/ghidza.v2i1.10256
- Jamaluddin J, Widodo A, Mufliha N. 2018b. Vitamin A of eel fish (Anguilla marmorata) from palu river and poso lake. Jurnal Gizi dan Kesehatan. 2(1):24–30. http://jurnal.untad.ac.id/jurnal/index.php/ghidza.
- Jamaluddin J, Widodo A, Setiawan N. 2020. Studi Perbandingan Kandungan Vitamin B Ikan Sidat (Anguilla marmorata (Q.) Gaimard) Fase Yellow Eel dan Silver Eel Asal Sungai Palu Sulawesi Tengah.Ghidza. Ghidza: Jurnal Gizi dan Kesehatan. 4(2):120–130. doi:10.22487/ghidza.v4i2.36.
- Kahoun D, Fojtíková P, Vácha F, Čížková M, Vodička R, Nováková E, Hypša V. 2022. Development and validation of an LC-MS/MS method for determination of B vitamins and some its derivatives in whole blood. PLoS One. 17(7):e0271444. doi:10.1371/journal.pone.0271444.
- Kelly C, Salinas I. 2017. Under pressure: Interactions between commensal microbiota and the teleost immune system. Front Immunol. 8:559. doi:10.3389/fimmu.2017.00559.
- Kieu TH, Nguyen QL. 2020a. Nutrition composition and lipid distribution in the flesh of species Anguilla marmorata natural mining in Thua Thien Hue, Vietnam. Hue Univ J Sci. 129:3C. http://jos.hueuni.edu.vn/index.php/HUJOS-ARD/article/view/5848.
- Kieu TH, Nguyen QL. 2020b. Phylogenetic analysis of Anguilla marmorata population in Thua Thien Hue, Vietnam based on the cytochrome C oxidase I (COI) gene fragments. AMB Express. 10(122). doi:10.1186/s13568-020-01059-7
- Kieu TH, Vo DN, Tran NN, Truong VD, Vo VP, Tran QD, Nguyen QL. 2020. Using DNA barcodes based on mitochondrial COI and 16S rRNA genes to identify Anguilla eels in Thua Thien Hue province, Vietnam. Genet Mol Res. 19(4):gmr18722. doi:10.4238/gmr18722.
- Lee K, Kim YJ, Yang KH, Song MY, Lee WO, Hwang KT. 2020. Lipid content and fatty acid composition of freshwater eels Anguilla japonica caught in different seasons and locations in South Korea. Fish Sci. 86:573–580. doi:10.1007/s12562-020-01421-5.
- Leonard JB, Summers RG. 1976. The ultrastructure of the integument of the American eel, Anguillu rostratu. Cell Tissue Res. 171:1–30. doi:10.1007/BF00219697.
- Maton A, Hopkins J, McLaughlin CW, Johnson S, Warner MQ, LaHart D, Wright JD. 1993. Human biology and health. Englewood Cliffs, NJ: Prentice Hall.
- Ministry of Science and Technology of Vietnam. 2011a. TCVN 8972-1:2011 (Foodstuffs – determination of vitamin A by high-performance liquid chromatography – Part 1: Measurement of all-trans-retinol and 13-cis-retinol).
- Ministry of Science and Technology of Vietnam. 2011b. TCVN 8973:2011, Foodstuffs – determination of vitamin D by high performance liquid chromatography – Measurement of cholecalciferol (D3) or ergocalciferol (D2).
- Ministry of Science and Technology of Vietnam. 2011c. TCVN 8977:2011 (EN 14130:2003), Foodsuffs – determination of vitamin C by high-performance liquid chromatography (HPLC).
- Ministry of Science and Technology of Vietnam. 2012. TCVN 8764:2012 (ISO 13903:2005) for Animal feeding stuffs – determination of amino acids content.
- Ministry of Science and Technology of Vietnam. 2013a. TCVN 9969:2013 (ISO 15885:2002) Milk fat – determination of the fatty acid composition by gas-liquid chromatography.
- Ministry of Science and Technology of Vietnam. 2013b. TCVN 9969:2013 (ISO 15885:2002), Milk fat – determination of the fatty acid composition by gas-liquid chromatography.
- Ministry of Science and Technology of Vietnam. 2020. TCVN 13263-2:2020, Fertilizers – Part 2: Determination of vitamin B group content by high performance liquid chromatographic method.
- Nafsiyah I, Nurilmala M, Abdullah A. 2018. Nutrient composition of eel Anguilla bicolor bicolor and Anguilla marmorata. Jurnal Pengolahan Hasil Perikanan Indonesia. 21(3):504–512. DOI: 10.17844/jphpi.v21i3.24733.
- Pickering AD. 1974. The distribution of mucous cells in the epidermis of the Brown Trout, Sulmo trutu (L.) and the char Salvelinus alpinus (L.). J Fish Biol. 6:111–118. doi:10.1111/j.1095-8649.1974.tb04531.x.
- Preedy VR, Watson R. 2020. Olives and olive Oil in health and disease prevention. Cambridge, MA: Academic Press.
- Reverter M, Tapissier-Bontemps N, Lecchini D, Banaigs B, Sasal P. 2018. Biological and ecological roles of external fish mucus: a review. Fishes. 3:41. doi:10.3390/fishes3040041.
- Salinas I, Zhang YA, Sunyer JO. 2011. Mucosal immunoglobulins and B cells of teleost fish. Dev Comp Immunol. 35:1346–1365. doi:10.1016/j.dci.2011.11.009.
- Sari NP, Jamaluddin AW. 2019. Vitamin A Sidat fish (Anguilla bicolor) from Lake Poso, Central Sulawesi Ghidza. Jurnal Gizi dan Kesehatan. 3(2):62–66.
- Seo JS, Choi JH, Seo JH, Ahn TH, Chong WS, Kim SH, Ahn JC. 2013. Comparison of major nutrients in eels Anguilla japonica cultured with different formula feeds or at different farms. Fish Aqua Sci. 16(2):85–92.
- Smith DG. 1999. Anguillidae. Freshwater eels. In: Carpenter KE, Niem VH, editors. FAO species identification guide for fishery purposes. The living marine resources of the Western Central Pacific. Volume 3. Batoid fishes, chimaeras and bony fishes part 1. Rome: FAO; p. 1630–1636.
- Sridhar A, Manikandan DB, Palaniyappan S, Sekar RK, Ramasamy T. 2021. Correlation between three freshwater fish skin mucus antiproliferative effect and Its elemental composition role in bacterial growth. Turkish J Fish Aquat Sci. 21:233–244. doi:10.4194/1303-2712-v21_5_03.
- Stanley SS, Claude S. 2001. Role of vitamins in skin care. Nutrition. 17(10):839–844. doi:10.1016/S0899-9007(01)00660-8.
- Swain P, Dash S, Sahoo PK, Routray P, Sahoo SK, Gupta SD, Meher PK, Sarangi N. 2007. Nonspecific immune parameters of brood Indian major carp Labeo rohita and their seasonal variations. Fish Shellfish Immunol. 22:38–43. doi:10.1016/j.fsi.2006.03.010.
- Tagami M, Kuwahara J. 2020. Evaluation of antioxidant activity and amino acids in the mucus of mackerel for cosmetic applications. J Oleo Sci. 69(9):1133–1138. doi:10.5650/jos.ess20029.
- Tasleem F, Amjad R, Sherazi SDZ, Saddique A, Saba A, Zainab SN, Masood S. 2020. Physiology and biochemical properties of fish mucus particular emphasizes as a body defense system. Haya Saudi J Life Sci. 5(12):274–280. DOI: 10.36348/sjls.2020.v05i12.002.
- Tesch FW. 2003. The eel. Oxford: Blackwell Science.
- Whitear M. 1986. The skin of fish, including cyclostomes. In: J. Bereiter-Hahn, A.G. Matolsy, K.S. Richards, editors. Biology of the Integument, 2. Vertebrates. Berlin: Springer Verlag; p. 8–64. doi:10.1007/978-3-662-00989-5_2
- Widyasari RHE, Kusharto CM, Wiryawan B, Wiyono ES, Suseno SH. 2014. Pemanfaatan limbah ikan sidat Indonesia (Anguilla bicolor) sebagai tepung pada industri pengolahan ikan di palabuhanratu, kabupaten sukabumi. J Gizi dan Pangan. 8(3):215–220.
- Wijayanti I, Setiyorini ESS. 2018. Nutritional content of wild and cultured Eel (Anguilla bicolor) from southern coast of central java. ILMU KELAUTAN: Indonesian J Marine Sci. 23(1):37–44. doi:10.14710/ik.ijms.23.1.37-44.
- World Health Organization. 2005. Vitamin and mineral requirements in human nutrition, 2nd ed. World Health Organization; p. 341. https://apps.who.int/iris/handle/10665/42716.
- Yu S, Derr J, Etherton TD, Kris-Etherton PM. 1995. Plasma cholesterol-predictive equations demonstrate that stearic acid is neutral and monounsaturated fatty acids are hypocholesterolemic. Am J Clin Nutr. 61:1129–1139. doi:10.1093/ajcn/61.5.1129.
- Zaccone G, Kapoor BG, Fasulo S, Ainis L. 2001. Structural, histochemical and functional aspects of the epidermis of fishes. Adv Mar Biol 40:253–348. doi:10.1016/S0065-2881(01)40004-6.
