ABSTRACT
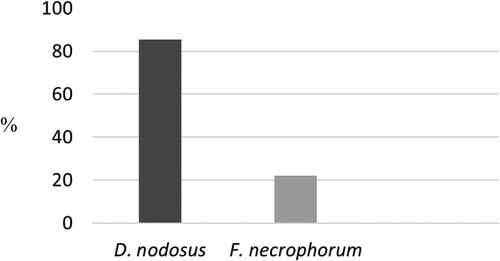
The aim of this study was to determine the prevalence of bacteria Dichelobacter nodosus (D. nodosus) and Fusobacterium necrophorum (F. necrophorum) in non-lame dairy cows on eight Slovak dairy herds. In total, 1,631 Holstein Friesian dairy cows were included in the study. Information of the cows was collected from on-farm software included cow ID, lactation number (heifer/cow), year milk yield (kg), and DIM on collection date. The PCR method detected D. nodosus and F. necrophorum on the feet of 1,394 (85.5%) and 373 (22.1%) dairy cows, respectively (p < 0.05). No dairy farm could be found without positive samples for D. nodosus and the majority of the farms were free or had very low prevalence of F. necrophorum (0–9.1%). Statistical difference for the prevalence of D. nodosus and F. necrophorum in different Slovak regions was detected for both strains. Logistic regression revealed an association between both D. nodosus and F. necrophorum identification and annual milk production (odds ratios = 0.69 and 0.32, respectively). Our data demonstrates that D. nodosus is present on the feet of almost every dairy cow what means a potential risk for cattle to develop foot rot. Furthermore, both bacteria are associated with decreased annual milk production.
Introduction
Lameness is an important negative phenomenon that affects the welfare and productivity of dairy cows worldwide (Jurkovich Citation2007; Huxley Citation2013). Claw disorders are responsible for 92% of lameness (Murray et al. Citation1996) and they can be classified as infectious (e.g. interdigital dermatitis and digital dermatitis) and noninfectious (e.g. sole ulcer and white line disease), and each claw disorder has a specific effect on cow performance.
Foot rot (interdigital phlegmon) is a significant health disorder of dairy cattle caused by bacteria D. nodosus and F. necrophorum (Roberts and Egerton Citation1969; Langworth Citation1977; Wani and Samanta Citation2006; Bennett et al. Citation2009b). Foot rot usually occurs as a sporadic infection of cattle. The herd incidence per lactation is generally below 5%, but also prevalence rates of up to 25% have been reported ((David Citation1993; DeFrain et al. Citation2013). Interestingly, in a study investigating the prevalence of claw disease in two dairy herds in Slovakia, zero cases of foot rot were detected (Mudroň Citation2016). In many cases, it could cause a critical condition for breeders due to fast onset of severe clinical signs, such as debilitating pain, varying degrees of lameness and in the end a long-term recumbency of the animal (Reinöhl-DeSouza and Kofler Citation2006). Affected animals have a reduced feed intake which has a negative influence/impact on the farm economics, and thus, it is necessary to detect and diagnose these animals immediately (Hernandez et al. Citation2002). Confirmation of the presence of foot rot in the farm should result in the rapid application of therapy/prevention measures both to heal affected animals and to protect other animals from infection (Morck et al. Citation1998; Reinöhl-DeSouza and Kofler Citation2006; Apley Citation2015; Osová et al. Citation2017).
F. necrophorum is an obligatory anaerobic gram negative bacterium, pleomorphic or bacillary shaped. It is a part of the natural microflora of the gastrointestinal tract of animals and humans (Langworth Citation1977; Narayanan et al. Citation2002; Nagaraja et al. Citation2005; Bennett et al. Citation2009a; Bennett et al. Citation2009b). Exotoxin – leukotoxin (lktA), produced by F. necrophorum subspecies necrophorum, is a major virulence factor within biotype A and it is a primary toxin to ruminant leucocytes. It can induce apoptosis of immune cells and at higher concentrations damage leucocytes, additionally it is more active against polymorph nuclear leucocytes than to lymphocytes (Narayanan et al. Citation2002; Nagaraja et al. Citation2005). The lktA gene appears to be unique to F. necrophorum, as it is reportedly not present in other Fusobacterium species (Oelke et al. Citation2005), D. nodosus is a bacillary shaped gram negative, obligatory anaerobic bacteria. The mechanism explaining the synergistic relationships of F. necrophorum and D. nodosus can run through the production of lktA by F. necrophorum (Narayanan et al. Citation2001), which is involved in assisting in the bacterias proliferation by creating an immunocompromised environment of the skin. This is optimal for growth and activity of D. nodosus. The Leukotoxin – mediated effect is the likely cause of foot rot, since D. nodosus is known to be proficient at attacking the immune system (Roberts and Egerton Citation1969). In addition, the virulent strains of D. nodosus are able to produce a proteolytic enzyme, causing hydrolysis of keratin which results in disruption of the skin structure (Thomas Citation1962). The disrupted skin is a medium enriched with nutrients and growth factors suitable for further propagation of F. necrophorum that leads to necrotic tissue breakdown (Bennett et al. Citation2009a; Citation2009b). In previous decades a fast, accurate method of reverse dot blot hybridization and PCR was discovered and introduced for the detection of anaerobic bacteria (La Fontaine et al. Citation1993; Zhou et al. Citation2001). F. necrophorum and D. nodosusus are frequently found on the claws of lame dairy cattle and may be associated with lameness (Bennett et al. Citation2009a).
Therefore, the purpose of this study was to determine the prevalence of bacteria D. nodosus and F. necrophorum in non-lame dairy cows in a selection of Slovak dairy herds.
Material and methods
Animals and sampling
Samples were collected on eight Slovak dairy farms (F1 – F8). Three farms were located in Eastern Slovakia (F1, F2, F3), two in West Slovakia (F4, F5), and three in Central Slovakia (F6, F7, F8). In total, 1,631 Holstein Friesian dairy cows were included in the study. The farms did not differ in farm hygiene protocols (free-stalls with lying boxes) or feeding regime (TMR twice a day). The lying boxes with concrete surfaces were bedded with straw. The forage component of the diet included maize and grass silage. Cow information collected from on-farm software included cow ID, lactation number (heifer/cow), year milk yield (kg), and DIM on the collection date. The annual milk yield ranged from 7,143 (Farm 6) to 12, 686 (Farm 5) kg. The dairy cows on the farms underwent regularly claw trimming twice per year by professional trimmers. The use of foot-baths was not used regularly or consecutively as part of the management on these farms.
Sample collection was performed by two authors of this study (PM and AMO), each one visiting farms separately but following a standardized methodology. For sampling swabs Amies Agar Gel Medium Transport Swabs (Sarstedt) were used. The swabs were obtained from healthy dairy cows from the plantar interdigital space of one of the pelvic limbs (‘Surface’ swab) during milking in the milking parlour, not preceded by cleaning or other action performed on the feet before sampling. This method is very quick and easily enforced even during milking without the need of limb fixation (Osová et al. Citation2018).
Sample processing and DNA extraction
The swabs were incubated in 1.5 ml anaerobic broth (AB – Anaerobic Basal broth, HiMedia) for 48 h at 37° C under anaerobic conditions created by the Anaerocult (Merck). Immediately after the incubation the extraction of DNA was processed by freezing and boiling. Briefly, after a centrifugation (Eppendorf Centrifuge 5418) of the samples (5000 xg for 5 min at room temperature) the resulting sediment was resuspended in 500 µl of saline and re-centrifuged (5000 xg for 5 min at room temperature). After the removal of the supernatant, the sediment (pellet) was resuspended in 100 µl distilled water. Subsequently, the sample was frozen at −70°C for 10 min and boiled for 5 min at 100°C. Finally, the samples were centrifuged at 13900 xg for 5 min at room temperature. The supernatant containing the DNA was then transferred to a clean tube. The DNA concentration in the samples was measured with a NanoDrop™ 8000 Spectrophotometer, Thermo Scientific. The purity of isolated nucleic acids was determined by the ratio of the measured absorbance A260/280. The DNA sample without contamination of proteins and other organic substances was considered pure when the ratio of the measured absorbance was between 1.8 and 2.0. A value below 1.8 indicates sample contamination. Subsequently, the sample with the isolated DNA was stored at −20° C until the testing.
PCR and electrophoresis
For the diagnostics of D. nodosus specific primers were designed for the detection of 16S rRNA gene encoding, which is by its sequence specific to the bacterial species. Diagnostics of F. necrophorum were performed with primers specifically designed for the gene region leucotoxin A (lktA). Based on the known gene sequences and the applications of general principles of selecting the specific primers, the primers were designed with the use of the software Primer3 (SAS EMBnet node, EMBnet Slovakia). The primers were synthesized and delivered by Generi Biotech (Hradec Králové, Czech Republic, and ). Conditions of the amplification and the composition of the reactive solution were recently presented (Osová et al. Citation2018).
Table 1. Primer sequention for Dichelobacter nodosus (16S rRNA).
Table 2. Primer sequention for Fusobacterium necrophorum (lktA).
DNA used for the PCR optimization and also as a positive control was isolated from anaerobic cultures of lyophilized bacterial strains D. nodosus (DSM 20708) from collections in Germany (Leibniz Institute DSMZ – German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany) and F. necrophorum (CCM 5981) from the collection in Brno (CCM Czech Collection of Microorganisms, Brno, Czech Republic). Extraction of DNA was done with an isolation kit ROCHE. As a negative control for PCR qH2O was used.
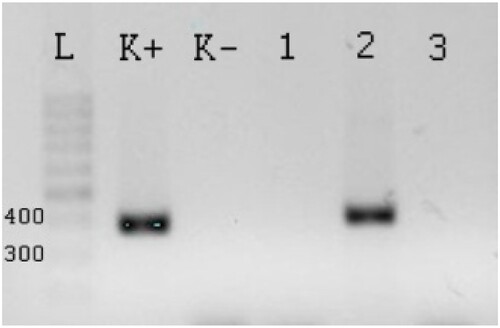
PCR was evaluated by agarose electrophoresis. DNA separation was performed in 2% agarose gel and the DNA was visualized by the GelRed ™ (Biotium, Inc.) flouroscein dye. The amplicon resulting from the PCR of the 16S rRNA transcription of the D. nodosus was detected in 295 bp and the lktA for F. necrophorum was detected in 362 bp. 50 bp GeneRuler DNA Ladder was used (Thermo Fisher Scientific) for molecular weight standards ().
Figure 1. The PCR product of lktA F. necrophorum generated from field samples. L – Ladder 50 bp, K+ – positive control, K− – negative control with qH2O. Column 1–3 samples for F. necrophorum, column 1- the method of freezing and boiling from PBS is negative, lane 2 – the method of freezing and boiling from AB is positive, lane 3 – the method with high pure PCR template preparation kit is negative.
Statistical evaluation
All statistical analyses were performed using R software version 3.6.3 (R Core Team Citation2021). The outcome of interest was the bacterial status (negative or positive). The Chi-squared test was used to assess differences between the prevalence of D. nodosus and F. necrophorum in dairy cows and among the farms and regions. Differences were considered significant when P < 0.05.
We studied the association of bacteria in a multinomial logistic regression model. The farm, region, lactation number, and DIM had no effect on the results and were not included in the final model. The outcome of the model was bacterial contamination with D. nodosus and F. necrophorum and the variables were farm, region, lactation number, year milk yield, DIM, and bacterial contamination of both bacteria. The year milk yield and DIM were transformed by taking the natural logarithm to achieve normally distributed data.
Results
The mean size of the herd samples was 204 (range: 110–310), the herd mean 305-day milk production was 9,019 kg (range: 7,143–12,686 kg), and the mean herd sampling DIM was 197 (range: 193–200). The total final sample size for all eight farms is 1,631 sampled dairy cows. The PCR method detected D. nodosus and F. necrophorum in 1,394 (85.5%) and 373 (22.1%) animals, respectively (p < 0.05; ).
The authors compared the prevalence of the bacteria among herds with no difference in housing and management conditions. There was a statistical difference in for the prevalence of D. nodosus existed between the herds with over 80 and below 55%. However, this lower prevalence could only be observed only in two dairy herds. In addition, none of the dairy farms in this study could be found without positive samples for D. nodosus in our study. The majority of the dairy farms were free from or had a very low prevalence of F. necrophorum (0–9.1%). They differed statisticaly from the three herds with the prevalence of Fusobacterium necrophorum over 43%. In contrast to total positivity for D. nodosus in more than 30% of dairy farms F. necrophorum could not be detected ().
Table 3. Numbers and frequencies (%) of dairy cows positive for D. nodosus and F. necrophorum.
Farms have been sampled across all geographical regions of Slovakia. In Eastern Slovakia, 564/711 (79.3%) D. nodosus positive cows were observed, while 450/460 (97.8%) cows were positive for D. nodosus in West Slovakia and 380/460 (83%) in the Central Slovakia. Statistical difference for the prevalence of D. nodosus in different regions existed only for West Slovakia with the highest prevalence. In 1,631 dairy cows, Fusobacterium necrophorum was detected 113/711 (16%) in the Eastern Slovakia, 150/460 (32.6%) in the West Slovakia, and 110/460 (24.2%) in the Central Slovakia. Statistical difference in the prevalence of F. necrophorum in different regions existed only between Eastern and West Slovakia with the lowest and highest prevalence, respectively ().
Table 4. Numbers and frequencies (%) of dairy cows positive for D. nodosus and F. necrophorum in regions.
The authors investigated the association of farm, region, lactation number, year milk yield, DIM, and bacterial contamination with the bacterial species detected by PCR (). D. nodosus was distinctively associated with annual mik yield and F. necrophorum detection (OR = 0.69, p < 0.05; OR = 2.37, p < 0.05), respectively. Simirarly, F. necrophorum was negatively associated with annual mik yield and D. nodosus detection (OR = 0.32, p < 0.05; OR = 4.55, p < 0.05), respectively. Effect plots were made for the interaction terms: F. necrophorum positivity (FN) x milk yield (lnkg) and D. nodosus positivity (FN) x lnkg ( and ). shows the association between milk yield and D. nodosus positivity for distinct positivity for F. necrophorum. When a cow is FN positive, the risk of being DN positive increases with an decreasing milk yield. A similar effect could be observed for D. nodosus positivity (FN) x lnkg (). A decrease in milk yield was associated with an increased risk of of being FN positive were only in cows that were DN negative.
Figure 3. Effect plot of the interaction milk yield x F. necrophorum positivity. Milk yield (kg): natural logarithm.
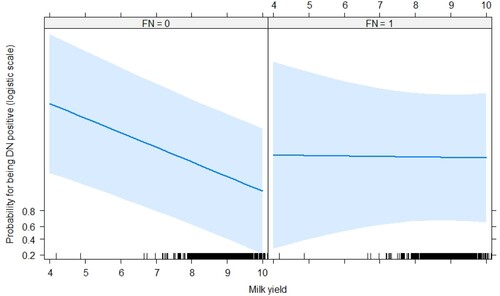
Figure 4. Effect plot of the interaction milk yield x D. nodosus positivity. Milk yield (kg): natural logarithm.
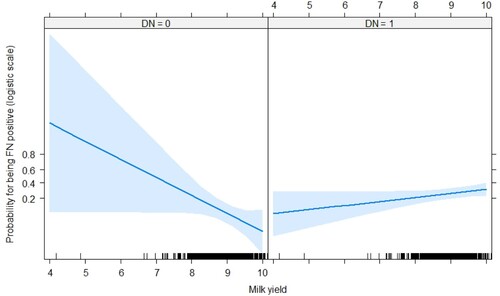
Table 5. The multinomial logistic regression model for the association of presence of bacteria and the final model variables in 1,631 Slovak dairy cows.
Discussion
Currently there is a general consensus that D. nodosus and F. necrophorum are anaerobic bacteria responsible for the development of foot rot in ruminants. Both are commonly detected in samples obtained from animals suffering not only from interdigital phlegmon but also from other claw diseases, including digital dermatitis, sole ulcers, or toe ulcers, with a prevalence of up to 100% (Zhou et al. Citation2009; Bennett et al. Citation2009a; Knappe-Poindecker et al. Citation2013; Citation2014). It is not surprising as all of these conditions are associated with the necrotic tissue lesions.
At the farm level, it still remains to be clarified whether D. nodosus and F. necrophorum are widely spread in the healthy population of dairy cattle. In the first study on healthy cattle, a 1.5 to 18.9% prevalence of D. nodosus has been demonstrated (Laing and Egerton Citation1978). Based on those results, severe lesions were considered to be exceptional sequela under normal conditions. In another study of claw diseases, D. nodosus was identified in 12.5% of the cows with healthy claws (Rasmussen et al. Citation2012). On the contrary, in the study with a strict definition of foot rot, all the sheep flocks without foot rot were negative for D. nodosus (Monaghan et al. Citation2021). Their results may confirm the hypothesis that D. nodosus, the causative agent of foot rot in sheep, is not present in flocks where there is no foot rot. However, in the study on sheep digital dermatitis D. nodosus was detected on the feet of healthy, control animals (Staton et al. Citation2021). In the Norwegian study that included 14 dairy herds with a total of 633 cows, D. nodosus was detected in 66.0% of cows with healthy feet, in 97.1% of the cows with interdigital dermatitis, in 36.4% with heel horn erosions, in all cows with both interdigital dermatitis and heel horn erosions, and in all cows with digital dermatitis (Knappe-Poindecker et al. Citation2013). In the present study, we analysed interdigital space swabs from non-lame dairy cows in eight herds in Slovakia. The herds were typical for modern dairy herds performing routine claw trimming and animal health registrations. The study was performed in three different regions of the country and it was stated that every dairy farmer was able to participate. The combination of 1,631 analysed swab samples and the regionally different herds suggests that the results of the study are likely to be robust. In agreement with the findings of Knappe-Poindecker et al. (Citation2013), most of the cows and all the herds were positive. In our study, 1394 (85.5%) cows and 100% of herds were positive for D. nodosus. Our analysis on the farm level has demonstrated a 100% prevalence of D. nodosus on three farms. Although the current study has highlighted a high level of exposure to D. nodosus, the prevalence is comparable to those reported for other regions. Similar to our results, a cross-sectional study done in Switzerland by Ardüser et al. (Citation2020) also showed that D. nodosus is common in many cattle farms. They did not detect virulent D. nodosus in cattle, but the prevalence of the benign form was 88.5%. As the difference in herd prevalence of D. nodosus in our study must be assumed to be high, it can be concluded that the herd level prevalence is very significant based on the definition of a herd being positive if at least one animal is tested positive. From this point on, the detected significant differences in the prevalence of D. nodosus among the farms are of importance. The density of sheep or wild ruminants surrounding the farm may have a role to play. In the field study in Germany on 9,000 sheep D. nodosus was detected in 42.9% of the animals (Storms et al. Citation2021). In another clinical study findings and the coexistence of the same serogroups of D. nodosus in co-grazing sheep and cattle indicated cross-infection (Rogdo et al. Citation2012). We can assume that the farms might differ in certain risk factors for the growth of these bacteria such as varying degrees of moisture and warmth in the environment, increased parity and milk yield, inappropriate housing conditions and infrastructures, inadequate hygiene status, imbalanced nutrition, insufficient claw care, and lameness control plans which should include targeted implementation of claw trimming and footbathing, and the continuous training of farming personnel. Milk yield showed a statistically significant association with the detection of both bacteria in our study, implying that lower milk production was associated with a higher risk of positive swabbing. Hernandez et al. (Citation2002) found that cows that had claw diseases during the lactation had a decreased milk yield compared to those that were not lame throughout the whole lactation. However, this was only the case in animals having interdigital phlegmon; in those presenting with digital dermatitis or claw horn disruption, no statistically significant association with 305-d milk yield was shown.
In this study, F. necrophorum was found as the less frequent pathogen in comparison with D. nodosus. F. necrophorum is an obligate anaerobic, Gram-negative bacterium, pleomorphic or bacillus-shaped and it is part of the gastrointestinal microbiome of humans and animals (Nagaraja et al. Citation2005). In a study of infectious agents associated with interdigital phlegmon, a small number of fusobacteria were detected on healthy claws (Johnson et al. Citation1969). This finding is consistent with a recent study of sheep digital dermatitis etiology where F. necrophorum bacteria were found in animals with no foot lesions ((Staton et al. Citation2021). In the Finnish study including 22 dairy herds with a total of 228 cows, D. nodosus was (27.1%) but F. necrophorum was not detected on the skin of healthy claws, even when a severe foot rot outbreak was evident in the herd (Konturi et al. Citation2020). Based on those results, they concluded that F. necrophorum does not colonize the intact skin in large numbers. Inappropriate housing or poor level of claw care have been mentioned as predisposing factors for interdigital phlegmon in previous studies (DeFrain et al. Citation2013). In contrast, in the present study, F. necrophorum was detected in the interdigital space of healthy claws in 22.1% of dairy cows with a significant difference in the herd prevalence. Of eight farms, F. necrophorum was not detected on three of them. Two of these three farms are located in the Eastern Slovakia, therefore, this region had shown the lowest prevalence. Our study was not performed on dairy farms with strong variations in herd-level factors possibly affecting the skin claw condition of dairy cows. However, we agree with the conclusion of the Canadian study that herd-level factors can explain most of the disease probability of claw diseases, including infectious diseases, heel erosion, and hemorrhages (Arango-Sabogal et al. Citation2020). The positive results of F. necrophorum detection can depend on various factors. Unexpectedly, in repeated sampling, fusobacteria were cultivated from foot rot lesions even though cows had been treated with antimicrobials and interdigital phlegmone was at the healing stage ((Konturi et al. Citation2020). The antimicrobial resistance of F. necrophorum should not play an important role, as another study reported that this resistance is not characteristic of F. necrophorum (Knappe-Poindecker et al. Citation2013). In a study of digital dermatitis contaminants, a low herd prevalence of F. necrophorum (0.0–0.25%) was detected when the affected bovine claws were sampled (Rasmussen et al. Citation2012). Bennett et al. (Citation2009a) reported a much higher prevalence of F. necrophorum with 53.4 and 100% in cows with claw diseases. In the study of the F. necrophorum variants, eight of the nine samples from cattle were positive for a variant that matched the type strain of F. necrophorum subsp. necrophorum and of the 14 samples from sheep, 13 were positive for lktA, but none of these matched the known type strains (Zhou et al. Citation2009). The authors concluded that none of the foot rot infections carried multiple variants of lktA, suggesting that only one strain of F. necrophorum is present in each case.
Conclusions
The results of this study show that the foot rot causative bacteria D. nodosus and F. necrophorum are common in dairy herds across Slovakia. Despite a very high prevalence of D. nodosus, there is no evidence of a high number of foot rot cases in dairy cows in the country. Our data demonstrates that D. nodosus is present on the feet of almost every dairy cow. Thus, the potential risk for cattle to develop foot rot is present and hence the farmers must always be aware of the potential for future outbreaks.
Acknowledgment
The technical assistance of Ľ. Tkáčiková and D. Stasinková is gratefully acknowledged.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Apley MD. 2015. Clinical evidence for individual animal therapy for papillomatous digital dermatitis (hairy heel wart) and infectious bovine pododermatitis (foot rot). Vet Clin North Am Food Anim Pract. 31:81–95.
- Arango-Sabogal JC, Desrochers A, Lacroix R, Christen AM, Dufour S. 2020. Prevalence of foot lesions in Québec dairy herds from 2015 to 2018. J Dairy Sci. 103:11659–11675.
- Ardüser F, Moore-Jones G, Gobeli Brawand S, Dürr S, Steiner A, Ryser-Degiorgis MP, Zanolari P. 2020. Dichelobacter nodosus in sheep, cattle, goats and South American camelids in Switzerland—Assessing prevalence in potential hosts in order to design targeted disease control measures. Prev Vet Med. 178:104688.
- Bennett G, Hickford J, Sedcole R, Zhou H. 2009b. Dichelobacter nodosus, Fusobacterium necrophorum and the epidemiology of foot rot. Anaerobe. 15:173–176.
- Bennett G, Hickford J, Zhou H, Laporte J, Gibbs J. 2009a. Detection of Fusobacterium necrophorum and Dichelobacter nodosus in lame cattle on dairy farms in New Zealand. Res Vet Sci. 87:413–415.
- David GP. 1993. Severe foul-in-the-foot in dairy cattle. Vet Rec. 132:567–568.
- DeFrain JM, Socha MT, Tomlinson DJ. 2013. Analysis of foot health records from 17 confinement dairies. J Dairy Sci. 96:7329–7339.
- Hernandez J, Shearer JK, Webb DW. 2002. Effect of lameness on milk yield in dairy cows. J Am Vet Med Assoc. 220:640–644.
- Huxley JN. 2013. Impact of lameness and claw lesions in cows on health and production. Livest Sci. 156:64–70.
- Johnson DW, Dommert AR, Kiger DG. 1969. Clinical investigations of infectious foot rot of cattle. J Am Vet Med Assoc. 155:1886–1891.
- Jurkovich V. 2007. Prevalence of claw disorders in dairy herds. Magy Allatorvosok. 129:468–473.
- Knappe-Poindecker M, Gilhuus M, Jensen TK, Klitgaard K, Larssen RB, Fjeldaas T. 2013. Interdigital dermatitis, heel horn erosion, and digital dermatitis in 14 Norwegian dairy herds. J Dairy Sci. 96:7617–7629.
- Knappe-Poindecker M, Gilhuus M, Jensen TK, Vatn S, Jørgensen HJ, Fjeldaas T. 2014. Cross-infection of virulent Dichelobacter nodosus between sheep and co-grazing cattle. Vet Microbiol. 170:375–382.
- Konturi M, Junni R, Kujala-Wirth M, Malinen E, Seuna E, Pelkonen S, Soveri T, Simojoki H. 2020. Acute phase response and clinical manifestation in outbreaks of interdigital phlegmon in dairy herds. Comp Immun Microb Infect Dis. 68:101375.
- La Fontaine S, Egerton JR, Rood JI. 1993. Detection of Dichelobacter nodosus using species-specific oligonucleotides as PCR primers. Vet Microbiol. 35:101–117.
- Laing EA, Egerton JR. 1978. The occurrence, prevalence and transmission of Bacteroides nodosus infection in cattle. Res Vet Sci. 24:300–304.
- Langworth BF. 1977. Fusobacterium necrophorum: its characteristics and role as an animal pathogen. Bacteriol Rev. 41:373–390.
- Monaghan EM, Prosser NS, Witt J, Lewis KE, Nabb E, Keeling MJ, Purdy KJ, Green LE. 2021. Impact of strain variation of Dichelobacter nodosus on disease severity and presence in Sheep Flocks in England. Front Vet Sci. 8:713927.
- Morck DW, Olson ME, Louie TJ, Koppe A, Quinn B. 1998. Comparison of ceftiofur sodium and oxytetracycline for treatment of acute interdigital phlegmon (foot rot) in feedlot cattle. J Am Vet Med Assoc. 212:254–257.
- Mudroň P. 2016. Effects of manure bedding on the rate of claw diseases in dairy cows. Folia Vet. 60:14–19.
- Murray RD, Downham DY, Clarkson MJ, Faull WB, Hughes JW, Manson FJ, Merritt JB, Russell WB, Sutherst JE, Ward WR. 1996. Epidemiology of lameness in dairy cattle: description and analysis of foot lesions. Vet Rec. 138:586–591.
- Nagaraja TG, Narayanan SK, Stewart GC, Chengappa MM. 2005. Fusobacterium necrophorum infections in animals: pathogenesis and pathogenic mechanisms. Anaerobe. 11:239–246.
- Narayanan SK, Nagaraja TG, Chengappa MM, Stewart GC. 2001. Cloning, sequencing, and expression of the leukotoxin gene from fusobacterium necrophorum. Infec Immun. 69:5447–5455.
- Narayanan SK, Nagaraja TG, Chengappa MM, Stewart GC. 2002. Leukotoxins of gram-negative bacteria. Vet Microbiol. 84:337–356.
- Oelke AM, Nagaraja TG, Wilkerson MJ, Stewart GC. 2005. The leukotoxin operon of fusobacterium necrophorum is not present in other species of fusobacterium. Anaerobe. 11:123–129.
- Osová A, Benito Pilipčincová Segurado, Király I, Dolník J, Mudroň M. 2018. Assessment of two different methods for sampling and detection of Dichelobacter nodosus and Fusobacterium necrophorum in dairy cows in Eastern Slovakia. J Appl Anim Res. 46:1452–1456.
- Osová A, Mihajlovičová X, Hund A, Mudroň P. 2017. Interdigital phlegmon (foot rot) in dairy cattle - an update. Wien Tierärztl Monat – Vet Med Austria. 104:209–220.
- Rasmussen M, Capion N, Klitgaard K, Rogdo T, Fjeldaas T, Boye M, Jensen TK. 2012. Bovine digital dermatitis: possible pathogenic consortium consisting of Dichelobacter nodosus and multiple Treponema species. Vet Microbiol. 160:151–161.
- R Core Team. 2021. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing.
- Reinöhl-DeSouza C, Kofler J. 2006. Infektiöse Interdigitalnekrose (infektiöse Interdigitalphlegmone) bei 66 Rindern. Teil 1, Klinische Befunde Tierärztl Prax Großtiere. 34:5–14.
- Roberts DS, Egerton JR. 1969. The aetiology and pathogenesis of ovine foot-rot: II. The pathogenic association of Fusiformis nodosus and F. necrophorus. J Comp Pathol. 79:217–227.
- Rogdo T, Hektoen L, Schau Slettemeås J, Jørgensen HJ, Østerås O, Fjeldaas T. 2012. Possible cross-infection of Dichelobacter nodosus between co-grazing sheep and cattle. Acta Vet Scand. 54:19.
- Staton GJ, Angell JW, Grove-White D, Clegg SR, Carter SD, Evans NJ, Duncan JS. 2021. Contagious Ovine digital dermatitis: a Novel Bacterial Etiology and Lesion Pathogenesis. Front Vet Sci. 8:722461.
- Storms J, Wirth A, Vasiliadis D, Brodard I, Hamann-Thölken A, Ambros C, Moog U, Jores J, Kuhnert P, Distl O. 2021. Prevalence of dichelobacter nodosus and ovine foot rot in German sheep flocks. Animals. 11:1102.
- Thomas JH. 1962. The differential diagnosis of foot rot in sheep. Aust Vet J. 38:159–163.
- Wani SA, Samanta I. 2006. Current understanding of the aetiology and laboratory diagnosis of foot rot. Vet J. 171:421–428.
- Zhou H, Bennett G, Hickford JG. 2009. Variation in Fusobacterium necrophorum strains present on the hooves of foot rot infected sheep, goats and cattle. Vet Microbiol. 135:363–367.
- Zhou H, Hickford JG, Armstrong KF. 2001. Rapid and accurate typing of Dichelobacter nodosus using PCR amplification and reverse dot-blot hybridisation. Vet Microbiol. 80:149–162.