ABSTRACT
The investigation of genetic differentiation of subspecies and breeds is nowadays fundamental for understanding their adaptation to specific environments and for facilitating selection processes for production purposes. These aspects allow us both to safeguard their biodiversity and to protect the products with a high nutritional value that derive from them and to which consumers today pay particular importance.
In this study, a genomic approach by RAPD-PCR and DNA-barcoding was used to identify possible genetic markers to discriminate Italian Mediterranean Buffalo (IMB) and Romanian Buffalo (RB). The two molecular tools have been widely used in this study not only to clarify genetic differences between breeds but also to avoid food frauds, given the great importance of Italian buffalo mozzarella recognized all over the world and protected by Denomination of Protected Origin (DPO).
The research did not reveal any difference between the two breeds, despite belonging to two geographically different areas.
1. Introduction
In recent years, several studies have analyzed the distribution of the world buffalo population and identified the genotypic relationships between the different populations (Iamartino et al. Citation2017; Colli et al. Citation2018; Vohra et al. Citation2021; Khan et al. Citation2022). As regards European buffaloes, in particular the breeds belonging to the river buffalo (Bubalus bubalis bubalis, 2n = 50) (Borghese et al. Citation2011), the largest population is currently present only in Italy and Romania, classified as Mediterranean breeds (Noce et al. Citation2021), Italian Mediterranean Buffalo (IMB) and Romanian Buffalo (RB), respectively (Borghese Citation2005). However, although these two breeds belong to the same subspecies, they are phenotypically similar and mostly used for milk and meat in both countries, they have been reported in two different clusters as described by Noce et al. (Citation2021).
Specifically, as for the RB breed, it was acclimatized and reproductively isolated in the cold and humid Carpathian climate region and the largest part is in Transylvania. The Romanian breed was recognized and approved as Romanian buffalo starting with 1987 (Vidu et al. Citation2014) when the herd book was established. In 2009, the Association of Transylvanian Buffalo Breeders was created, it has been accredited since 2015 for the determination of genetic quality in buffaloes, performance testing and genetic evaluation, by controlling milk production performances. Today the Romanian buffalo population is about 15000 heads, and it is a dual-purpose breed raised for milk and meat production, with an increasing trend for the production and marketing of milk and milk products. In the family farms the buffaloes are still used for draught and carry. The traditional products obtained in Romania from buffalo milk are yogurt, butter and typical Romanian cheese. In recent years, several small mozzarella production factories have developed but only for domestical consumption.
Regarding the IMB, it is an indigenous water buffalo breed in Italy, officially recognized by the Italian Ministry of Agricultural Food and Forestry Policies (MIPAAF), controlled through a herd book, established in 1980, and the National Association of Bufalina Species Breeders (CitationANASB) established in 1979. The IMB, actually with a number of animals consisted of 431.985 (CitationVETINFO), is one of the three main breeds of river buffalo highly selected for milk purposes (Borghese et al. Citation2016) to produce a typical soft cheese, containing about 45% of water, named mozzarella produced in central and southern Italy, especially the Campania, Lazio, Puglia and Molise.
Compared to other international buffalo dairy products called mozzarella, the Italian buffalo mozzarella is a cheese with a high nutritional value recognized throughout the world, whose production is regulated by the production specification of the Denomination of Protected Origin (DPO), assigned by the EU (CitationCommission Regulation EC No 103/2008). The attribution of the DPO to ‘mozzarella di bufala’ is assigned exclusively to the cheese produced in specific geographical areas, indicated above, from fresh whole buffalo milk with certain requirements including that of belonging to the Italian Mediterranean buffalo breed. Furthermore, the milk used to produce PDO buffalo mozzarella, must be exclusively from Italian buffaloes, must have a fat composition of 7.2%, a minimum of 4.2% protein, provide for delivery and processing within 60 h of the first milking.
The production of Italian buffalo mozzarella is particularly important for the national economy due to the remarkably high demand both domestically and from many foreign countries. Unfortunately, this fresh dairy product is mainly consumed in spring and summer (Borghese & Mazzi, Citation2005) in opposition to the natural milk production of buffaloes whose maximum peak occurs in winter periods. In fact, standard lactation of buffaloes reaches a maximum production about 60 days after giving birth, which in 90% of cases takes place in late summer and early autumn (Sabia et al. Citation2015). So, the lesser availability of IMB milk when the demand for mozzarella increases, could lead to use of other milk than what is required by the production specification. To prevent and counter any food fraud involving this product and to meet market demands, various experimental approaches have been used, among which the one to highlight the possible use of cow's milk (Russo et al. Citation2012; Trimboli et al. Citation2019; Cutarelli et al. Citation2021) or the use of non-Mediterranean buffalo milk through the protein differentiation of species and/or different breeds (Caira et al. Citation2019; Gunning et al. Citation2019). To date, no genomic data have been reported that have made it possible to discriminate against the presence of non-Italian Mediterranean buffalo milk, as required by the MIPAAF Decree (CitationCommission Regulation EC No 103/2008).
Two of the several genomic approaches that can discriminate populations, races and/or subpopulations, as reported in the literature, are random amplified polymorphic DNA (rapd pcr) and mtDNA barcode. The RAPD-PCR is a very effective methodology because it allows to identify specific biomarkers through the study of single base polymorphisms present in varied species or breeds without having any upstream information of the genome under examination (Koh et al. Citation1998; Paraguison et al. Citation2012; Mhuka et al. Citation2017). Furthermore, the mtDNA bracoding, in particular cytochrome c oxidase subunit I (COI) allows to identify animal species and/or food products derived from these. A study conducted by Hassan et al. (Citation2018) demonstrated the efficacy of COI barcode groups of genetically close buffaloes, such as two river buffalo breeds. Both methods have been used extensively to clarify genetic differences between breeds and food fraud (Khatun et al. Citation2012; Galimberti et al. Citation2013; Barcaccia et al. Citation2016; Rossetti et al. Citation2021).
Therefore, in this study, a genomic approach was used to screen the IMB breed and the RB breed belonging to the same species and to the same breed strain, with a similar milk composition, present in territories belonging to the European community but whose production milky, as mentioned, must be used for different products.
2. Materials and methods
2.1. Ethics statement
All samples work has been conducted according to the national and international guidelines for animal welfare. Sampling was approved by the Institutional Review Board of Research and Development Institute for Bovine (protocol code nr.4498/28.10.2019), some samples were taken respecting animal welfare because they were collected during the company's regular prophylaxis activities.
2.2. Selection, collection and shipping of specimens
A total of sixty milk and blood samples of domestic river buffalo, breed Mediterranean buffaloes, were collected and analyzed. Samples were collected from buffalo cows between 3 and 6 years of age. The animals were all lactating females, reared under semi-intensive management. The buffaloes were selected in two farms located one in Campania (southern Italy) and one in Sercaia (central Romania), and respectively thirty were taken from each farm. From each animal, blood samples were collected by Vacutainer EDTA (ethylene-di-amine-tetra-acetic acid) and samples of milk (100 ml) were collected in sterile tubes. All samples were transported at 4°C to Animal Cytogenetics and Genomics laboratory of CNR-ISPAAM in Naples, and they were processed within 24 h.
2.3. Samples DNA extraction and purification
Both the genomic DNA from blood and milk samples were isolated by method for liquid-phase extraction protocol.
The DNA from blood samples were processed from 1 ml of blood, according to the method described by Pauciullo et al. (Citation2012).
The genomic DNA isolated from somatic cells of milk samples was obtained starting from a volume of 50 ml and centrifuged at 2500 rpm for 30 min at 4°C. Once the surface represented by the fat was eliminated, the recovered pellet was resuspended in 25 ml of 1X PBS and centrifuged as above for several times. The cell pellet was resuspended in 860 microliters dilution buffer (10 mM tris, 50 mM NaCl, 2 mM EDTA, 1x SDS) with the addition of 5M guanidine and 20 ng/microliters of Proteinase K. After an incubation at 55°C overnight, DNA was extracted with a phenol–chloroform method and precipitated with cold isopropanol.
DNA extracted from blood and milk samples was resuspended in sterile water and quantified using NanoDrop™ 2000/2000c spectrophotometers (Thermo Fisher). Per each sample we corrected the concentration and OD260/280 ratio to 100 ng/ microliters.
2.4. Selected primer and random amplification of polymorphic DNA (RAPD-PCR)
In this study, ten different primers were selected from the literature, in the size range of 10-mer and a with 60 and 70% GC content (), to identify those which presented a greater number of reproducible bands. The first six primers were used because they had already been tested in our laboratory for a previous study (Rossetti et al. Citation2021). They were also found to be suitable for the buffalo species. Primers from P7 to P10 have been selected based on a literature review, as presented in because they are employed on several ruminant species.
Table 1. List of the random gene primers, their nucleotide sequence and GC % contents.
The RAPD-PCR was performed as described by Rossetti et al. (Citation2021). The amplified products obtained were analyzed by electrophoretic run with 2% agarose gel in 1X TBE buffer. A 3000 bp ladder (100 bp DNA Ladder Ready to Loadwas-Solis Biodyne) used to identify the different band sizes generated by the PCR. The gel was observed, captured and analyzed by GEL DOC EZ System (Biorad) with its Image Lab Touch Software dedicate.
2.5. Mitochondrial DNA amplification end sequencing
A specific region of the mitochondrial DNA (mtDNA) segment of buffalo was analyzed to find ancestral differences between the breeds under examination. As suggested by Hassan et al. (Citation2018) to amplify mtDNA, we used a set of primers (F: 5’-TCTCAACCAACCATAAAGATATCGG-3’; R: 5’-TATACTTCAGGGTGTCCGAAGAATCA-3’), approximately 709 bp from the 5’ half of the mitochondrial cytochrome c oxidase subunit I (COI) gene.
The PCR was performed in a final volume of 25 microliters containing: DNA template (100 ng/microliters),1X PCR Buffer, MgCl2 (1.5 mM), forward and reverse primers (1 mM each), 1U Taq DNA polymerase (Microgem 01-01-02000) and dNTP mix (0.2 mM). The amplification conditions were as follows: 1 min for denaturation at 94 °C, 2 min for annealing at the appropriate temperature for primer set and 2 min for extension at 72 °C, for 35 cycles, and a final extension at 72 °C for 7 min. The amplified product was examined on 1.5% agarose gel with an electrophoretic run as previously described. COI amplicon was sequenced by Ceinge-Biotecnologie Avanzate, a consortium company, according to the requested sampling conditions. For greater accuracy of the result, given the length of the COI gene amplicon, a double sequencing was performed both with the forward primer and the reverse primer.
2.6. mtDNA multiple sequence alignment
The data obtained from sequencing, were analyzed. The FINCHTV software (CitationFinchTV 1.4 Download (Free) - Informer Technologies) was used to evaluate the sequences obtained both from the forward primer and the reverse primer, and then, to manually assemble and correct all the buffalo sample amplicon.
COI was investigated by CitationBasic Local Alignment Search Tool (BLAST) programme to verify the homology sequences of Bubalus bubalis breed Mediterranean mitochondrion complete genome. Therefore, the multiple alignment of all sequences was performed by CitationMultiple Sequence Alignment CLUSTALW (Clustalw programme).
3. Results
The study, performed on sixty DNA samples of Italian and Romanian river buffalo, presented a genetic screening by RAPD-PCR method on two European river buffalo breeds. To set up the experimental design by this molecular tool, both genomic DNAs, from blood and milk samples isolated, were evaluated. Furthermore, for each population the ten selected primers () were used to verify their efficacy (number of bands and reproducibility) and the absence of correlation between banding pattern and variable age. Analyzing both groups, Italian and Romanian, no variables related to the age difference constituting the sampling emerged.
From the analysis of the obtained data, the P1, P2, P3 primers were eliminated because they presented non-homogeneous patterns within the same population, as well as P7 and P10 primers because they showed few bands compared to the other primers. P4, P5, P6, P8, P9 primers, instead, were considered in our analysis due to the higher number of presented bands. In particular, starting from the primer showed more bands: P6 nine bands whose range is between 1000 bp to 255 bp, P8 with only eight bands amplified of which the highest band to 1200 bp while the lowest is about 325 bp, P9 seven bands with a pattern ranging from 900 bp to 350 bp and P5 six bands displayed the same range of P9 (). The P4 was more suitable, featuring nine specific and repeatable bands with a pattern (). In fact, the lowest band was at about 200 bp and the highest at about 900 bp ().
Figure 1. Representative banding pattern of RAPD-PCR products in IMB and RB: white no band; light gray homogeneous band; dark gray polymorphic band.
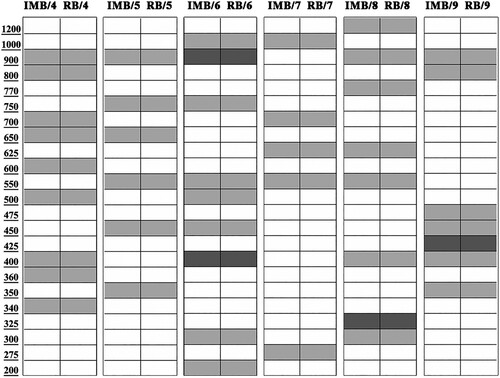
Figure 2. Electrophoretic pattern of the P4 primer in IMB (lanes 1–9) and RB (lanes 10–17); DNA Ladder 100 bp to 1.5 kb (100 bp Opti-marker G016, Applied Biological Materials Inc. Canada).
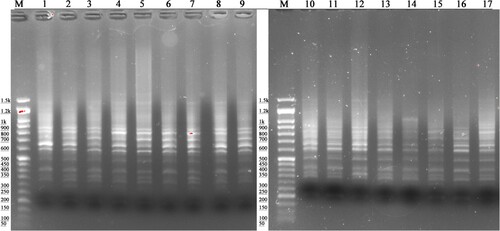
Table 2. List of the mtDNA COI primers, their nucleotide sequence.
This situation prompted the use of mtDNA barcoding, given its low intraspecific variability between species and its high interspecies variability (Hassan et al. Citation2018; Ali et al. Citation2021).
For this purpose, PCR technique was used to obtain a specific sequence of mtDNA barcoding: the region generated was a 709 bp amplicon, of which about 650 bp were recovered by sequencing analysis. The amplicon was verified in BLAST databases confirming for COI gene 100% homology with the Mediterranean mitochondrion of the Bubalus bubalis, complete genome. (MN756622.1).
To ensure a correct interpretation of the multiple alignment of the amplicons COI gene amplified from Italian and Romanian buffaloes, we subsequently compared them by CLUSTALW programme.
The programme did not show nucleotide differences within the same population and between the two populations analyzed.
4. Discussion
In the recent years, numerous studies have been conducted on buffaloes populations to understand their origin, geographical distribution over time and their ability to adapt to environmental conditions. Furthermore, many of these have been genetically selected to obtain food products with a high biological and nutritional value. This condition has sanctioned very stringent production rules and has regulated the raw materials to be used. A very complex challenge is therefore linked to the identification of biomarkers capable of distinguishing phylogenetically close species or breeds and to screen the products deriving from them. In this study, the IMB and RB breed, two different subpopulations but belonging to the Mediterranean buffalo, were genetically examined using different molecular approaches.
The use of RAPD-PCR allows to recognize specific species or breed polymorphisms without a particular knowledge of the genome; however, no difference was identified between analysed samples, probably because of their similar genealogy.
To investigate in more detail any genomic differences, mtDNA barcoding, a molecular technique, was used. mtDNA barcoding allows us to uniquely identify different biological subjects, as it is based on the genetic diversity found in specific regions of the genome (Galimberti et al. Citation2013). In fact, mtDNA gene was preferred, belonging to a haploid genome, because they show high copy number, lack introns, has low recombination and it is maternally inherited (Barcaccia et al. Citation2016). In this study, the barcodes COI was amplified and sequenced. Also in this case, the obtained results did not show any difference between the two populations.
The COI gene results reported by Hassan for river buffalo, show several variable nucleotide sites. In the present study it was not possible to trace this data. It is probable that a smaller number of samples or a probable genetic relatedness between the subpopulations analyzed influenced the results obtained.
This screening, despite what is reported in the literature in belonging to different clusters (Noce et al. Citation2021), did not reveal genetic differences between IMB and RB. This implies the great difficulty in obtaining markers or tests capable of performing a genomic screening on the milk of these subpopulations. This involves, in our opinion, the impossibility of total compliance with the criteria of the specification for the production of Italian PDO mozzarella.
It is hoped that further investigations could be conducted in the future to confirm or implement the information currently in possession to create, safeguard and incentivize not only the buffalo subpopulations, but also and above all the markets related to their products.
5. Conclusions
To implement genomic studies on buffalo breeds in this research we have screening two breeds, RB and IMB, belonging to the same genealogical strain, characterized by genomic methods to highlight specific markers of breed.
Although several primers were used by RAPD-PCR, the banding patterns obtained did not allow identifying specific breed bands. Similarly, during mtDNA barcoding analysis, to study the matrilineal ancestry (maternal line) of the two races, no difference was found. Further investigations are needed to find the keystone to screen and differentiate not only species differences but also and above all those of breed.
Author contributions
Conceptualization, methodology, data curation, investigation, Rossetti and Genualdo.; software, Genualdo and Incarnato; resources, validation, Rossetti, Genualdo, Nicolae and Perucatti; writing original draft preparation, Rossetti, Genualdo, Nicolae. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
The authors wish to thank Mr. Raffaele Pappalardo, Mr. Giuseppe Grazioli and Mr Giuseppe Auriemma (CNR ISPAAM) for the technical support, the buffalo dairy farm ‘La Pagliara’, Pollena Trocchia, Naples, and to Mr. Adrian Bota, Research and Development Station for buffalo, Sercaia, Brasov, Romania, for providing the blood and milk samples.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ali F, Ahmad I, Ali MI, et al. 2021. Mitochondrial phylogenetic and diversity analysis in Azi-Kheli buffalo. Trop Anim Health Prod. 53:512. doi:10.1007/s11250-021-02949-z.
- ANASB. 01/07/2022. https://www.anasb.it/.
- Barcaccia G, Lucchin M, Cassandro M. 2016. Dna barcoding as a molecular tool to track down mislabeling and food piracy. Diversity (Basel). 8(2). doi:10.3390/d8010002.
- Basic Local Alignment Search Tool. 1/12/2022. https://blast.ncbi.nlm.nih.gov/Blast.cgi.
- Borghese A. 2005. Buffalo production and research. FAO Ed REU Tech Ser. 67:1–315. doi:10.4081/ijas.2006.203.
- Borghese A. 2011. Situation and perspectives of buffalo in the world. Europe and Macedonia. Mac J Anim Sci. 1:281–229. doi:10.54865/mjas112281b.
- Borghese A, Mazzi M. 2005. Buffalo population and strategies in the world. In: A Borghese, editor. Buffalo production and research. Rome, Italy: FAO; p. 1–40.
- Borghese A, Moioli B. 2016. Buffalo: Mediterranean region. Ref Module Food Sci. B845-0-08-100596-5.21232-8. doi:10.1016/B978-0-08-100596-5.21232-8.
- Caira S, Pinto G, Nicolai MA, Novi G, Addeo F, Scaloni A. 2019. A non-canonical phosphorylation site in β-casein a from non-Mediterranean water buffalo makes quantifiable the adulteration of Italian milk with foreign material by combined isoelectrofocusing-immunoblotting procedures. Food Chem 277:195–204. doi:10.1016/j.foodchem.2018.10.076.
- Colli L, Milanesi M, Vajana E, Iamartino D, Bomba L, Puglisi F, Del Corvo M, Nicolazzi EL, Ahmed SSE, Herre-ra JRV, et al. 2018. New insights on water buffalo genomic diversity and post-domestication migration routes from medium density SNP chip data. Front Genet. 2(9):53. doi:10.3389/fgene.2018.00053.
- Commission Regulation EC No 103/2008. 1/09/2022. http://www.agricoltura.regione.campania.it/tipici/pdf/disciplinare-mozzarella-bufala.pdf.
- Cutarelli A, Fulgione A, Fraulo P, Serpe FP, Gallo P, Biondi L, Corrado F, Citro A, Capuano F. 2021. Droplet digital PCR (ddPCR) analysis for the detection and quantification of cow DNA in buffalo mozzarella cheese. Animals (Basel). 11(5):1270. doi:10.3390/ani11051270.
- FinchTV 1.4 Download (Free) - Informer Technologies, Inc. 1/12/2022. https://finchtv.software.informer.com/1.4/.
- Galimberti A, De Mattia F, Losa A, Bruni I, Federici S, Casiraghi M, Martellos S, Labra M. 2013. DNA barcoding as a new tool for food traceability. Food Res Int. 50(1):55–63. ISSN0963-9969. doi:10.1016/j.foodres.2012.09.036.
- Güneren G, Akyüz B, Ertuğrul O. 2010. Use of RAPD-PCR for genetic analyses on the native cattle breeds in Turkey. Ankara Üniversitesi Veteriner Fakültesi Dergisi. 57:167–172. doi:10.1501/Vetfak_0000002370.
- Gunning Y, Fong LKW, Philo M, Kemsley EK. 2019. Quantitative authenticity testing of buffalo mozzarella via αs1-casein using multiple reaction monitoring mass spectrometry. Food Control. 101:189–197. doi:10.1016/j.foodcont.2019.02.029.
- Hassan AAM, Balabel EA, Oraby HAS, Darwish SA. 2018. Buffalo species identification and delineation using genetic barcoding markers. J Genet Eng Biotechnol. 16(2):499–505. doi:10.1016/j.jgeb.2018.07.006.
- Iamartino D, Nicolazzi EL, Van Tassell CP, Reecy JM, Fritz-Waters ER, Koltes JE, Biffani S, Sonstegard TS, Schroeder SG, Ajmone-Marsan P, et al. 2017. Design and validation of a 90K SNP genotyping assay for the water buffalo (Bubalus bubalis). PLoS One. 12(10):e0185220. doi:10.1371/journal.pone.0185220.
- Khan A, Singh K, Jaiswal S, Raza M, Jasrotia RS, Kumar A, Gurjar AKS, Kumari J, Nayan V, Iquebal MA, Angadi UB. 2022. Whole-genome-based web genomic resource for water buffalo (Bubalus bubalis). Front Genet. 13:809741. doi:10.3389/fgene.2022.809741.
- Khatun MM, Hossain KM, Mahbubur Rahman SM. 2012. Molecular characterization of selected local and exotic cattle using RAPD marker. Asian-Australas J Anim Sci. 25(6):751–757. doi:10.5713/ajas.2011.11331.
- Koh MC, Lim CH, Chua SB, Chew ST, Phang ST. 1998. Random amplified polymorphic DNA (RAPD) finger-prints for identification of red meat animal species. Meat Sci. 48(3-4):275–285. doi:10.1016/S0309-1740(97)00104-6.
- Mhuka C, Chatiza FP, Chidzwondo F, Sithole-Niang I, Makuza SM, Mlambo SS. 2017. Use of RAPD-PCR for breed/genotype identification in Zimbabwean cattle. J Comput Biol. 2:131–137. doi:10.3233/JCB-15033.
- Multiple Sequence Alignment by CLUSTALW. 1/12/2022. http://www.genome.jp/tools/clustalw/.
- Noce A, Qanbari S, González-Prendes R, Brenmoehl J, Luigi-Sierra MG, Theerkorn M, Fiege MA, Pilz H, Bota A, Vidu L, et al. 2021. Genetic diversity of bubalus bubalis in Germany and global relations of its genetic background. Front Genet. 11:610353. doi:10.3389/fgene.2020.610353.
- Paraguison RC, Faylon MP, Flores EB, Cruz LC. 2012. Improved RAPD-PCR for discriminating breeds of water buffalo. Biochem Genet. 50(7-8):579–584. doi:10.1007/s10528-012-9502-8.
- Pauciullo A, Cosenza G, Steri R, Coletta A, Jemma L, Feligini M, Di Berardino D, Macciotta NP, Ramunno L. 2012. An association analysis between OXT genotype and milk yield and FLOW IN Italian Mediterranean river buffalo. J Dairy Res. 79(2):150–156. doi:10.1017/S0022029911000914.
- Rossetti C, Perucatti A, Mottola F, Incarnato D, Genualdo V. 2021. Genetic investigation for the characterization of three indigenous pig breeds of southern Italy: advantages and prospects. Anim Sci Pap Rep. 39:141–150.
- Russo R, Severino V, Mendez A, Lliberia J, Parente A, Chambery A. 2012. Detection of buffalo mozzarella adulteration by an ultra-high performance liquid chromatography tandem mass spectrometry methodology. J Mass Spectrom. 47(11):1407–1414. doi:10.1002/jms.3064.
- Sabia E, Napolitano F, Claps S, Braghieri A, Piazzolla N, Pacelli C. 2015. Feeding, nutrition and sustainability in dairy enter-prises: the case of Mediterranean Buffaloes (Bubalus bubalis). In: A Vastola, editor. The sustainability of agro-food and natural resource Sys-tems in the Mediterranean basin. Cham, Switzerland: Springer International Publishing; p. 57–64.
- Sekena AA, Lamiaa S, Mohamed H, Karima M. 2010. Genetic analysis between and within three Egyptian water buffalo populations using RAPD-PCR. J Am Sci. 6:217–226.
- Trimboli F, Costanzo N, Lopreiato V, Ceniti C, Morittu VM, Spina A, Britti D. 2019. Detection of buffalo milk adultera-tion with cow milk by capillary electrophoresis analysis. J Dairy Sci. 102(7):5962–5970. doi:10.3168/jds.2018-16194.
- VETINFO. https://www.vetinfo.it/j6_statistiche/#/report-pbi/11.
- Vidu L, Diaconescu C, Nicoleta-Alina U, Vasile B, Dana P, Popa R, Mirela S. 2014. The buffalo -part of animal biodiversity in romania and the importance for the bioeconomy. International multidisciplinary scientific geoconference DGEm, 14th SGEM GeoConference on Ecology, Economics, Education and Legislation, section Ecology and Environmental Protection; 1. 665 + 672.
- Vohra V, Singh NP, Chhotaray S, Raina VS, Chopra A, Kataria RS. 2021. Morphometric and microsatellite-based comparative genetic diversity analysis in Bubalus bubalis from north india. PeerJ 9:e11846. doi:10.7717/peerj.11846.
