?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Alstonia scholaris (L.) R.Br., a traditional herbal medicine, exhibits many biological activities in humans. Several activities have been confirmed either in vitro or in vivo. The plant might also provide health benefits in other living things. Its pharmacological activities let us expect the essential effects on fish species. To date, A. scholaris activity in Channa striata (Bloch, 1793), an economic farmed fish, has not yet been studied. In this study, we aimed to determine the effects of the plant’s flower extract on growth performance and serum biochemical indices in C. striata. The experimental fish were fed with a control diet, comprising 0.3, 0.6 and 0.9 mg/kg extract supplement for 90 days. The results showed that the growth indices became significantly different (P < 0.05) after 60 days of the trial. Growth parameters; weight gain, average daily gain, specific growth rate, feed conversion ratio, and protein efficiency ratio tended to be improved in a dose-dependent manner. Serum ALT and triglyceride were significantly decreased in all treatment groups. Serum glucose was also affected by the extract. The results revealed that A. scholaris flower extract exhibits a beneficial effect on C. striata growth and health. The plant could be further determined as a valuable commodity for the aquaculture industry.
Introduction
Over the past decades, the world aquaculture sector has faced disease outbreaks caused by viruses, bacteria, fungi, and parasites, resulting in enormous economic losses. Various chemical substances have been used against these pathogenic microorganisms. However, they rendered satisfying results only at the beginning of the treatment period, as chemical resistance has been consequently developed (Zhang et al. Citation2014; He et al. Citation2016; Uchida et al. Citation2016). The chemical contamination in the environment and aquatic animals are other matter of concern. In safety aquaculture, organic farming technologies are becoming a favourable trend. Many products from natural origins, especially herbal products or herbal extracts are popularly used in aquaculture. Most scientific documents have demonstrated that various kind of herbs can be applied for disease prevention (Kumar et al. Citation2010; Pratheepa and Sukumaran Citation2014) and to promote growth performance in fish (Tan et al. Citation2017; Panase et al. Citation2018; Panase and Tipdacho Citation2018; Sutthi et al. Citation2020).
Alstonia scholaris (L.) R.Br. belongs to the family Apocynaceae. Its common names are devil’s tree or blackboard tree (Pandey et al. Citation2020). In Thai, the plant is called ‘Phaya sattaban’ or ‘Teen pet’. This plant is distributed across Eastern and Southeast Asia as well as in Southern India (Nadkarni Citation1976; Khare Citation2007). From the literature review, it is clear that alkaloids, flavonoids, saponins, steroids, reducing sugars, and phenolic compounds are major bioactive compounds in A. scholaris (Pandey et al. Citation2020), and the plant has numerous medicinal applications. Some reports revealed that this plant has been used in traditional medicine as an antidiabetic, antibacterial, antianxiety, anticancer, anti-inflammatory, and hepatoprotective agent (Pankti et al. Citation2012; Pratap et al. Citation2013). The flower of A. scholaris, as well as the stem bark, stem, and leaves, also contains many bioactive compounds (Thakamani et al. Citation2011). The methanolic extract of A. scholaris flower was tested for its phytochemical constituents and the results indicated the existence of alkaloids, carbohydrates, phenols, tannins, terpenoids, cardiac glycosides, and fixed oils (Thakamani et al. Citation2011). These results corresponded to those of our preliminary phytochemical screening tests. To date, the activity of A. scholaris flower has not been biologically tested in aquatic animals, including Channa striata, an economic farmed fish in Thailand and Southeast Asia.
Channa striata (Bloch, 1793), snakehead fish or ‘Pla chon’ (or chorn) in Thai, is a voracious carnivorous species that is a native freshwater fish species in Thailand. This species can be found in rivers, dams, swamps, creeks, ponds, canals, lakes, and rice fields. C. striata is very economically important in Thailand and other countries in Southeast Asia because it is favoured as a local food. This kind of fish provides higher economic value than that of Nile tilapia (Oreochromis niloticus) in the food market. It is well known that in its natural environment, snakehead fish grow faster and became stronger compared to those cultured in confined conditions, such as cement fishponds or net cages. This may be due to the lower density, lower stress, and lower incidence of infectious diseases. Therefore, the fast growth rate and healthy conditions are major requirements of C. striata farming in which the desired environment cannot be rendered. Dietary supplements are one choice for this approach. Cost savings, easy application, environmental friendliness, and non-synthetic substances are expected. On account of the positive biological activities of A. scholaris, this plant might also be a promising product. The methanolic extract of A. scholaris flower, therefore, was selected for this purpose. This research was the first approach to studying the effects of A. scholaris flower extract on growth performance and biochemicals in C. striata. This work aimed to evaluate the effects of methanolic extract obtained from the A. scholaris flower on growth performance and serum biochemistry in C. striata. Our assumptions are that the extract would promote growth performance without negative effects on serum biochemicals in C. striata. In this research, growth performance was determined by weight gain (WG), average daily gain (ADG), specific growth rate (SGR), feed conversion ratio (FCR), and protein efficiency ratio (PER). Survival rate (SR) and serum biochemicals, i.e. alanine transaminase (ALT), glucose, total protein, albumin, globulin, triglyceride, cholesterol, and calcium were also measured. It was found that the growth indices in the treatment groups receiving the extract supplement diet were better than in the control group. Meanwhile, serum ALT and triglyceride in C. striata receiving the 0.3, 0.6, and 0.9 mg extract/kg fish diet were significantly lower than in the control group. This research revealed that methanolic A. scholaris flower extract can help promote growth and health conditions in C. striata. The extract might be further applied to the culturing of other fish species.
Materials and methods
Plant material and preparation of A. scholaris flower extract
Inflorescences of A. scholaris were collected from the local area around the University of Phayao, Phayao province, Thailand, from October to November. The samples were washed with tap water, dried at 50°C in a hot air oven for 20–24 h, and ground using a grinder. The extract was prepared using a standard extraction procedure. First, the powder was twice macerated using 99.7% methanol at room temperature for 48 h. The mixture was then filtered through Whatman no. 1 filter paper. Finally, the filtrates were concentrated under reduced pressure in a rotary evaporator at 45°C. The extract was kept at 8°C until use.
Preliminary phytochemical screening
Phytochemical screening tests of A. scholaris flower extract were performed using universal methods as shown in .
Table 1. Preliminary phytochemical screening tests.
Supplemental diet preparation
Throughout this research, commercial fish feed pellets (2 mm, diameter) (ACCO FEEDS 82) containing 30% crude protein, 4% lipid, 12% moisture, and 6% fiber were used. The ingredients of the commercial fish feed consisted of fish meal, soybean meal, corn, broken rice, vitamins, and minerals. The feed was prepared for four groups of experimental fish: (1) control group; the pellets were sprayed with distilled water without any A. scholaris flower extract, (2)–(4) treatment groups; the pellets were sprayed with low-dose (0.3 mg/100 ml), medium-dose (0.6 mg/100 ml), and high-dose (0.9 mg/100 ml) A. scholaris flower extract in distilled water, respectively. The ratio of distilled water or diluted A. scholaris flower extract and pellets was 100 ml:1 kg feed. While spraying, the mixture was thoroughly mixed using a pan coating machine with air blower (CM/Thai, CMCA-10 model). After that, the pellets were coated with 4% agar solution and air-dried again (Sutthi et al. Citation2020). Finally, the pellets contained different doses of the plant extract and were kept separately in sterile containers at room temperature. A new batch of pellets was prepared every 7 days.
Experimental fish and the conditions for acclimatization
Juvenile C. striata were purchased from a private fish farm in Phayao province, Thailand. Experimental fish were transported to the fish farm and acclimatized in fiberglass fishponds (1 × 1.5 × 0.5 m) containing 400 l of water for 2 weeks under a natural photoperiod. A continuous aeration and water recirculation system was maintained weekly. Water quality was maintained by setting up the following parameters: temperature (28.6 ± 3.5°C), dissolved oxygen concentration (7.50 ± 1.6 mg/l), and pH (7.2 ± 1.50) (multi-probes, HORIBA, U50 series). During the acclimatization period, C. striata were fed twice a day at 8 am and 5 pm with the commercial fish feed without A. scholaris flower extract at 5% of individual body weight per day (30% crude protein).
Experimental design
After the acclimatization, healthy fish with an average body weight of 7.4 ± 10.6 g were placed in 12 net cages (1 × 2 × 0.8 m; mesh size, 2.5 mm2) in four triplicate groups; each group was in a 3 × 5 × 1 m cement pond. This was done at a stocking density of 20 fish/net cage. All groups were fed with the diet prepared specifically for them at 4% of their body weight, twice daily for 90 days. The observed water quality parameters were as follows: water temperature was 26.8–29.5°C, dissolved oxygen concentration was 6.3–7.82 mg/l, pH was 7.23–8.79 and total dissolved solids was 0.20–0.57 g/l.
Growth performance and survival evaluations
The experimental fish in each group were weighed at 15-day intervals to adjust the feed volume; growth parameters were then calculated, with the results being put in a report at 30-day intervals. Growth indices: WG, ADG, SGR, FCR, PER, and SR were determined using the following equations (Bagenal Citation1978):
Serum biochemistry study
Serum biochemical analysis was done at the end of our experiment; 9 fish from each group (3 fish per replication) were randomized for blood collection. Blood samples (0.8 ml/fish) were collected from caudal veins using non-heparinized syringes. For serum collection, blood samples were transferred into micro-centrifuge tubes and allowed to clot for 4 h at room temperature, then spun down at 5000 rpm for 15 min at 25°C. Supernatants were stored in sterile serum tubes at −20°C until use. The frozen serum samples were sent to the Chiang Mai veterinary laboratory centre limited partnership, Chiang Mai, Thailand, in a medical transport cooler box. Serum analysis had been done within 72 h after freezing. Serum indices, i.e. ALT, glucose, total protein, albumin, globulin, triglyceride, cholesterol, and calcium were investigated using an analytical biochemistry analyzer (P400 and PC400, HORIBA, Japan).
Statistical analysis
The normality and homogeneity of variance were tested before the analysis. Statistical analysis of data involved a one-way analysis of variance (ANOVA) followed by a Tukey’s test at a significance level of 95% (P < 0.05). Statistical analyses were done using IBM SPSS Statics 23. All data were presented as mean ± SD.
Results
Preliminary phytochemical analysis
The results from preliminary phytochemical analyses are shown in . Alkaloids, terpenoids, lactones, and tannins are present in the methanolic A. scholaris flower extract while other chemicals were not detected.
Table 2. Preliminary phytochemical screening of methanolic flower extract from Alstonia scholaris.
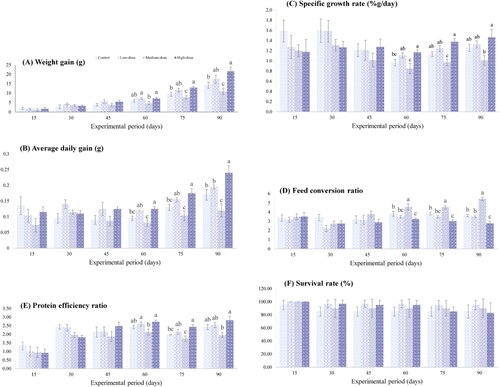
Growth indices and survival rate
It was found that C. striata in the medium-dose group represented the lowest growth rate at day 60, 75, and 90. At the end of the study, the significantly different parameters in this group compared to other treatment groups were WG, ADG, and FCR (P < 0.05), while SGR and PER were not different from the control group or the low-dose group (A–E). The SGR and PER in the high-dose group were comparable to those obtained from the low-dose group at day 60, 75, and 90 (C,E). The WG and ADG in the high-dose group were significantly higher than in other groups at day 90 (A,B). Meanwhile, the FCR of this group was the lowest compared to others (D). The SGR and PER significantly differed between the high-dose group and control group at day 75; however, at the end of the study these values were not different between the two groups. Throughout the experimental period, there was no significant difference (P > 0.05) in SR between all groups (F).
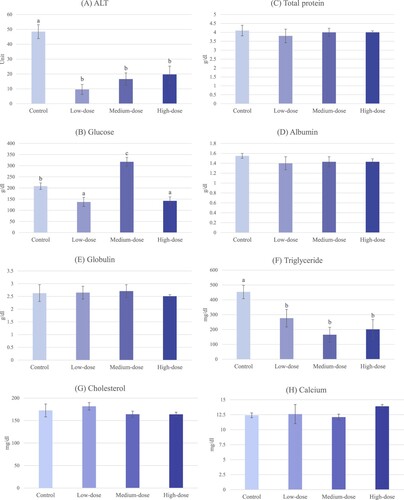
Serum biochemical indices
In this study, levels of serum total protein, albumin, globulin, cholesterol, and calcium were not significantly different (P > 0.05) in all groups. The ranges of serum total protein, albumin, globulin, cholesterol, and calcium were 3.8–4.1 g/dl, 1.4–1.55 g/dl, 2.51–2.71 g/dl, 163.8–181.7 mg/dl, and 12.1–13.9 mg/dl, respectively (C–E, G, H). Surprisingly, A. scholaris flower extract at all doses significantly reduced ALT and triglyceride compared to the control group. However, there was no significant difference between treatment groups (A,F). Serum glucose was also affected by the plant extract significantly. Compared to the control group, higher serum glucose was found in the medium-dose group, while the lower values were observed in the low-dose and high-dose groups (B).
Discussion
As stated, A. scholaris is a herbal medicine that has been used for a long time due to its diverse pharmacological activeness combined with the requirements of C. striata farming. This study was, therefore, performed. This research investigated the effects of A. scholaris flower extract on growth performance and biochemicals in the cultured fish, C. striata.
According to the results, the plant extract could promote growth performance in C. striata. Growth indices, i.e. WG and ADG, significantly increased in C. striata in the high-dose group. These results were contrary to FCR. SR was not significantly different across all groups. In the medium-dose group, the overall growth performance was worse than in the control group. These unexpected outcomes might be the result of the differences in fish body size and weight. Fish in this group developed distinctive body size and weight differences after day 30 of the experimental period. The bigger fish exploited their size and stole the feed from the smaller ones, resulting in an inadequate diet for proper growth promotion. This occurrence was not observed in other groups.
The better growth indices discovered in the low-dose and high-dose groups might be the result of chemical components in A. scholaris flower extract. There were many studies which reported the existence of alkaloids, tannins, terpenoids, and cardiac glycosides in A. scholaris. Many reports have revealed the positive effects of these bioactive compounds on growth performance, biochemical indices, and immune responses in fish, such as increased feed intake levels (Munglue et al. Citation2019; Adeli et al. Citation2021), improved growth indices (Panase et al. Citation2018), increased digestive enzymes (Gabriel et al. Citation2017; Shabana et al. Citation2019), increased survival rate (Shabana et al. Citation2019), and enhanced growth hormone and insulin-like growth factor I gene expressions (Guo et al. Citation2018).
As well as other chemical substances, not only efficacy but also safety data of herbal extract should be of concern. In this work, we expected that the plant extract would promote growth performance in C. striata without negative physiological effects. Therefore, serum biochemical indices were also investigated. Serum glucose in the low-dose and high-dose groups was lower than in the control group, while the glucose level in the medium-dose group was higher than in the control and other treatment groups. This might be caused by the different body sizes and growth performance of the experimental fish in the medium-dose group. Glucose is closely related to cortisol hormone, and this is the most common stress indicator (Iwama et al. Citation1992). This might be the reason why the glucose level of the medium-dose group was high. Some fish in this group might be stressed from the feeding scramble and encroachment leading to high serum glucose levels to cope with the incremental energy demands (Montero et al. Citation1999). Besides, serum ALT and total triglycerides in all treatment groups were significantly decreased when compared to the control group. These results indicated the hepatoprotective, hypoglycemic, and hypolipidemic effects of A. scholaris flower extract. Some chemical constituents in the extract might potentially possess one or more activity. The natural origin substances in various classes represent their potential biological effects, as aforementioned. A. scholaris flower extract contained alkaloids, terpenoids, and tannins which are predominant classes of bioactive phytochemicals. There was a report of the hepatoprotective compound, α-amyrin, a pentacyclic triterpene, in A. scholaris flowers (Khyade et al. Citation2014). The compound was reported to offer comparable hepatoprotective effects against drug-induced liver damage to the positive control by the decrement of serum AST, ALT, GSH, and the histological score of liver damage (Oliveira et al. Citation2005). α-amyrin isolated from A. scholaris bark also exhibited the modulatory potential effects in hepatic oxidative stress. The recovery of liver function was determined nearly to a normal level and comparable to silymarin, a well-known hepatoprotective agent (Singh et al. Citation2015). Furthermore, the compound showed antihyperglycemic and hypolipidemic activity in a study of Santos et al., in 2012. α-amyrin tended to reduce blood glucose levels in normal mice and significantly reduced blood glucose levels in diabetic mice. These results revealed the ability to normalize blood glucose levels of the compound. This bioactive substance also reduced the levels of triglycerides and total cholesterol in mice fed on a high-fat diet (Santos et al. Citation2012). Apart from α-amyrin, other chemical constituents in A. scholaris flower extract might be incorporated in the same or different pharmacological mechanisms of the anti-diabetic and anti-hyperlipidemic properties.
From the results on growth performance, A. scholaris flower extract tended to improve growth performance in a dose-dependent manner, without side effects on serum biochemicals. In addition, the extract exhibited other health benefits in C. striata via serum ALT, glucose, and triglyceride lowering effects. From our results, it can be concluded that A. scholaris flower extract displayed positive effects on growth performance and biochemical indices in C. striata, especially with regard to the dietary incorporation of 0.9 mg/kg, which has the potential to significantly improve growth performance. However, phospholipids and high-density lipoprotein-cholesterol should be investigated to confirm our findings since they are classified as the main transporters of cholesterol and triglycerides. The anti-diabetic and other properties of the flower extract from this plant need to be further studied as well.
CRediT authorship contribution statement
Sontaya Sookying: Investigation, Resources, Software, Methodology, Writing – original draft. Arporn Panase: Supervision, Validation, Visualization. Phanit Srisuttha: Supervision, Validation, Visualization. Apirak Chaophothun: Investigation, Resources, Paiboon Panase: Conceptualization, Data curation, Funding acquisition, Project administration, Writing – original draft.
Ethics statement
The scientific procedures were conducted according to guidelines for the animal care and use of the University of Phayao and approved by the Committee of Institutional Animal Care, University of Phayao, Thailand (ID: 640104034)
Acknowledgements
We would like to thank the School of Agriculture and Natural Recourses and School of Pharmaceutical Sciences, at the University of Phayao, Thailand, for providing facilities for the present research. Moreover, the authors would like to thank all the staff and students in the fisheries programme and the research project in pharmacy for their help with plant extraction, phytochemical analysis and data collection.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Adeli A, Shamloofar M, Akrami R. 2021. Dietary effect of Lemon Verbena (Aloysia triphylla) extract on growth performance, some haematological, biochemical, and non-specific immunity and stocking density challenge of rainbow trout juveniles (Oncorhynchus mykiss). J Appl Anim Res. 49(1):382–390. doi:10.1080/09712119.2021.1990069.
- Bagenal T. 1978. Methods for the assessment of fish production in fresh waters. 3rd ed. Hoboken (NJ): Blackwell Scientific Publication.
- Gabriel NN, Qiang J, Ma XY, Xu P, Nakwaya DN. 2017. Effects of dietary Aloe vera crude extracts on digestive enzyme activities and muscle proximate composition of GIFT tilapia juveniles. S Afr J Anim Sci. 47(6):904–913. doi:10.4314/sajas.v47i6.18.
- Guo H, Lin W, Hou J, Wang L, Zhang D, Wu X, Li L, Li D. 2018. The protective roles of dietary selenium yeast and tea polyphenols on growth performance and ammonia tolerance of juvenile Wuchang bream (Megalobrama amblycephala). Front Physiol. 9:1371. doi:10.3389/fphys.2018.01371.
- Hartanti D, Cahyani AN. 2020. Plant cyanogenic glycosides: an overview. Farmasains J Farm Il Kes. 5(2):1–6. doi:10.22219/farmasains.v5i1.10047.
- He X, Deng M, Wang Q, Yang Y, Yang Y, Nie X. 2016. Residues and health risk assessment of quinolones and sulfonamides in cultured fish from Pearl River Delta, China. Aquaculture. 458(1):38–46. doi:10.1016/j.aquaculture.2016.02.006.
- Iwama GK, McGeer JC, Bernier NJ. 1992. The effect of stock and rearing history on the stress response in juvenile Coho salmon (Oncorhynchus kisutch). ICES Mar Sci Symp. 194(1):67–83.
- Khare CP. 2007. Indian medicinal plants: an illustrated dictionary. Heidelberg (HDB): Springer.
- Khyade MS, Kasote DM, Vaikos NP. 2014. Alstonia scholaris (L.) R. Br. and Alstonia macrophylla Wall. ex G. Don: A comparative review on traditional uses, phytochemistry and pharmacology. J Ethno. 153(1):1–18. doi:10.1016/j.jep.2014.01.025.
- Kumar S, Malhotra R, Kumar D. 2010. Euphorbia hirta: its chemistry, traditional and medicinal uses, and pharmacological activities. Pharmacogn Rev. 4(7):58–61. doi:10.4103/0973-7847.65327.
- Montero D, Izquierdo MS, Tort L, Robaina L, Vergara JM. 1999. High stocking density produces crowding stress altering some physiological and biochemical parameters in gilthead sea bream, Sparus aurata, juveniles. Fish Physiol Biochem. 20(1):53–60. doi:10.1023/A:1007719928905.
- Munglue P, Rattana K, Sangchanjiradet S, Jankam A, Dasri K. 2019. Growth performance and intestinal morphology of hybrid catfish (Clarias microcephalus × Clarias gariepinus) fed diet supplemented with rice paddy herb (Limnophila aromatica) extract. Asia Pac J Sci Technol. 24(2):APST–AP24. doi:10.14456/apst.2019.17.
- Nadkarni KM. 1976. Indian materia medica. 3rd ed., Vol. 1. Mumbai, MUM: Popular Prakashan.
- Oliveira FA, Chaves MH, Almeida FRC, Lima Jr. RCP, Silva RM, Maia JL, Brito GAAC, Santos FA, Rao VS. 2005. Protective effect of α- and β-amyrin, a triterpene mixture from Protium heptaphyllum (Aubl.) March. trunk wood resin, against acetaminophen-induced liver injury in mice. J Ethnopharmacol. 98(1-2):103–108. doi:10.1016/j.jep.2005.01.036.
- Panase P, Kamee B, Moungmor S, Tipdacho P, Matidtor J, Sutthi N. 2018. Effects of Euphorbia hirta plant leaf extract on growth performance, hematological and organosomatic indices of hybrid catfish, Clarias macrocephalus × C. gariepinus. Fish Sci. 84(6):1025–1036. doi:10.1007/s12562-018-1234-1.
- Panase P, Tipdacho P. 2018. Preliminary use of Polygonum minus Linn. leaf extract on growth performance, feed utilization, and some hematological indices of Anabas testudineus (Bloch, 1792). Comp Clin Pathol 27(1):1–6. doi:10.1007/s00580-014-1916-8.
- Pandey K, Shevkar C, Bairwa K, Kate AS. 2020. Pharmaceutical perspective on bioactives from Alstonia scholaris: ethnomedicinal knowledge, phytochemistry, clinical status, patent space, and future directions. Phytochem Rev. 19(1):191–233. doi:10.1007/s11101-020-09662-z.
- Pankti K, Payal G, Manodeep C, Jagadish K. 2012. A phytopharmacological review of Alstonia scholaris: a panoramic herbal medicine. Int J Res Ayurveda Pharm. 3(3):367–371.
- Pant DR, Pant ND, Saru DB, Yadav UN, Khanal DP. 2017. Phytochemical screening and study of antioxidant, antimicrobial, antidiabetic, anti-inflammatory and analgesic activities of extracts from stem wood of Pterocarpus marsupium Roxburgh. J Intercult Ethnopharmacol. 6(2):170–176. doi:10.5455/jice.20170403094055.
- Pratap B, Chakraborthy GS, Mogha N. 2013. Complete aspects of Alstonia scholaris. Int J Pharm Tech Res. 5(1):17–26.
- Pratheepa V, Sukumaran N. 2014. Effect of Euphorbia hirta plant leaf extract on immunostimulant response of Aeromonas hydrophila infected Cyprinus carpio. PeerJ. 2:e671. doi:10.7717/peerj.671.
- Santos FA, Frota JT, Arruda BR, Sousa deMelo T, da Silva A, Brito G AAC, et al AC. 2012. Antihyperglycemic and hypolipidemic effects of α, β-amyrin, a triterpenoid mixture from Protium heptaphyllumin mice. Lipids Health Dis. 11(1):98. doi:10.1186/1476-511X-11-98.
- Shabana MS, Karthika M, Ramasubramanian V. 2019. Effect of dietary Citrus sinensis peel extract on growth performance, digestive enzyme activity, muscle biochemical composition, and metabolic enzyme status of the freshwater fish, Catla Catla. J Basic Appl Zool. 80(1):51. doi:10.1186/s41936-019-0119-x.
- Shaikh JR, Patil MK. 2020. Qualitative tests for preliminary phytochemical screening: An overview. Int J Chem Stud. 8(2):603–608. doi:10.22271/chemi.2020.v8.i2i.8834.
- Singh D, Arya PV, Sharma A, Dobhal MP, Gupta RS. 2015. Modulatory potential of α-amyrin against hepatic oxidative stress through antioxidant status in wistar albino rats. J Ethno. 161(1):186–193. doi:10.1016/j.jep.2014.12.025.
- Sutthi N, Panase A, Chitmanat C, Sookying S, Ratworawong K, Panase P. 2020. Effects of dietary leaf ethanolic extract of Apium graveolens L. on growth performance, serum biochemical indices, bacterial resistance and lysozyme activity in Labeo chrysophekadion (Bleeker, 1849). Aquac Rep 18:100551. doi:10.1016/j.aqrep.2020.100551.
- Tan X, Sun Z, Huang Z, Zhou C, Lin H, Tan L, Xun P, Huang Q. 2017. Effect of dietary hawthorn extract on growth performance, immune responses, growth- and immune-related genes expression of juvenile of golden pompano (Trachinotus ovatus) and its susceptibility to Vibrio harveyi infection. Fish Shellfish Immunol. 70(1):656–664. doi:10.1016/j.fsi.2017.09.041.
- Thakamani VL, Joel James JJ, Veetil AKT, Sagadevan LDM. 2011. Phytochemical screening and anti-microbial activity of Alstonia Scholaris Flowers (L) R. BR. Fam:Apocynaceae. Int J Pharm Res Dev. 3(3):172–178.
- Uchida K, Konishi Y, Harada K, Okihashi M, Yamaguchi T, Do MHN, Thi Bui L, Duc Nguyen T, Do Nguyen P, Thi Khong D, et al. 2016. Monitoring of antibiotic residues in aquatic products in urban and rural areas of Vietnam. J Agric Food Chem. 64(1):6133–6138. doi:10.1021/acs.jafc.6b00091.
- Zhang Q, Yu H, Tong T, Tong W, Dong L, Xu M, Wang Z. 2014. Dietary supplementation of Bacillus subtilis and fructooligosaccharide enhance the growth, non-specific immunity of juvenile ovate pompano, Trachinotus ovatus and its disease resistance against Vibrio vulnificus. Fish Shellfish Immunol. 38(1):7–14. doi:10.1016/j.fsi.2014.02.008.