ABSTRACT
Heavy metals and their genotoxic effects in captive Indian Peafowl (Pavo cristatus) residing in various regions of Punjab, Pakistan, specifically, Wildlife Park Bahawalpur (WPB), Jallo Wildlife Park Lahore (JWPL), and Wildlife Park Murree (WPM) were evaluated in blood, feathers, eggshell and egg content samples. The Single-cell gel electrophoresis (Comet) assay was performed to evaluate DNA damage. The results showed that the concentration of Cr was significantly high (P < 0.05) in Blood (3.79 µg/g), Feather (4.87 µg/g), Egg shell (51.02 µg/g) and Egg Content (13.59 µg/g) samples of Jallo Wildlife Park Lahore followed by Pb, Mn, Ni and Co. The highest (P < 0.05) metal accumulation was found in eggshell samples due to its porous structure as compared to other samples. Likewise region-wise analysis showed that Jallo Wildlife Park Lahore appeared to be more polluted than WPB and WPM. Indian Peafowl kept at WPM and JWPL exhibited higher levels of genotoxicity compared to the birds kept at WPB. This disparity can be attributed to the increased exposure to pollution and heightened stress experienced by the peafowl in the former two locations. This study concluded that among all the three study sites of Punjab, the WPB is most suitable for housing captive animals and birds.
Introduction
Pheasants are widely regarded as one of the most distinctive avian species due to their remarkable characteristics and significant ecological contributions. Their visual appeal and pivotal role within ecosystems are primary factors that underline their importance. They serve as valuable indicators for assessing the environmental quality of their respective habitats, as highlighted by Ali et al. (Citation1987). Pavo cristatus, commonly referred to as the common or blue peafowl, is a species belonging to the pheasant family. It is recognized as the largest flying bird within the pheasant family and is classified under the order Galliformes and family Pheasianidae, as noted by Shahbaz (Citation2020).
In Pakistan, Pavo cristatus is predominantly found in the extreme of Sindh province and the north-eastern border areas of the Punjab province, which include the border belt in district Narowal and northern Punjab (Roberts Citation1991; Azam and Shafique Citation2005). However, its population has experienced local extinction in certain areas within this historical distribution range, and it has become rare in the wild due to several threats to its survival. The primary factor posing risk to the existing peafowl population include habitat loss and degradation (Anwar et al. Citation2015; Jose and Nameer Citation2020). To overcome these threats, measures have been taken to protect the Indian Peafowl, and hunting of P. cristatus is explicitly prohibited under the Punjab Wildlife Act-1974 (Mushtaq-ul-Hassan et al. Citation2012).
For the conservation of P. cristatus, some individuals are maintained in captivity, which serves as a preventive measure against extinction (El-Shahawy Citation2010). Captivity, in this context, refers to the confinement of both domestically bred and wild-caught birds in cages and enclosures (Akram et al. Citation2019). While captivity plays a crucial role in animal conservation, it also entails significant consequences. The restricted living spaces subject the birds to stress, leading to a decline in their overall fitness (Parveen and Sidra Citation2018). Additionally, captivity-induced stress can exacerbate the problem of parasitic infections, posing a serious threat to this endangered species. Moreover, captivity also decrease the resistance of species to disease (Akram et al. Citation2019).
The zoos, which were constructed outside the cities now come in the center of the towns and cities due to the unplanned expansion of towns with increase in the human population. In addition to experiencing captivity-induced stress, wild animals kept in protected zones also are confronted with the mounting problem of environmental pollution, which poses adverse effects to their health and well-being. The increasing levels of pollution in these environments disrupt the natural ecological balance and can compromise the overall health and comfort of the captive wildlife (Gupta and Bakre Citation2013). Especially, heavy metal pollution is one of the major threats to health, since these are particularly hazardous and dangerous elements of contamination (Gworek et al. Citation2016). A small amount of specific metals such as Co, Cu, Fe, Mn and Zn plays an important role for the normal metabolism of organisms. On the other hand, their excessive amount leads to toxicity by bio-accumulation (Lenntech Citation2012). Organisms show variations in the accumulation of heavy metals, influenced by factors such as size, dietary preferences, age, gender, and other internal or external parameters. These diverse characteristics can lead to differential levels of heavy metal uptake and storage in different species or individuals within a population (Anbazhagan et al. Citation2021). Some metals are highly toxic like Pd. Cd, Hg and As. Even their small dose can lead to serious problems (Aragay and Merkoçi Citation2012; Yasmeen et al. Citation2020). Heavy metal contamination from anthropogenic activities displays a possible risk to health through bio-accumulation in the food chain (Sheppard et al. Citation2009).
Heavy metals can induce various toxic effects in birds, such as reduced reproductive rates, eggshell thinning, slowed growth, compromised immunocompetence, and developmental deformities and malformations. Consequently, the bird population experiences declines (Dauwe et al. Citation2006). As a result, public concern has escalated concerning heavy metal contamination. It is now imperative to monitor, assess, manage, and remediate the biological and ecological damage caused by heavy metal pollution (Movalli Citation2000; Naccari et al. Citation2009). Numerous bird tissues have been used for monitoring bird’s exposure to heavy metals and evaluating risk mainly feathers, liver and more recently, blood is used. Feathers, blood, and eggs can be obtained without any difficulty and repetitively from the same individual if required, and without losing the bird therefore they are preferable (Alvárez et al. Citation2013). Furthermore, this study aims to evaluate whether the combination of captivity-related stresses and excessive metal contamination can negatively impact the DNA of P. cristatus. Understanding the potential impacts on their DNA will contribute to a comprehensive assessment of the risks faced by these birds in captive environments and aid in the development of appropriate conservation strategies.
This research aimed to evaluate and contrast the buildup of metallic elements and their impacts on DNA damage in captive P. cristatus.
Materials and methods
Study area
This study was conducted at three geographically different sites of Punjab, Pakistan that are Wildlife Park Bahawalnagar (WPB), Jallo Wildlife Park Lahore (JWPB) and Wildlife Park Murree (WPM). These sites have different environmental and management conditions. Jallo Wildlife Park was established in 1978. It is situated 20 km east to Lahore city which is a semi- aired hot plane area (Sarfaraz et al. Citation2014) and have an elevation of 217 m with average annual rain fall 628 mm. The minimum temperature of Lahore is 20 °C in winter while in summer it exceeds up to 40 °C. It covers an area of 461 acres or 187 ha. Wildlife Park Bahawalnagar was established in 1986–1988 under the developmental program of Captive Breeding of Blackbuck at Bahawalnagar. It has an area of fifteen acre. It is 4 Km from Railway station, Bahawalnagar which has semi-arid climate (Sarfaraz et al. Citation2014). The annual rainfall is 194.4 mm. The minimum temperature recorded is 7-12°C and 38-42°C is the maximum range. Wildlife Park Murree was established in 1986–1992 under the developmental program of ‘Development of Wildlife Park, Bansra Gali, Murree’. It is 70 Km from Rawalpindi close to Lawrence College, Bansra Gali, Murree. It has an area of two hundred forty acres. The annual rainfall is 1789.3 mm. The climate remains charming throughout the year having four seasons of spring, summer, autumn and winter. The minimum temperature recorded is −10 °C and 22–25 °C is the maximum range (Sarfaraz et al. Citation2014, ).
Species selection
P. cristatus is a common species that may be found in parks and zoos throughout the Pakistan. It has a high capacity to adapt to changing environments in a wide range of habitats. The number of selected birds were counted and recorded from all the selected sites. Selected number of birds were used to compare the heavy metal accumulation and DNA damage.
Collection of samples
Blood samples
About 2-3 ml of blood sample (20 samples from each site) were collected from the avian brachial vein into EDTA tubes (10 samples for Single-cell gel electrophoresis) and serum separator tubes (10 samples from each site for screening of heavy metal bio-accumulation). These tubes were wrapped in a cloth towel and placed in bags of handling with ice packs (Samour et al. Citation2010).
Feather samples
The tail feathers were collected from the peafowls’ cages of each site because these feathers have constant supply of blood and are also in direct contact with the environment contaminants. Only molten feathers of birds were collected, with no interaction or injury to the birds. The feathers were properly packed in sterile ziplock bags with proper labelling (Anbazhagan et al. Citation2021).
Egg samples
During the breeding season of P. cristatus from April to May (Naseer et al. Citation2018),10 eggs were collected from each site. The collected eggs were labelled with nest numbers before being put in glass jars washed with acetone (Ashkoo et al. Citation2020)
Screening for heavy metal concentration
Digestion of samples
Blood samples in serum vacutainers were collected and allowed to clot in plain bottles. The clotted blood samples were subjected to centrifugation at 2000 rpm for 10 min to separate the serum from the unwanted red blood cells, as this method efficiently increases the volume of serum per unit of blood. Subsequently, one millilitre of serum was diluted with ten millilitres of de-ionized water (Akan et al. Citation2014).
The feather samples were washed with acetone and then rinsed three times with de-ionized water. Then the feathers were dried in the oven for 48 h at 60 0 C and cut into smaller pieces. One gram of crushed feathers from each sample underwent treatment with a reagent consisting of 5 ml of nitric acid and 5 ml of hydrogen peroxide on a hot plate set at 70 °C until the acid digestion process was fully completed. The resulting extract was then allowed to gradually reach room temperature and then filtered afterwards using Whatman filter paper. The filtered solution was further diluted with 25 ml of de-ionized water (Anbazhagan et al. Citation2021).
In order to eliminate the adhering exterior pollutants, eggs were washed with acetone and then with de-ionized water. Then eggs’ content was removed with the help of toothpick and poured in petri dishes, while the egg shells were placed in other petri dishes. The samples were dried in an oven to obtain a consistent dry weight. The dried samples of eggshells and egg contents were homogenized separately. Erlenmeyer flasks were used and 0.5 g of homogenized powder from each egg sample was added in the flasks together with 10 ml of nitric acid. Erlenmeyer flasks were heated at 140°C until a clear solution was obtained (Miri et al. Citation2017). After cooling at room temperature, the digested acidic samples were filtered by using Whatman filter paper and the solution was diluted with 25 ml of de-ionized water into 25 ml polypropylene volumetric flask. All digested samples were maintained in the refrigerator at 4 °C before the chemical analysis of heavy metals by Atomic Absorption Spectrophotometry (Ashkoo et al. Citation2020).
Chemical analysis of samples
All the prepared solutions of feather, blood, and egg samples were subjected to chemical analysis for heavy metals using Atomic Absorption Spectrometry (AAS). The concentraitons of, Pb, Cr, Co, Ni and Mn were measured at different wavelengths using this analytical technique at 357.9, 217.0, 232.0, 240.7 and 403.0 nm, respectively (Yasmeen et al. Citation2020).
Calculation of metal concentration
The concentrations of metals were calculated by using the following formula:
In current study, the dilution factor was 25 ml for 1 g feather, egg shell and egg content samples and 10 ml for 1 ml blood serum as mentioned above in the digestion of samples.
DNA Damage examination
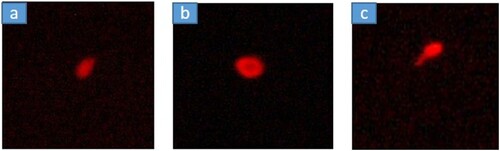
The Single-Cell gel electrophoresis (SCGE) or Comet assay determines the DNA damage caused by heavy metals contamination. The assay was conducted following the protocols recommended by Singh et al. (Citation1988). DNA damage in different parts of the cells were analyzed and recorded.
Statistical analysis
All statistical analyses were conducted using IBM SPSS Statistics 21 and Statistics 8.1 software. ANOVA (Analysis of variance) was employed to compare the concentrations of heavy metals and DNA damage based on different sites (Steel et al. Citation1997). Post hoc Tukey's range test was utilized to compare means among different groups.
Results
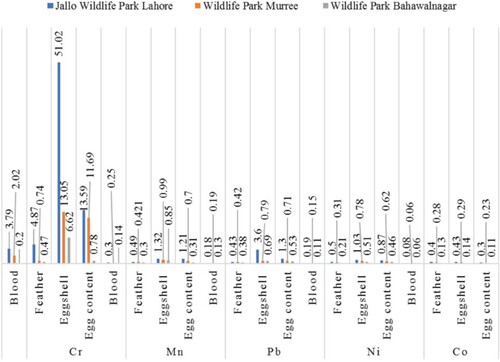
demonstrates that the mean concentration of heavy metals significantly varied (P < 0.05) in blood, feather, eggshell and egg content samples of P. cristatus kept at WPM, JWPL and WPB. The Cr contamination was significantly higher in blood, feather, eggshell and egg content samples of all locations followed by Mn, Pb and finally Ni and Co.
Table 1. Comparisons of Means ± SD, (µg/g) of heavy metals in the blood and feather samples of P. cristatus kept at three wildlife parks on wet weight basis.
Overall, heavy metals accumulation pattern in samples of all sites were egg shells > Egg contents > Feathers > Blood. JWPL was polluted as indicated by the levels of all heavy metals compared to WPM and WPB ().
Figure 2. Comparative concentration of all selected heavy metals(µg/g) in the blood, feather and egg samples of P. cristatus kept at three wildlife parks on wet weight basis.
The results obtained from the single-cell gel electrophoresis indicated significant differences in the mean values of LHead, LTail, LComet, Head DNA, and Tail DNA at a significance level of P < 0.05. However, OTM and TM values were observed to be consistent across all sites, displaying only minimal deviations. Values for LHead, LTail, LComet, Head DNA, and Tail DNA were the highest in WPM followed by JWPL, and lowest in WPB ().
Table 2. Assessment of Comet parameters in blood of captive P. cristatus at three wilflife parks.
The Post Hoc Tukey test for between-group comparison revealed that DNA mutations in P. cristatus kept at WPM and JWPL were nearly similar. However, the DNA variations in P. cristatus kept at WPB differed significantly from the previous groups ().
Discussion
In this research work, blood, feathers and egg samples of P. cristatus were utilized as a non-destructive technique to investigate the concentration of heavy metal bio-accumulation. Actually, heavy metal concentrations in blood samples were correlated to the distance from source of pollution (Berglund Citation2018) and captivity stress (Yasmeen and Asif Citation2022). Lahore is densely populated, has a heavy traffic load, and is more industrialized as compared to Murree and Bahawalnagar therefore the concentration of Cr is higher in Lahore. The mean concentration of Cr (0.20–3.78 μg/g) in blood was below the threshold level in all the sites as compared to the study reported by Riaz et al. (Citation2021). In the latter study, the mean concentration of Chromium (Cr) in the blood samples of scavenger birds from three waste disposal sites, namely Lakhodair landfill Lahore, Mehmood booti waste dumping site Lahore, and Muhammad wala Faisalabad waste dumping site, was found to be 25 µg/g. The mean concentration of Mn was lower in blood samples in our study as compared to the study of Van Wyk et al. (Citation2001). Khan et al. (Citation2015) reported the mean concentrations of Ni in blood samples of poultry chicken gallus domesticus in three selected cities of Pakistan and showed that Ni concentration in blood samples of Karrachi, Hyderabad and Thatta were 0.543, 0.885 µg/g and 0.402 μg/g, respectively. And this concentration was higher than the concentration determined in our study and its possible reason might be site and species differences. In contrast, the concentration of Co that was measured in our research work was higher compared to that found in the research work of Van Wyk et al. (Citation2001). Cr concentration determined in feather samples of JWPL exceeded the threshold level (2.8 μg/g) similar to work reported by Burger and Gochfeld (Citation2000) and it might be linked to the anthropogenic source of contamination. In current study the mean concentration of Mn was very low in feather samples of P. cristatus kept at WPM (0.42 μg/g), JWPL (0.49 μg/g) and WPB (0.30 μg/g) as compared to the study of Malik and Zeb (Citation2009), in which the mean concentration of Mn in the urban and rural feather samples of Bubulcus ibis was 9.65 and 8.79 μg/g respectively. The mean concentration of Pb (0.38 μg/g −0.43 μg/g) measured in the feather samples of P. cristatus in study area were much lower than that reported by Tasneem et al. Tasneem et al. who reported concentrations of Pb within 31.62 ± 9.80 μg/g in the tail feathers of A. grayii in a research work performed in the outskirts of Lahore, Pakistan and its possible reason might be the type of feather. Ni concentrations in the current research work in feather samples of P. cristatus kept at WPM, JWPL and WPB were 0.50, 0.31, and 0.21 μg/g respectively, which were much lower than those found in previous studies, in Pakistan. For example, Abdullah et al. (Citation2015) reported that the concentration of Ni in feathers of birds was 30-47.5 μg/g in Lahore and 77–89 μg/g in Sialkot. In current study work the mean concentration of Cr was very high in eggshell samples of P. cristatus, kept at WPM (13.05 µg/g), JWPL (51.01 µg/g) and WPB (6.62 µg/g). Mn concentration (0.84 μg/g −1.32 µg/g) and Pb concentration (0.69 μg/g −3.61 µg/g) was lower and concentration of Ni (0.51 μg/g −1.02 µg/g) was higher than the concentrations reported by Hashmi et al. (Citation2013), who found that the mean concentration of Cr, Mn, Pb and Ni concentration in the eggshell samples of cattle egret (Bubulcus ibis) and little egret (Egretta garzetta) from the Punjab province, Pakistan ranged from 0.35-0.8μg/g; 0.47-3.98μg/g; 1.05-5.45μg/g and 0.01-0.08μg/g, respectively. For both species, variations in heavy metal concentrations at different sites might represent different exposures due to local variances in pollutant concentrations (Burger et al. Citation2009), distance from the contaminants source, and habitat characteristics. In the current study, the concentration of Cr in egg content was 13.59 μg/g at JWPL,11.69 µg/g at WPM and 0.79 µg/g at WPB that was much higher than the study reported by Ashkoo et al. (Citation2020), where the concentration of Cr in egg content of seabirds Lesser (Thalasseus bengalensis) and Greater Crested Tern (Thalasseus bergii) were below the detection limit. In our study the concentration range of Pb and Mn in egg content samples of P. cristatus kept at JWPL, WPM and WPB were 0.53 μg/g −1.30 µg/g and 0.31–1.21 μg/g, respectively, similar to the study reported by Kim and Oh (Citation2014), who measured the concentration of heavy metals in egg content samples of black-tailed gull (Larus crassirostris) from Korea and found that concentration of lead and Mn were 0.92 µg/g and 1.99 μg/g, respectively. These values were within the range of our research work. The concentration of Ni and Co in egg content samples of P. cristatus in our research work were 0.87 and 0.29 µg/g at Jallo Wildlife Park Lahore,0.62 µg/g and0.23 µg/g at WPM and 0.46 and 0.11 µg/g at WPB. The only possible reason might be Lahore is more industrial area as compared to Murree and Bahawalnagar.
The Comet Assay has proven to be a sensitive and efficient technique for assessing DNA damage in birds and animals. This study aimed to measure the relationship between ecological damage and genotoxicity, with a particular focus on heavy metal exposure in captive P. cristatus at JWPL, WPB, and WPM.
The results of the current study indicated that Lahore and Murree exhibited higher levels of heavy metal pollution compared to Bahawalnagar. Additionally, the DNA damage was greatest in the birds of WPM and JWPL. These findings are supported by Bonisoli-Alquati et al. (Citation2010), who studied DNA damage in Hirundo rustica exposed to low-level radioactive contamination in the Chernobyl region. Their results suggested that birds in contaminated areas exhibited more DNA damage than those in distant regions, which aligns with our study's findings.
A similar study by Gomes et al. (Citation2018) assessed the impact of pollution on DNA impairment in Geophagus brasiliensis fish before and after a mining company's tailings influx disaster in Mariana. Indications of genetic harm in fish were also observed previously due to the presence of local industries discharging effluents containing toxic heavy metals. Similarly, Naz et al. (Citation2020) reported DNA damage in captive P. cristatus species due to long-term captivity, which is consistent with our study's findings.
In the present study, captive P. cristatus experienced stress from captivity, and exposure to pollutants like heavy metals, which could lead to DNA damage. The results demonstrated that DNA damage increased with increasing levels of heavy metal exposure in captive P. cristatus. As Lahore exhibited the highest level of heavy metal exposure, the DNA damage was higher in the captive birds kept at WPM and JWPL.
Conclusions
This study demonstrates that captivity plays a significant role in conserving P. cristatus; however, environmental pollution have detrimental effects on captive animals. Among the three wildlife parks studied, Wildlife Park Bahawalnagar appears to be the most suitable for captivity, as it exhibits the least accumulation of heavy metals. Chromium was found to be the most accumulated metal compared to others. DNA damage was observed in P. cristatus kept at Wildlife Park Murree and Jallo Wildlife Park Lahore, which can be attributed to their increased exposure to pollution and stress. To safeguard this species, proper management and control of environmental pollutants are imperative. Such investigations will aid in implementing effective conservation strategies and mitigating potential threats to wildlife in captivity.
Ethical statement
The Committee on Animal Rights and Welfare, GC University Faisalabad, Pakistan approved this study (DZ/122/2019).
Acknowledgements
We extend our appreciation to the Researchers Supporting Project (no. RSP2023R191), King Saud University, Riyadh, Saudi Arabia.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Abdullah M, Fasola M, Muhammad A, Malik SA, Bostan N, Bokhari H, Kamran MA, Shafqat MN, Alamdar A, Khan M, et al. 2015. Avian feathers as a non-destructive bio-monitoring tool of trace metals signatures: a case study from severely contaminated areas. Chemosphere. 119:553–561. doi:10.1016/j.chemosphere.2014.06.068.
- Akan JC, Sodipo OA, Liman Y, Chellube ZM. 2014. Determination of heavy metals in blood, urine and water samples by inductively coupled plasma atomic emission spectrophotometer and fluoride using ion-selective electrode. J Anal Bioanal Tech. 5(9):1–7.
- Akram MZ, Zaman MA, Jalal H, Yousaf S, Khan AY, Farooq MZ, Hussain T. 2019. Prevalence Of gastrointestinal parasites of captive birds In punjab, Pakistan. Pak Vet J. 39(1):132–134. doi:10.29261/pakvetj/2018.123.
- Ali S, Ripley SD, Dick JH. 1987. Compact handbook of the birds of India and Pakistan, 2nd ed. Delhi: Oxford University Press.
- Alvárez CR, Moreno MJ, Alonso LL, Gómara B, Bernardo FG, Martín-Doimeadios RR, González MJ. 2013. Mercury, methylmercury, and selenium in blood of bird species from Doñana National Park (Southwestern Spain) after a mining accident. Environ Sci Pollut Res. 20(8):5361–5372. doi:10.1007/s11356-013-1540-1.
- Anbazhagan V, Partheeban EC, Arumugam G, Selvasekaran V, Rajendran R, Paray BA, Al-Sadoon MK, AR A-M. 2021. Avian feathers as a biomonitoring tool to assess heavy metal pollution in a wildlife and bird sanctuary from a tropical coastal ecosystem. Environ Sci Pollut Res. 28:1–11. doi:10.1007/s11356-021-13371-1.
- Anwar M, Mahmood A, Rais M, Hussain I, Ashraf N, Khalil S. 2015. Population density and habitat preference of Indian peafowl (Pavo cristatus) in Deva Vatala National park, Azad Jammu and Kashmir, Pakistan. Pakistan J. Zool. 47:1381–1386.
- Aragay G, Merkoçi A. 2012. Nanomaterials application in electrochemical detection of heavy metals. Electrochim Acta. 84:49–61. doi:10.1016/j.electacta.2012.04.044.
- Ashkoo A, Amininasab SM, Zamani-Ahmadmahmoodi R. 2020. Bioaccumulation of heavy metals in eggshell and egg content of seabirds: Lesser (Thalasseus bengalensis) and greater crested tern (Thalasseus bergii). Mar Pollut Bull. 154:111126. doi:10.1016/j.marpolbul.2020.111126.
- Azam MM, Shafique CM. 2005. Birdlife in Nagarparkar, district Tharparkar, Sindh. Rec Zool Surv Pak. 16:26–32.
- Berglund ÅM. 2018. Evaluating blood and excrement as bioindicators for metal accumulation in birds. Environ Pollut. 233:1198–1206. doi:10.1016/j.envpol.2017.10.031.
- Bonisoli-Alquati A, Voris A, Mousseau TA, Møller AP, Saino N, Wyatt MD. 2010. DNA damage in barn swallows (Hirundo rustica) from the Chernobyl region detected by use of the comet assay. Compar Biochem Physiol Part C Toxicol Pharmacol. 151(3):271–277. doi:10.1016/j.cbpc.2009.11.006.
- Burger J, Gochfeld M, Jeitner C, Burke S, Volz CD, Snigaroff R, Snigaroff D, Shukla T, Shukla S. 2009. Mercury and other metals in eggs and feathers of glaucous-winged gulls (Larus glaucescens) in the Aleutians. Environ Monit Assess. 152(1):179–194. doi:10.1007/s10661-008-0306-6.
- Burger J, Gochfeld M. 2000. Metal levels in feathers of 12 species of seabirds from Midway Atoll in the northern Pacific Ocean. Sci Total Environ. 257(1):37–52. doi:10.1016/S0048-9697(00)00496-4.
- Dauwe T, Janssens E, Eens M. 2006. Effects of heavy metal exposure on the condition and health of adult great tits (Parus major). Environ Pollut. 140:71–78. doi:10.1016/j.envpol.2005.06.024.
- El-Shahawy IS. 2010. Eimeria pavoaegyptica sp. nov.(Apicomplexa: Eimeriidae) in faeces of Indian peacocks, Pavo cristatus Linnaeus, 1758 (Galliformes: Phasianidae) from Egypt. Mem Inst Oswaldo Cruz. 105(8):965–969. doi:10.1590/S0074-02762010000800003.
- Gomes LC, Chippari-Gomes AR, Miranda TO, Pereira TM, Merçon J, Davel VC, Barbosa BV, Pereira AC, Frossard A, Ramos JP. 2018. Genotoxicity effects on Geophagus brasiliensis fish exposed to Doce River water after the environmental disaster in the city of Mariana, MG, Brazil. Braz J Biol. 79:659–664. doi:10.1590/1519-6984.188086.
- Gupta V, Bakre P. 2013. Mammalian feces as bioindicator of urban air pollution in captive mammals of Jaipur Zoo. World Environ. 3(2):60–65.
- Gworek B, Dmuchowski W, Koda E, Marecka M, Baczewska AH, Brągoszewska P, Sieczka A, Osiński P. 2016. Impact of the municipal solid waste Łubna landfill on environmental pollution by heavy metals. Water. 8(10):470. doi:10.3390/w8100470.
- Hashmi MZ, Malik RN, Shahbaz M. 2013. Heavy metals in eggshells of cattle egret (Bubulcus ibis) and little egret (Egretta garzetta) from the Punjab province, Pakistan. Ecotoxicol Environ Saf. 89:158–165. doi:10.1016/j.ecoenv.2012.11.029.
- Jose VS, Nameer PO. 2020. The expanding distribution of the Indian Peafowl (Pavo cristatus) as an indicator of changing climate in Kerala, southern India: A modelling study using MaxEnt. Ecol Indic. 110:105930. doi:10.1016/j.ecolind.2019.105930.
- Khan MZ, Perween SH, Gabol K, Khan IS, Baig N, Kanwal R, Jabeen T. 2015. Concentrations of heavy metals in liver, meat and blood of poultry chicken Gallus domesticus in three selected cities of Pakistan. Can J Pure Appl Sci. 9(1):3313–3324.
- Kim J, Oh JM. 2014. Trace element concentrations in eggshells and egg contents of black-tailed gull (Larus crassirostris) from Korea. Ecotoxicology. 23(7):1147–1152. doi:10.1007/s10646-014-1256-0.
- Lenntech BV. 2012. Heavy metals, Available at Website periodic. Periodic chart.
- Malik RN, Zeb N. 2009. Assessment of environmental contamination using feathers of Bubulcus ibis L., as a biomonitor of heavy metal pollution, Pakistan. Ecotoxicology. 18(5):522–536. doi:10.1007/s10646-009-0310-9.
- Miri M, Akbari E, Amrane A, Jafari SJ, Eslami H, Hoseinzadeh E, Zarrabi M, Salimi J, Sayyad-Arbabi M, Taghavi M. 2017. Health risk assessment of heavy metal intake due to fish consumption in the Sistan region, Iran. Environ Monit Assess. 189(11):1–10. doi:10.1007/s10661-017-6286-7.
- Movalli PA. 2000. Heavy metal and other residues in feathers of laggar falcon Falco biarmicus jugger from six districts of Pakistan. Environ Pollut. 109(2):267–275. doi:10.1016/S0269-7491(99)00258-4.
- Mushtaq-ul-Hassan M, Ali Z, Arshad MI, Mahmood S, Mahmood-ul-Hassan M. 2012. Effects of mating sex ratios in Indian peafowl (Pavo cristatus) on production performance at Wildlife Research Institute, Faisalabad (Pakistan). Iran J Vet. 13(2):143–146.
- Naccari C, Cristani M, Cimino F, Arcoraci T, Trombetta D. 2009. Common buzzards (Buteo buteo) bio-indicators of heavy metals pollution in Sicily (Italy). Environ Int. 35:594–598. doi:10.1016/j.envint.2008.11.002.
- Naseer J, Anjum KM, Munir MA, Nazir MA, Yousaf MZ, Naseer O, Anjum A, Khan AU, Akbar MT. 2018. A study on Indian peafowl (Pavo cristatus) emphasising breeding season and feeding behaviour in captivity. Indian J Anim Res. 52(11):1664–1666.
- Naz S, Muazzam S, Sagheer A, Tanveer A, Khan NA, Ali Z, Chand N, Khan RU. 2020. Captivity stress influences the DNA damage of Pavo cristatus under environmental conditions of Faisalabad, Pakistan. Environ Sci Pollut Res. 27(5):5636–5639. doi:10.1007/s11356-019-07307-z.
- Parveen Z, Sidra S. 2018. Diet preferences and general behavior of peafowls in captive environment. Punjab Univ J Zool. 33:16–21. doi:10.17582/pujz/2018.33.1.16.21.
- Riaz A, Said N, Riaz MA, Ilyas M, Ashfaq UA, Rehman A. 2021. Heavy metals accumulation trends in scavenger birds from different landfill and dumping sites of Punjab. Pure Appl Biol. 11:109–115. doi:10.19045/bspab.2022.110012.
- Roberts TJ. 1991. The birds of Pakistan. Non-passeriformes. Volume 1. Karachi: Oxford University Press.
- Samour J, Naldo J, Rahman H, Sakkir M. 2010. Hematologic and plasma biochemical reference values in Indian peafowl (Pavo cristatus). J Avian Med Surg. 24(2):99–106. doi:10.1647/2008-019.1.
- Sarfaraz SA, Arsalan MH, Fatima HI. 2014. Regionalizing the climate of Pakistan using Köppen classification system. Pak Geogr Rev. 69(2):111–132.
- Shahbaz M. 2020. Prevalence of endoparasites in Indian peafowl under captive facilities in the punjab, Pakistan. Biologia (Lahore, Pakistan). 66:155–161.
- Sheppard SC, Grant CA, Sheppard MI, de Jong R, Long J. 2009. Risk indicator for agricultural inputs of trace elements to Canadian soils. J Environ Qual. 38(3):919–932. doi:10.2134/jeq2008.0195.
- Singh NP, McCoy MT, Tice RR, Schneider EL. 1988. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 175:184–191. doi:10.1016/0014-4827(88)90265-0.
- Steel RGD, Torrie JH, Dicky DA. 1997. Principles and procedures of statistics. A biometrical approach, 3rd ed. New York: McGraw Hill, Inc. Book Co.
- Van Wyk E, Van der Bank FH, Verdoorn GH, Hofmann D. 2001. Selected mineral and heavy metal concentrations in blood and tissues of vultures in different regions of South Africa. S Afr J Anim. 31(2):57–64.
- Yasmeen R, Asif L. 2022. Heavy metal exposure and behavioral assessment of vultures in a captive environment. Environ Sci Pollut Res. 29(45):68096–68102. doi:10.1007/s11356-022-20656-6.
- Yasmeen R, Shaheen S, Khan BN, Bokhari SS, Rafi U, Qurashi AW. 2020. Faecal matter of spotted deer (Axis axis) acts as bioindicator of heavy metals contamination in the Air. Pak J Zool. 52(2):813–816. doi:10.17582/journal.pjz/20181214041244.