?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The development of gastrointestinal tracts in goats after birth is critical for establishment of digestion and absorption function, growth, immunity and health of adults. However, this process from birthday to adulthood is lacking. This study was conducted to determine the anatomical, morphological, physiological and microbiome development of the gastrointestinal tract of Leizhou goats. The weights of rumen, reticulum, omasum and abomasum increased with age. The weights of each intestinal segment increased with age. The length and width of rumen papilla, muscular thickness, cuticle thickness and wall thickness were significantly increased with developmental stage. Consistently, the villus length and width and crypt depth of the small intestine increased significantly with age. The volatile fatty acids (VFAs) concentration in both rumen and caecum increased with ages. Specifically, VFAs were not detected at birthday rumen, while they showed in caecum. The activities of digestive enzymes in both rumen and caecum had a similar pattern with VFAs. The microbial diversity and composition were distinct between the rumen and caecum after birth. We observed a rapid influx of microbes in rumen and caecum at birthday and gradually changed from 1 month of age to adulthood. This study provides insights into feeding and health maintenance strategies.
Highlights
This study systematically determined the growth and development of Leizhou goats from birth to adulthood.
The developments of the gastrointestinal (GI) tracts including digestive enzyme activities, VFAs and microbiome matured with age.
The microbiome in the GI-tracts of goats were associated with the host phenotypes.
Introduction
Goats (Capra hircus), one of the most important livestock species, provide an important protein source for human requirements (Wang et al. Citation2021). Due to the fast economic growth, the demands for improving the quality and productivity of goats are urgent. In young ruminants, vast changes in the digestive tract, including morphological, biochemical and ultrastructural development, impact the performance of adult ruminants (Abdelsattar et al. Citation2022). Good development of the gastrointestinal tract has a lifelong influence on nutrient digestion, absorption and metabolism, as well as production performance in goats (Bi et al. Citation2019). The knowledge of the temporal gastrointestinal physiology from birth to adulthood could allow us understand anatomical physiology and functional development for decreased chances of disorders in livestock (Zhuang et al. Citation2020). Therefore, it is critical to investigate the transitions of the anatomical, morphological and physiological development of the gastrointestinal tract of goats.
The rumen is one of the most important organs where plant materials convert to volatile fatty acids (VFAs) such as acetate, propionate and butyrate, which could provide energy for animal uses (Zhuang et al. Citation2020). During the period that animals suckled breast milk, the rumen in goat kids is not well developed and not involved in digestion. Then, the rapid development of the rumen is observed as the structure and physiological characteristics change significantly with age. The rumen microbiome and its fermentation products are strongly associated with the rumen papillae developments (Jiao et al. Citation2016; Yang et al. Citation2018; Xie et al. Citation2020). The small intestine is the primary site for the digestion and absorption of nutrients. The digestion of goat kids shifts from the intestine to the rumen during the transition of diets from milk to solid feed (Li et al. Citation2019). The caecum is another vital fermentation organ for animals. Most of the chyme that has not been fermented completely by the rumen microbiota will be fermented to produce metabolites (e.g. VFAs) in the caecum and then are absorbed and utilized in the caecum (Li et al. Citation2012). In goat kids, several studies started investigating the rumen and intestinal microbiome in young ruminants (Li et al. Citation2019; Zou et al. Citation2020; Abdelsattar et al. Citation2022). However, the development of gastrointestinal structure, digestive enzyme, VFAs and microbiome from birth to adult is not systematically studied (Wang et al. Citation2017).
The Leizhou goat is a famous local breed in China due to its prolificacy. However, to the best of our knowledge, there has been no data published about the anatomical and microbial development of the digestive tract of the Leizhou goat from birthday to adulthood. Therefore, in this study, gastrointestinal development from birthday to adulthood was measured, and rumen and caecal microbiome were sequenced to find the host–microbiome symbiosis.
Materials and methods
Experimental design
A total of 24 healthy male Leizhou goat kids with similar birth weights were selected for this experiment in the goat pilot base of the Guangdong Ocean University. Four ages (birthday, 1 month of age, 2 months of age and adult (about 12 months of age)) were selected, and each age had six replicates. Goat kids lived with their dams and consumed breast milk from birthday to 60 days of age. Goats were supplemented with a solid concentrate (nutrient level in Table S1) starting on day 10. After weaning, goat kids grazing on pasture were supplemented with concentrate.
Measurement of the developments of the gastrointestinal tract
Six goat kids of each age were taken to the experimental abattoir for slaughter on the respective deadline to determine the developments of the gastrointestinal tract. On birthday, newborn kids were selected after they suckled colostrum. The goats were anaesthetized using 30 mg kg−1 bodyweight sodium pentobarbitone (3% concentration; BioChemParter company), killed by exsanguination from the jugular vein, skinned, and eviscerated according to the method described by (Chai et al. Citation2018). The empty weight of rumen, reticulum, omasum, abomasum, duodenum, jejunum, ileum, caecum, colon and rectum were recorded. The volume of the stomach was recorded by filling the stomach with water. The length of each fragment of both the small and large intestine was measured. Furthermore, the relative weight and volume were calculated by the weight or volume of each stomach divided by the weight or volume of the forestomach parts. Similarly, the relative weight and length of the intestine followed similar equation.
Histomorphological examination
Rumen and small intestine (six replicates for duodenum, jejunum and ileum, respectively) tissue samples were collected for histomorphological examination. Tissue samples (about 1 cm2) were separated with a sharp scalpel from the nonsqueezed parts of the organ, washed with prechilled phosphate buffer solution (pH = 7.1), placed in an embedding box, and immersed in a 10% formalin solution immediately. After fixation, samples were dehydrated by a TSJ-II fully automatic closed tissue dehydrator (Zhongwei Electronic Instrument Co., Ltd.). The samples were trimmed and embedded in paraffin blocks using a BMJ-III type embedding machine (Zhongwei Electronic Instrument Factory). The formed blocks were cut on a rotary microtome (Leica-2016) and stained with haematoxylin and eosin (Chengdu Lilai Biological Technology Co., Ltd.). The histomorphological examination was done using a BA400 digital trinocular micro-camera (McAudio Industrial Group Co., Ltd.) and Image-Pro-Plus 6.0 software (American Media Cybernetics). Six pictures were taken for each sample, including 2 objective lenses of 40× and 100×. Ten sets of data were measured for each slice number. The small intestine indicators included villus height, crypt depth and muscle layer thickness by lens 40×, as well as the epithelial thickness by lens 100×. The rumen indicators had rumen papillae height, rumen papillae width, lamina propria thickness, muscle layer thickness (40× lens), epithelial thickness and stratum corneum thickness (40× lens).
VFA determination
Rumen fluid at approximate the 10 mL level was filtered through four layers of gauze, placed in a 15 mL centrifuge tube, and immediately frozen at −20°C for the analysis of rumen fermentation. Caecal contents about 0.10 g were weighted, added deionized water according to the weight volume ratio of 1:9 (g mL−1), and mixed well after complete thawing at 4°C. VFA concentration in the rumen fluid was quantified by gas chromatography (GC) using methyl valerate as the internal standard in an Agilent 6890 series GC equipped with a capillary column (HP-FFAP19095F-123, 30 m, 0.53 mm diameter and 1 mm thickness, Agilent Technologies, Santa Clara, CA, USA) (Makkar et al. Citation1982).
Determination of digestive enzyme activity of gastrointestinal contents
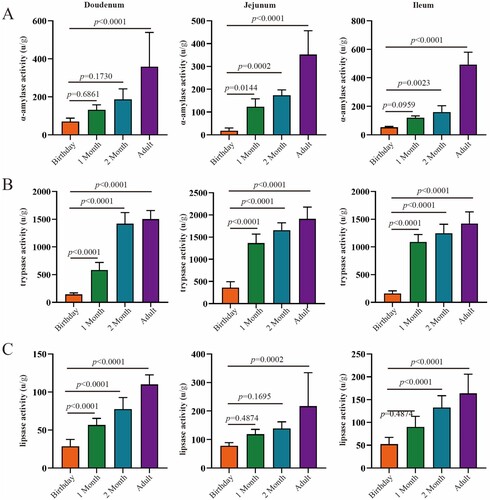
After slaughter, content of rumen, duodenum, jejunum and ileum were collected. The rumen fluid was centrifuged at 3000 r min−1 for 10 min at 4°, and the supernatant was broken by ultrasonic wave 3 times at 400 W. After 30 s each time, the activities of lipase, α-amylase, cellulase and pectinase were measured based on methods described by (Rey et al. Citation2012). The intestinal contents of about 0.10 g were weighted, added homogenate medium according to the weight volume ratio of 1:9 (g mL−1), crushed and homogenized. Then, the homogenate was centrifuged at 2500 r min−1 for 10 min at 4°, and the supernatant was taken to determine lipase, trypsase and α-amylase.
DNA extraction and 16S sequencing of rumen and caecum
Three replicates of each age were used for next-generation sequencing. A DNeasy PowerSoil Kit (Qiagen, Valencia, CA, USA) was used to extract total genomic DNA from content samples of the rumen and caecum. The DNA quality was checked using a Thermo NanoDrop 2000 UV microphotometer and 2% agarose gel electrophoresis. The V3-V4 region was amplified using universal primers linked with specific adaptors. The diluted genomic DNA was then used as a template, and PCR was performed using a high-fidelity enzyme in the KAPA HiFi Hotstart ReadyMix PCR kit to ensure the accuracy and efficiency of the amplification. Library quality was checked using a Thermo NanoDrop 2000 UV microphotometer and 2% agarose gel electrophoresis. A Qubit 2.0 Fluorometer (Thermo Fisher Scientific, Waltham, MA, USA) was used to quantify the library. Amplicon libraries were sequenced using an Illumina Miseq PE250 platform (Wekemo Technology Co., Ltd, Shenzhen, China).
The sequencing files obtained from the Illumina sequencer were pre-processed, quality filtered (Qscore > 20) and analysed using the QIIME2 (2021.4 release) software (Bolyen et al. Citation2019). Deblur algorithm was used for sequence trimming, denoizing, chimaera removal and features binning at amplicon sequence variants (ASV) (Amir et al. Citation2017). Naive Bayes classifier was employed to assign all sequences into bacterial taxonomy using the Greengenes (v13_8 clustered at 99% identity) reference database.
Data analysis
Firstly, the data of gastrointestinal weight, length, VFAs concentration and enzyme activities were tested standard and different for normality. Then, the data analysis was performed by one-way ANOVA using IBM SPSS Statistics 22 (SPSS Inc.). The statistical formula was as follows:
where Yi is the dependent variable, μ is the overall mean, Ti is the fixed effect of the group (4 time points), and ϵi is the error term. Statistical differences between the means of groups were tested using Duncan’s multiple range test for a calculated least significant range. In addition, the differences were considered significant at the level of P < 0.05.
Alpha diversity, including the Shannon Index and the number of Observed ASVs, was tested using a two-tailed Wilcoxon signed-rank test between two groups. Beta diversity based on Weighted and Unweighted Unifrac distances was tested using an analysis of similarity (ANOSIM). The outputs of diversity were visualized using the ‘ggplot2’ package in R (v4.1.2). The linear discriminant analysis (LDA) effect size (LEfSe), an analytical tool for discovering and interpreting biomarkers of high-dimensional data, was used to identify the signature bacteria associated with the growth stages and intestinal segments. LDA score > 2 was used as a criterion for judging the significant effect.
Results
The developments of the gastrointestinal tract
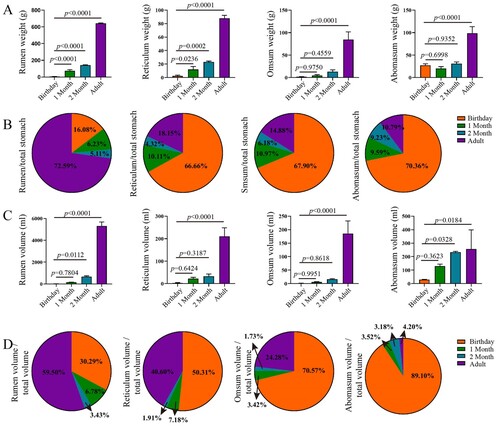
The weights of the rumen, reticulum, omasum and abomasum of Leizhou goats increased with age increasing (P < 0.05) ((A)). Notably, the weights of rumen and reticulum were significantly different at each growth stage (P < 0.05), while the weights of omasum and abomasum at adult stage were higher than those at other ages (P < 0.05). Correspondingly, the relative weight of rumen was greater at adult stage, while the relative weights of reticulum, omasum and abomasum were higher at birthday ((B)). The relative weight of rumen increased from 5.11% to 72.59% from birthday to adulthood. The volumes and relative volumes of the rumen, reticulum, omasum and abomasum changed with age had similar patterns compared to their weights ((C–D)).
Figure 1. Complex stomach weight and volume in goats from birthday to adult Differences between groups were labeled over bars.
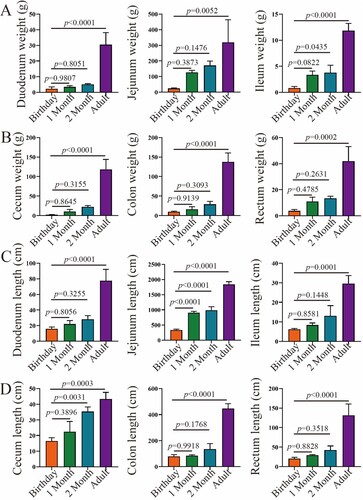
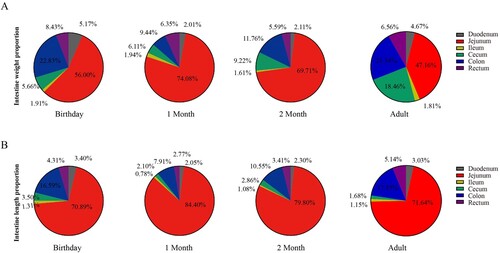
The weights of each segment of the intestine increased with age, and the duodenum, jejunum, ileum, caecum, colon and rectum at adults were significantly heavier than that at other ages (P < 0.05) ((A–B)). Similarly, the length of the small and large intestine increased with age ((C–D)). The relative weight of jejunum varied at ages (birthday 56.00%, 1 month 74.08%, 2 months 69.71% and adult 47.16%) ((A)). The relative weight of the caecum increased with age, especially in adulthood. The calculated weight of colon changed variably with age (birthday 22.83%, 1 month 9.44%, 2 months 11.76% and adult 21.34%). Interestingly, except the relative length of the caecum did not change with age, the calculated length of the jejunum and colon had a similar pattern compared to the changes in their relative weights.
Histomorphological examination
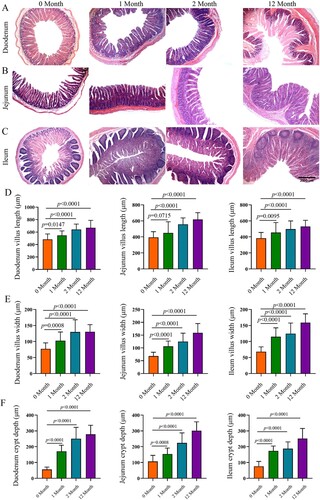
The developing rumen papillae were observed with age increasing ((A–C)). The density of rumen papilla at newborn, 1-month-old and 2 months old was 17.55, 7.84 and 4.70 times that of adult goats (P < 0.05) ((D)). The length and width of rumen papilla, muscular thickness, cuticle thickness and rumen wall thickness in Leizhou goats were significantly increased at different developmental stages (P < 0.05) ((E–I)). The length of rumen papilla in newborns, 1-month-old and 2-month-old goat kids were 9.60%, 27.82% and 36.85% of that in adult, and the width of rumen papilla was 2.70, 14.71 and 20.99 of that in adult Leizhou goats, respectively (P < 0.05).
Figure 4. Rumen morphology in goats from birthday to adult Differences between groups were labeled over bars.
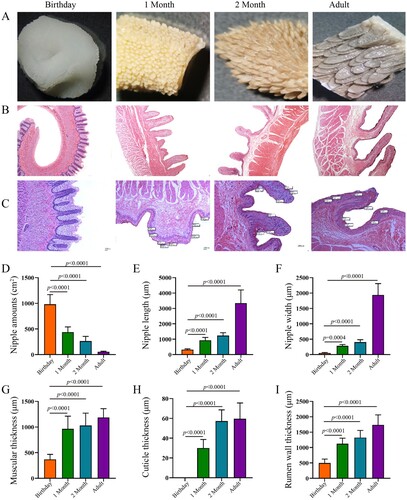
Consistently, significant development of the duodenum, jejunum and ileum with age was observed ((A–C)). With the increase of age, the villus length and width and crypt depth increased significantly (P < 0.05). Moreover, as we observed, the jejunum had more significant weight and length compared to the duodenum and ileum at the same growth stage.
Gut pH and fermentation parameters
During the trial, the pH of each part of the gastrointestinal tract changed, and there was no significant difference in the pH values of the rumen, abomasum, duodenum, jejunum, ileum and caecum between 2 months of age and adult (P > 0.05) (Figure S1). With the development of rumen, the pH of rumen contents in 2 months of age and adulthood was significantly lower than that in newborns (P < 0.05). The pH of the abomasum varied from 3.53 to 4.49, which was significantly lower in 2-month-old and adulthood than in newborn and 1-month-old (P < 0.05). The pH of the duodenum varied from 5.89 to 6.41, which was significantly higher at birth than at 1 month old (P < 0.05). There was no significant difference in the pH value of jejunum and caecum at different developmental stages (P > 0.05), while the pH of ileum increased with age increasing.
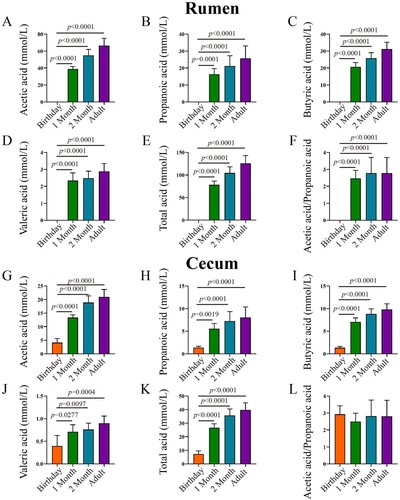
With the increase of age, the VFAs concentration in both rumen and caecum increased (). Specifically, acetic acid, propionic acid, butyric acid and valeric acid were not detected at birthday rumen, while they showed in caecum at the same age. The concentration of acetic acid in 2 months of age and adult caecum was 4.55 and 5.05 times higher than that in newborns, respectively. There was no significant difference in caecal butyric acid between 1-month and 2 months of age, no significant difference between 2 months of age and adult (P > 0.05), and 1-month-old was significantly lower than adult (P < 0.05). Regarding acetic acid / propionic acid, there were no significant changes in both rumen and caecum at different developmental stages (P > 0.05).
The digestive enzyme in rumen and caecum
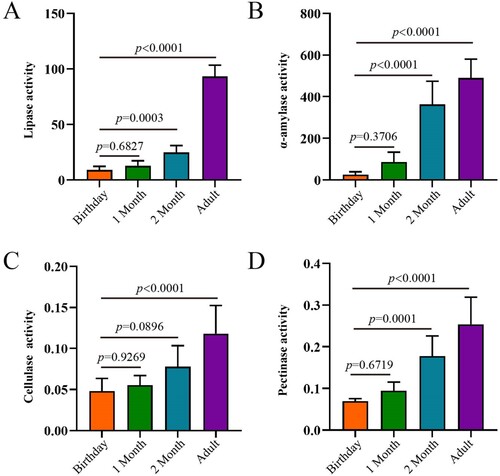
In the rumen, the activity of digestive enzymes, including lipase, α-amylase, cellulase and pectinase, increased with age (). Although these four enzyme activities were not different between birthday and 1 month of age, they started to be significantly higher from 2 months of age. Notably, the activity of α-amylase at 2 months of age and adult was significantly higher than that at birthday and 1 month of age, and lipase activity was hugely greater at adults compared to other ages.
Figure 7. The activities of digestive enzymes of the rumen in goats from birthday to adult Differences between groups were labeled over bars.
With the increase of age, as we observed, the digestive enzyme activity in the small intestine (duodenum, jejunum and ileum) increased gradually (). The activity of α-amylase in adulthood was significantly higher than birthday in duodenum, while α-amylase activity in jejunum and ileum were greater in 2 months of age and adulthood compared to birthday and 1 month of age. The trypsase reached peak at 2 months of age in the duodenum, while significantly increased and kept flat since 1 month of age. Lipase increased significantly with age in the duodenum, jejunum and ileum. Moreover, α-amylase activity was greater in ileum > jejunum and duodenum at adult stage, trypsase activity was higher in jejunum > ileum, and lipase activity was higher in jejunum > ileum > duodenum.
Microbial community in the gastrointestinal tract
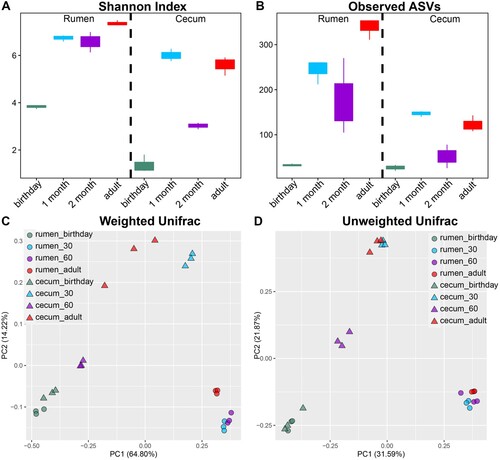
Significant increases in microbial diversity, including the Shannon Index and the number of Observed ASVs ((A–B)), were observed in both the rumen and caecum. In the rumen, both bacterial diversity and richness on birthday were lower compared to 1, 2 months of age and adulthood (p < 0.05), and alpha diversities at adulthood were greater than 1 and 2 months of age. Consistently, the same pattern of alpha diversity was observed in the caecal community, except for the caecal microbiome at 2 months of age was lower than that of 1 month of age and adulthood. Additionally, both diversity and richness in the caecum were lower compared to that in the rumen. Regarding beta diversity, significant shifts in community membership and structure of both rumen and caecum from birthday to adulthood were observed on the principal coordinate analysis (PCoA)-based Weighted ((C)) and Unweighted Unifrac ((D)) distances. Moreover, distinct microbial beta diversity between the rumen and caecum at the same age was found.
Figure 9. Alpha and beta diversities of rumen and caecum content in goats from birthday to adult Alpha-beta-genus-lefse. A,B. Alpha diversity (Shannon index and the number of observed ASVs) of the rumen and caecal microbiota. C,D. Beta diversity of the rumen and caecal microbiota based on Weighted and Unweighted Unifrac distance. One point represents one sample.
At the phylum level, Bacteroidetes, Proteobacteria and Firmicutes represented the top three highest abundances across all samples (Figure S2). In the rumen, Proteobacteria accounting for 62.45% was the predominant bacterial phylum at birthday, while the dominant bacteria from 1 month of age to adulthood were Bacteroidetes (80.45%). Firmicutes in the rumen decreased with age increasing. In the caecum, Proteobacteria was also dominant at birthday (80.20%), followed by 2 months of age (59.18%), adult (12.71%) and 1 month of age (8.02%). The dominant bacteria from 1 month of age to adulthood were Bacteroidetes phylum. Firmicutes and Verrucomicrobia in the caecum increased with age increasing. Moreover, differences in the major phyla between the rumen and caecum were also observed.
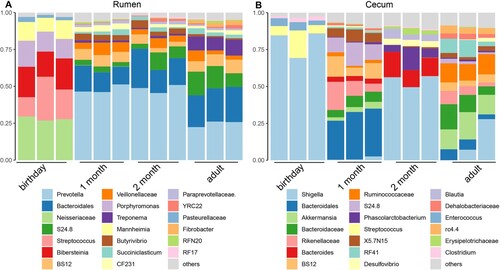
At the genus level, consistent genus changes with phyla were observed (). In the rumen, the major genera at birthday were Neisseriaceae unclassified (28.09%), Streptococcus (20.97%), Bibersteinia (19.57%), Porphyromonas (14.78%) and Mannheimia (11.60%), while Prevotella and Bacteroidales unclassified were dominant from 1 month of age to adult. In the caecum, Shigella and Streptococcus were over-presented at birthday, Bacteroidales unclassified and Rikenellaceae unclassified were top genera at 1 month of age, Shigella, Bacteroides and Phascolarctobacterium were enriched at 2 months of age, and Shigella, Akkermansia, Bacteroidaceae unclassified and Ruminococcaceae unclassified were major genera for adult goat caecum.
Figure 10. The microbial composition at the genus level for rumen and caecum samples The top 20 genera of the rumen (A) and caecum (B) in goats from birthday to adult are shown. Each column represents a sample, and each bar represents one bacterium.
To deeply understand the microbial evolution with age, growth stage-associated microbiota in the rumen and caecum were identified using LEfSe (Figure S3). Neisseriaceae unclassified, Streptococcus, Bibersteinia, Porphyromonas, Mannheimia, Pasteurellaceae unclassified and Moraxella were enriched in birthday rumen. Veillonellaceae unclassified, Butyrivibrio, Selenomonas, Pseudobutyrivibrio and Anaeroplasma had greater abundances at 1 month of the rumen. Prevotella, Desulfovibrionaceae unclassified and Ruminococcus were increased in the rumen at 2 months of age. S24_8, Treponema, Succiniclasticum and Fibrobacter were classified as rumen signatures at adult stage. Caecum at birthday was enriched with Shigella, Enterococcus, Clostidium, Butyricicoccus, Staphylococcus and Lactococcus. At 1 month of age, the caecal community had higher abundances of Bacteroidales unclassified, Rikenellaceae unclassified, Paraprevotellaceae unclassified and Christensenellaceae unclassified. In adult caecum, the microbiota, include Akkermansia, Bacteroidaceae unclassified, Ruminococcaceae unclassified, Clostridiales unclassified and Coprococcus.
Discussion
Although several studies investigated gastrointestinal developments (Gahlot and Kumar Citation2018; Koch et al. Citation2019; Abdelsattar et al. Citation2022), the knowledge of anatomical, morphological and physiological development of the gastrointestinal tract in Leizhou goats from birthday to adulthood have still lacked. This study measured both the rumen and intestinal development, the digestive enzyme activity, and the temporal dynamics of the gut microbiome. We found that the weight and morphology matured with age increasing. Moreover, the activity of digestive enzymes confirmed that the physiological functions of each fragment of the gastrointestinal tract reached maturity at a specific age. Additionally, the temporal dynamics of the rumen and the caecal microbiome from birthday to adulthood were investigated, and the early colonization, transition and maturity of microbial community were identified. Our results provided insights for growth development and feeding strategy of Leizhou goats.
Rumen develops rapidly after birth. In this study, rumen development in goats continued to increase significantly until adulthood. Previous study found that the rumen anatomic development was achieved after 2 months since they only measured from birthday to 70 days of age (Jiao et al. Citation2015). Although rumen reaches rumination phase, it continues to develop until adulthood. The weight and volume of the reticulum and abomasum showed a significant upward trend, but the increase was smaller than that of the rumen, and the relative weight change was related to the development of the rumen. Abomasum, the fourth and final stomach compartment in ruminants, is the stomach that produces hydrochloric acid and digestive enzymes such as pepsin (breaks down proteins) and receives digestive enzymes secreted from the pancreas such as pancreatic lipase (breaks down fats) to prepare proteins for absorption in the intestines. We found the abomasum had the highest relative weight at birth, which is because rumen did not develop at this time. Moreover, considering the small intestine is the primary site to absorb nutrition, the jejunum and ileum developed fast within 2 months after birth. Understandably, small intestine develops to obtain more nutrition for host growth. However, the speed increase of small intestine is still lower than rumen. Therefore, there is a priority for developing the rumen and its digestive function, which could affect the substantial growth of small ruminants (Paez Lama et al. Citation2014).
The digestion of nutrition in the rumen and small intestine is strongly associated with mucosal structure, especially the shape and size of rumen papillae and small intestinal villus and crypt depth (Thomsen et al. Citation2006). In the current study, the rumen papillae and the small intestinal villus increased significantly over age. Similar results of rumen papillae were observed in goats (Jiao et al. Citation2015; Abdelsattar et al. Citation2022) and cattle (Suarez et al. Citation2006; Malmuthuge et al. Citation2019). Although few studies have been conducted about intestine development in ruminants, our results of villus in the small intestine are consistent with a previous study using young goats (Abdelsattar et al. Citation2022). The papillae development of rumen is positively correlated with rumen-fermented VFAs (Zou et al. Citation2020). Hence, increases in VFA with age may promote rumen development. During the weaning transition stage, the main site of digestion and absorption is changed from the intestine to the rumen (Dias et al. Citation2018). The growth and development of the rumen and small intestine are influenced by multiple factors, such as age, strophic hormones, the chemical composition of diet, etc. (Chai et al. Citation2021). Accordingly, the increased metabolic energy intake and dietary forge increased ruminal and intestinal growth through cellular hyperplasia in lambs (Mcleod and Baldwin Citation2000). The villus height and crypt depth of the duodenum, jejunum and ileum were improved in calves fed alfalfa and starter diet (Cui et al. Citation2020). Therefore, age, accompanied by other factors, could regulate rumen and intestine epithelial proliferation (Baldwin et al. Citation2004).
The ability to obtain the essential nutrients from the feed benefits to grow or maintain health. Cellulase is one of the most important fibrolytic enzymes in ruminants (Arriola et al. Citation2017). This study found that cellulase activity increased with age, which indicated that the rumen fibre decomposition ability increased over age. The increases in activities of the other three enzymes stated the improvement of rumen functions. A previous study also found that rumen fibrolytic enzymes and amylase activity increased from birth to 83 days of age in calves (Rey et al. Citation2012). The small intestine plays a critical role in nutrient utilization (Liou Citation2013). With age increasing, the activities of α-amylase, trypsase and lipase increased, indicating that the function and physiology of the small intestine improve with age.
In ruminants, VFAs, fermentation products of the diets, play essential roles in rumen development and nutrient sources for the host, and rumen VFAs produce 70–80% of the energy requirements for the rumen epithelia (Lv et al. Citation2019). This study found that the rumen of newborn goat kids had no fermentation function, and the caecum fermentation parameters for acetate, propionate and butyrate were between zero and 5 mmol L−1, indicating that the caecum had certain functions at birth and that the caecum developed earlier than the rumen. The fermentation parameters of rumen were higher than those of caecum after 1-month-old, which means that rumen started to develop and had some functions in nutrient digestion. Increased rumen VFAs concentration from birth to 8 days of life was reported (Abecia et al. Citation2014). Changes in VFAs in gastrointestinal tracts not only are associated with gut development but also may be correlated with their microbial communities.
The rumen and caecum are two vital fermentation organs in ruminants. Investigation of their microbiome could allow us to better understand gut health, digestion and metabolism (Dias et al. Citation2018; Zhuang et al. Citation2020; Abdelsattar et al. Citation2022). We studied the temporal dynamic changes of the microbial community in the rumen and caecum from birth to adulthood and found that the diversity and structure of the microbial communities were distinct between ages and gut geographic sites after birth, although they were similar at birthday. The low alpha diversity of the microbiome in birthday goat kids represented the influx of microbes after receiving colostrum, which is consistent with previous studies (Stewart et al. Citation2018; Zou et al. Citation2020). Interestingly, rumen and caecum microbiome at birthday clustered closely. This indicated that the bacterial communities of the gastrointestinal tract at birthday displayed a high degree of similarity. After born, the factors, including age, gut development and diet consumption, cause the different microbiome in rumen and caecum (Chai et al. Citation2021). Moreover, the microbial changes affected by age were characterized. At birthday rumen, the genera, including Neisseriaceae unclassified, Bibersteinia and Mannheimia belonging to Proteobacteria, were the major bacteria. However, they were not detected or less in later growth stage. Many members in Mannheimia and Bibersteinia are pathogens. Mannheimia haemolytica and Bibersteinia trehalosi are the main causes of pneumonia in sheep and goats of all ages and can induce septicaemia in lambs or kids (Brogden et al. Citation1998; Rawat et al. Citation2019). This is possible because goat kids born in a natural environment might easily infect pathogenic microorganisms. The dominant genus Shigella in birthday caecum may also reflect the assumption. With age increasing, the major bacterium in the rumen was Prevotella which had lots of functions in nutrition (carbohydrates and proteins) degradation, which agreed with other reports (Dias et al. Citation2018; Chai et al. Citation2021; Hao et al. Citation2021). Additionally, different microbial composition between rumen and caecum was observed at different growth stage, which is due to the micro-environmental niche (Donaldson et al. Citation2015; Yeoman et al. Citation2018). Although changes in caecal microbiota with age were observed, it is surprising that caecal microbiome at 2 months of age was biased since its community was different compared to 1 month of age and adult. This might be due to the small sample size of microbiome analysis in our study. Future studies need to characterize more replicates and time points.
Conclusions
In this study, we characterized the development of gastrointestinal development in goats from birthday to adulthood. With age increasing, the weight of the gastrointestinal tracts, digestive enzyme activities of the rumen and small intestine, content VFAs and microbiome of the rumen and caecum matured. The physiological characteristics of the gastrointestinal tract in Leizhou goats at 2 months were significantly different from those at 1 month and were close to adult Leizhou goats. Through classification of microbiome changed with age in rumen and caecum, primary colonization and evolution of microbiota from birthday to adulthood were confirmed. The major bacteria of the newborn goat kids were rapidly absent, which might represent the next phase and adult of the microbiome of the gastrointestinal tract. This study provides insights for feeding and health-maintaining strategy.
3_supplemental_FINAL
Download MS Word (1.5 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Abdelsattar MM, Zhuang Y, Cui K, Bi Y, Haridy M, Zhang N. 2022. Longitudinal investigations of anatomical and morphological development of the gastrointestinal tract in goats from colostrum to postweaning. J Dairy Sci. 105:2597–2611. doi:10.3168/jds.2021-21056.
- Abecia L, Ramos-Morales E, Martínez-Fernandez G, Arco A, Martín-García AI, Newbold CJ, Yáñez-Ruiz DR. 2014. Feeding management in early life influences microbial colonisation and fermentation in the rumen of newborn goat kids. Anim Prod Sci. 54:1449–1454. doi:10.1071/AN14337.
- Amir A, Mcdonald D, Navas-Molina JA, Kopylova E, Morton JT, Zech Xu Z, Kightley EP, Thompson LR, Hyde ER, Gonzalez A, Knight R. 2017. Deblur rapidly resolves single-nucleotide community sequence patterns. mSystems. 2:e00191–e00116.
- Arriola KG, Oliveira AS, Ma ZX, Lean IJ, Giurcanu MC, Adesogan AT. 2017. A meta-analysis on the effect of dietary application of exogenous fibrolytic enzymes on the performance of dairy cows. J Dairy Sci. 100:4513–4527. doi:10.3168/jds.2016-12103.
- Baldwin RL, Mcleod KR, Klotz JL, Heitmann RN. 2004. Rumen development, intestinal growth and hepatic metabolism in the pre- and postweaning ruminant. J Dairy Sci. 87:E55–E65. doi:10.3168/jds.S0022-0302(04)70061-2.
- Bi Y, Cox MS, Zhang F, Suen G, Zhang N, Tu Y, Diao Q. 2019. Feeding modes shape the acquisition and structure of the initial gut microbiota in newborn lambs. Environ Microbiol. 21:2333–2346. doi:10.1111/1462-2920.14614.
- Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, Alexander H, Alm EJ, Arumugam M, Asnicar F, et al. 2019. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol. 37:852–857. doi:10.1038/s41587-019-0209-9.
- Brogden KA, Lehmkuhl HD, Cutlip RC. 1998. Pasteurella haemolytica complicated respiratory infections in sheep and goats. Vet Res. 29:233–254.
- Chai J, Diao Q, Zhao J, Wang H, Deng K, Qi M, Nie M, Zhang N. 2018. Effects of rearing system on meat quality, fatty acid and amino acid profiles of Hu lambs. Anim Sci J. 89:1178–1186. doi:10.1111/asj.13013.
- Chai J, Lv X, Diao Q, Usdrowski H, Zhuang Y, Huang W, Cui K, Zhang N. 2021. Solid diet manipulates rumen epithelial microbiota and its interactions with host transcriptomic in young ruminants. Environ Microbiol. 23:6557–6568. doi:10.1111/1462-2920.15757.
- Cui Z, Wu S, Li J, Yang QE, Chai S, Wang L, Wang X, Zhang X, Liu S, Yao J. 2020. Effect of alfalfa hay and starter feeding intervention on gastrointestinal microbial community, growth and immune performance of yak calves. Front Microbiol. 11:994. doi:10.3389/fmicb.2020.00994.
- Dias J, Marcondes MI, Motta De Souza S, Cardoso Da Mata ESB, Fontes Noronha M, Tassinari Resende R, Machado FS, Cuquetto Mantovani H, Dill-Mcfarland KA, Suen G. 2018. Bacterial community dynamics across the gastrointestinal tracts of dairy calves during preweaning development. Appl Environ Microbiol. 84. doi:10.1128/AEM.02675-17.
- Donaldson GP, Lee SM, Mazmanian SK. 2015. Gut biogeography of the bacterial microbiota. Nat Rev Microbiol. 14:20–32. doi:10.1038/nrmicro3552.
- Gahlot PK, Kumar P. 2018. Histological, histochemical and ultra structural studies of ileum of goat (Capra hircus). J of Anim Res. 8:187–193.
- Hao Y, Guo C, Gong Y, Sun X, Wang W, Wang Y, Yang H, Cao Z, Li S. 2021. Rumen fermentation, digestive enzyme activity, and bacteria composition between pre-weaning and post-weaning dairy calves. Animals (Basel). 11:2527. doi:10.3390/ani11092527.
- Jiao J, Li X, Beauchemin KA, Tan Z, Tang S, Zhou C. 2015. Rumen development process in goats as affected by supplemental feeding v. grazing: age-related anatomic development, functional achievement and microbial colonisation. Br J Nutr. 113:888–900. doi:10.1017/S0007114514004413.
- Jiao J, Wu J, Zhou C, Tang S, Wang M, Tan Z. 2016. Composition of ileal bacterial community in grazing goats varies across non-rumination, transition and rumination stages of life. Front Microbiol. 7:1364.
- Koch C, Gerbert C, Frieten D, Dusel G, Eder K, Zitnan R, Hammon HM. 2019. Effects of ad libitum milk replacer feeding and butyrate supplementation on the epithelial growth and development of the gastrointestinal tract in Holstein calves. J Dairy Sci. 102:8513–8526. doi:10.3168/jds.2019-16328.
- Li B, Zhang K, Li C, Wang X, Chen Y, Yang Y. 2019. Characterization and comparison of microbiota in the gastrointestinal tracts of the goat (Capra hircus) during preweaning development. Front Microbiol. 10:2125. doi:10.3389/fmicb.2019.02125.
- Li S, Khafipour E, Krause DO, Kroeker A, Rodriguez-Lecompte JC, Gozho GN, Plaizier JC. 2012. Effects of subacute ruminal acidosis challenges on fermentation and endotoxins in the rumen and hindgut of dairy cows. J Dairy Sci. 95:294–303. doi:10.3168/jds.2011-4447.
- Liou AP. 2013. Digestive physiology of the pig symposium: G protein-coupled receptors in nutrient chemosensation and gastrointestinal hormone secretion. J Anim Sci. 91:1946–1956. doi:10.2527/jas.2012-5910.
- Lv X, Chai J, Diao Q, Huang W, Zhuang Y, Zhang N. 2019. The signature microbiota drive rumen function shifts in goat kids introduced to solid diet regimes. Microorganisms. 7:516. doi:10.3390/microorganisms7110516.
- Makkar HP, Sharma OP, Dawra RK, Negi SS. 1982. Simple determination of microbial protein in rumen liquor. J Dairy Sci. 65:2170–2173. doi:10.3168/jds.S0022-0302(82)82477-6.
- Malmuthuge N, Liang G, Guan LL. 2019. Regulation of rumen development in neonatal ruminants through microbial metagenomes and host transcriptomes. Genome Biol. 20:172. doi:10.1186/s13059-019-1786-0.
- Mcleod KR, Baldwin RLT. 2000. Effects of diet forage:concentrate ratio and metabolizable energy intake on visceral organ growth and in vitro oxidative capacity of gut tissues in sheep. J Anim Sci. 78:760–770. doi:10.2527/2000.783760x.
- Paez Lama S, Grilli D, Egea V, Fucili M, Allegretti L, Guevara JC. 2014. Rumen development and blood metabolites of Criollo kids under two different rearing systems. Livest Sci. 167:171–177. doi:10.1016/j.livsci.2014.06.018.
- Rawat N, Gilhare VR, Kushwaha KK, Hattimare DD, Khan FF, Shende RK, Jolhe DK. 2019. Isolation and molecular characterization of Mannheimia haemolytica and Pasteurella multocida associated with pneumonia of goats in Chhattisgarh. Vet World. 12:331–336. doi:10.14202/vetworld.2019.331-336.
- Rey M, Enjalbert F, Monteils V. 2012. Establishment of ruminal enzyme activities and fermentation capacity in dairy calves from birth through weaning. J Dairy Sci. 95:1500–1512. doi:10.3168/jds.2011-4902.
- Stewart CJ, Ajami NJ, O’brien JL, Hutchinson DS, Smith DP, Wong MC, Ross MC, Lloyd RE, Doddapaneni H, Metcalf GA, et al. 2018. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature. 562:583–588. doi:10.1038/s41586-018-0617-x.
- Suarez BJ, Van Reenen CG, Gerrits WJ, Stockhofe N, Van Vuuren AM, Dijkstra J. 2006. Effects of supplementing concentrates differing in carbohydrate composition in veal calf diets: II. Rumen development. J Dairy Sci. 89:4376–4386. doi:10.3168/jds.S0022-0302(06)72484-5.
- Thomsen LE, Knudsen KE, Hedemann MS, Roepstorff A. 2006. The effect of dietary carbohydrates and Trichuris suis infection on pig large intestine tissue structure, epithelial cell proliferation and mucin characteristics. Vet Parasitol. 142:112–122. doi:10.1016/j.vetpar.2006.05.032.
- Wang J, Fan H, Han Y, Zhao J, Zhou Z. 2017. Characterization of the microbial communities along the gastrointestinal tract of sheep by 454 pyrosequencing analysis. Asian-Australas J Anim Sci. 30:100–110. doi:10.5713/ajas.16.0166.
- Wang K, Liu X, Qi T, Hui Y, Yan H, Qu L, Lan X, Pan C. 2021. Whole-genome sequencing to identify candidate genes for litter size and to uncover the variant function in goats (Capra hircus). Genomics. 113:142–150. doi:10.1016/j.ygeno.2020.11.024.
- Xie B, Huang W, Zhang C, Diao Q, Cui K, Chai J, Wang S, Lv X, Zhang N. 2020. Influences of starter NDF level on growth performance and rumen development in lambs fed isocaloric and isonitrogenous diets. J Anim Sci. 98:skaa093. doi:10.1093/jas/skaa093.
- Yang B, Le J, Wu P, Liu J, Guan LL, Wang J. 2018. Alfalfa intervention alters rumen microbial community development in Hu lambs during early life. Front Microbiol. 9:574. doi:10.3389/fmicb.2018.00574.
- Yeoman CJ, Ishaq SL, Bichi E, Olivo SK, Lowe J, Aldridge BM. 2018. Biogeographical differences in the influence of maternal microbial sources on the early successional development of the bovine neonatal gastrointestinal tract. Sci Rep. 8:3197. doi:10.1038/s41598-018-21440-8.
- Zhuang Y, Chai J, Cui K, Bi Y, Diao Q, Huang W, Usdrowski H, Zhang N. 2020. Longitudinal investigation of the gut microbiota in goat kids from birth to postweaning. Microorganisms. 8:1111. doi:10.3390/microorganisms8081111.
- Zou X, Liu G, Meng F, Hong L, Li Y, Lian Z, Yang Z, Luo C, Liu D. 2020. Exploring the rumen and cecum microbial community from fetus to adulthood in goat. Animals (Basel). 10:1639. doi:10.3390/ani10091639.