?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Fishes are hosts to a range of taxonomically diverse parasites. This survey was intended to provide insight into the occurrence, distribution, prevalence, and intensity of parasites infesting the fingerlings of the tilapia, in Tigray. A total of 384 Oreochromis niloticus specimens were collected from Midmar consisting of 227 males and 157 females. External and internal organs were examined for parasite infection. Squash preparation of fish eyes was also examined to reveal the organisms through microscopic examination. The major groups of parasites were trematode, nematodes, protozoa and hirudinea. A total of 3762 genus Diplostomum were recorded affecting the health of 93% of fishes. Contracaecum species was the second most prevalent distributed majorly in the intestine (77.8%), pericardial cavity (19.1%), swim bladder (2.4%), gill (0.2%) and mouth (0.8%). The prevalence and mean intensity of Diplostomum spp., Contracaecum, Myxosporea, Clinostomum spp., Eustrongylides and Leeches was 93%(10.5), 62.2%(5.3), 39.3%(8.3), 2.1%(8.8),1.8%(5.7) and 1.6% (2.5), respectively. The mean temperature, dissolved oxygen, conductivity, and pH of the dam were 24.4°C, 7.55 mg/l, 849.5µs/cm, and 7.5, respectively. Therefore, responsible bodies in fisheries should perform a pre-stock assessment and provide fish farming extension services for optimum production and marketing of fish products with management and production enhancement methods.
1. Introduction
Fish infections with parasites cause production and economic losses through direct fish mortality; reduction in fish growth; replica and energy loss; the fish's increased susceptibility to disease and predators, as well as the expensive treatment (Cowx Citation1992; Shinn et al. Citation2015). Facts regarding the modes of transmission and potential intermediate hosts are usually crucial to selecting the foremost acceptable management action to reduce or eliminate the matter (Aken’ova Citation2000).
Ethiopia has massive water resources, with an approximated calculable surface area of 733k km2 of major lakes and reservoirs, 275 km2 of undersized small water bodies, and 7285 km long rivers inside the country (FAO Citation2005; Janko Citation2014; Ayalew Citation2018). As a result of these ecological variations, the Federal Democratic Republic of Ethiopia has been the house of extremely wide-ranging flora and fauna. Over 200 species of fish are best known to occur in lakes, rivers, and reservoirs in the Federal Democratic Republic of Ethiopia (Jerbe Citation2007). The country depends on its interior water bodies for the fish offered to its population.
Mostly, Nile tilapia (Oreochromis niloticus) has been introduced throughout the country because of its adaptational capabilities and its quality to match Ethiopian consumers’ preferences. It contributed roughly 40.9% of the 13,253 tons of commercial fish taken in 2007/2008 as a result of its natural incidence and introduction into entirely different water bodies (MoARD Citation2008; FAO Citation2014).
One of the issues of fishery management within the wild populations is parasites and disease conditions of fish. Parasitic diseases cut back fish production by poignant the traditional physiology and if left uncontrolled, it may result in mass mortalities or some cases, will serve as a supply of infection for humans and alternative vertebrates that consume fish (Ayotunde et al. Citation2007). The presence of an enormous variety of parasites in every fish may represent a true threat to the fish population and needs immediate action (Komar and Wendover Citation2007; Areda et al. Citation2019).
Parasites are the most common cause of infectious diseases in fish. Most of the time problems occur when infected fish are brought into the laboratory or an intensive culture like smaller dams and earthen ponds situations. Not only are the fish outstandingly stressed but they are conjointly sometimes crowded and therefore the reproducing parasites do not seem to be distributed as they are within the wild. This means, that the closer the proximity of fish to one another the greater the probability of infection and mortality. Moreover, parasitic diseases of fish are classified into protozoan, crustacean, and helminthic diseases. Generally, most crustaceans are external parasites causing severe diseases while protozoans cause either external or internal diseases according to their habitats. The majority of monogeneans and annelids are external parasitic diseases, while the majority of digeneans cause internal parasitic diseases. Nematode, spiny-headed worm (acanthocephalan) and cestode infestations are generally internal parasitic diseases. Nevertheless, many parasites with larval stages in freshwater fish have a piscivorous mammalian carnivore as their normal final host and can infect humans because of the low host specificity of the adult stage (Aly Citation2013).
Metacercariae of the digenean fluke Diplostomum are the common parasites of wild and cultivated fish in temperate and tropical climates (Chappell et al. Citation1994; Hoogendoorn Citation2020). Fish heavily infected with these digenetic trematodes might experience loss of vision, reduced growth, thinness (Niewiadomska Citation1996; Islam et al. Citation2023) or deformation of the spinal column(backbone), brain tumour, cellular mortification and death (Machado et al. Citation2005). It has been shown that these parasites can cut back fish crypsis and escape response and therefore increase fish vulnerability to bird predation (Seppälä et al. Citation2005, Citation2012).
Parasites are important components of host biology, population structure, and indeed ecosystem functioning. They can be found in any fish species and within any type of aquatic and culture system. They range from protozoans such as flagellates, ciliates, and apicomplexans to metazoans including myxozoans, trematodes, cestodes, acanthocephalans, nematodes, and crustaceans (Marcogliese Citation2004). In Ethiopia, different genera of fish parasites have been reported from different water bodies. Fish parasites including genera of Trematodes (Clinostomum spp., Diplostomum spp., Neascus, etc.); nematode (Contracaecum spp., Eustrongylides, Camallanus, etc.), cestodes (Ligula intestinalis, Proteocephalus), Monogenea (Cichlidogyrus spp.), Acanthocephala spp., Argulus spp. and Trichodina spp. were identified and reported in different studies (Florio et al. Citation2009; Gulelat et al. Citation2013; Amare et al. Citation2014; Jossy and Daniel, Citation2015; Mohammed et al., Citation2015).
The most vital requirement of fish production is the convenience of healthy fish fingerlings of tilapias. It is evident from the offered literature that parasitic diseases have caused important damage inside the nursery system of fishes mainly the poignant fry and fingerlings (Gopalakrishnan Citation1961; Kaminskas Citation2022). The parasitic community of fish shows sizable changes in the environmental conditions inside the fish's lives (Hossain et al. Citation2008). Fish harbour a diversity of parasites particularly, protozoa, cestodes, trematodes, and acanthocephalans (Ali Citation1990; Tessema Citation2020), and also the degree of damage by infection is influenced to an outsized extent by the type and number of parasites existing (Ambani Citation2014). Once the parasites exist in giant numbers, they can typically cause gross pathological changes and damage to the host (Heckman Citation1996; Hoffman Citation1998; Gunn and Pitt Citation2022). Several environmental conditions are a lot of contribute to illness among these water temperature is one of the important criteria associated with unwell natural events and ill health incidence. As per Mitra and Haldar's (Citation2004) 1st record of Chilodonella hexasticha in a province of India, parasites occur mainly within the colder months and in summer no infection was recorded. In addition, biological factors of the host, as well as the water quality, are responsible for a fish ectoparasitic infestation. The parasite infects adult fishes. Since the fingerlings are delicate, they are easily vulnerable to infection. The water temperature, pH scale, and dissolved oxygen are three water parameters that are related to disease infestation as they fluctuate more quickly (Banerjee and Bandyopadhyay Citation2010). Fish fingerlings become more vulnerable to infection because of their immature immune system (Anderson Citation1974; Ahmad and Kaur Citation2018), and it has been previously reported that shallow ponds and stagnant water favour the multiplication of ciliate like Trichodina become more prone to infection. A high stocking density of fingerlings is one more reason for ectoparasitic sickness eruption (Hossain et al. Citation2008). High stocking density increases the prospect of epizoan transmission from fish to fish simply. The provision of hosts for ectoparasitic infection increases with the increasing stocking density. Therefore, it was concluded that water quality has a great impact on the abundance of pathogens and their ability to survive on the host (Banerjee and Bandyopadhyay Citation2010). Furthermore, with high fish stocking densities under commercial fish production, parasite outbreaks will certainly increase (Michel Citation1989; Meyer Citation1991; Bondad-Reantaso et al. Citation2005).
Generally, fish is vital to the human population in trade and economy; it is required within the diet as a basis of protein for diverse countries, especially within the tropics and subtropics where undernourishment is a major drawback (Alune and Andrew Citation1996; Davies et al. Citation2006). Tilapia is becoming the foremost cultured fish native to Africa (Agbeko et al. Citation2014) and among various factors that affect the production of Cichlids notably Nile tilapia, one vital issue that is commonly unnoticed is parasitic infections and diseases. Parasitic infection and diseases are some of the factors obstructive high productivity in fish farming (Kayis et al. Citation2009). Diseases caused by parasites are widespread and cause loss of fish in the intensively stocked ponds and aquariums (Tokşen Citation2006; Koyuncu and Toksen Citation2010). Moreover, parasite-affected fish decrease food intake and lose weight. Growth is stunted and damage or injuries to fish organs reduce their productivity. These disease problems are more prevalent in fish production with high biomass over limited space (Okaeme et al. Citation1986; Mitiku et al. Citation2018).
In Ethiopia fish stocking and aquaculture practices are increasing from time to time as demand for fish protein is increasing, however, there is no preliminary study on the fingerlings and their original habitats whether they are free of ecto and endoparasites or not. In Tigray, the most suitable water body for seining fingerlings to stock in reservoirs and ponds is Midmar Reservoir. This is shallow and open for domestic use and human activities around its source and the eastern part of the reservoir. Almost entire stocking activities practiced by different organizations in Tigray were done from this reservoir. Tigray agricultural research centre, Tigray agriculture offices, and our university particularly our team. Up to now the results from earthen pond aquaculture were not satisfactory. The weight and length relationship and increment were small or retarded growth was observed. This might be due to health problems (the effects of ecto and endoparasites). In our visit to the dam at different years, we saw some parasites, particularly Ascaris affecting the physiological activities of fingerlings. As parasites can affect the growth and reproduction of fish it is advisable to take a preliminary study before stocking. Moreover, the stocking density and water quality should be maintained properly to avoid a parasitic infestation in the ponds and reservoirs we planned to stock.
So far, very few diseases have been described in fish in the Ethiopian water bodies. Moreover, there is no report on the disease and prevalence of parasitic infection in fish in Tigray, particularly in the Midmar reservoir. Therefore to fill this gap a preliminary study on the prevalence of parasites on fingerlings before stocking is advisable. Because stocking infected fingerlings leads to economic losses and allows other healthy species to be contaminated. Hence, this survey aimed to determine the prevalence, diversity, and infection intensity of ecto and endoparasites in Oreochromis niloticus fingerlings in Midmar reservoir.
2. Materials and method
2.1. Study area description
The study was conducted at Midmar reservoir (14o 12’23.30’’N latitude and 38o54’52.48’’E longitude) which is located at an altitude of 1986 metres above sea level. Midmar Reservoir is about 7 km far from the centre of Adwa town. It was constructed in 1997 by the Tigray Regional Government as a source of water supply for Adwa town (Eskendir Citation2015). The surface area of the reservoir is about 6km2 (NFLARRC Citation2002; Tigabu Citation2010) and it was primarily constructed for the Adwa town water supply but currently, it also serves as the water supply for Axum town ().
2.2. Study methodology
2.2.1. Sample collection
Fish samples (fingerlings) were collected using a seine net from the shallowest area from four representative sites. Sampling was done during January and April 2016. Collected fishes were transported in the icebox to Mekelle University Fishery and Aquatic Ecology Laboratory, for analysis. In the laboratory, total length (cm) and weight (g) were measured; the sex of the fish was determined by internal examination of testes and ovaries (Smyly Citation1957; Mohammed et al. Citation2015). The length (L) of the fish was taken from the tip of the snout to the posterior tip of the caudal fin and was measured to the nearest ± 0.1 cm. The weight of the fish was measured to the nearest gram using an electric (sensitive) balance (Mohammed et al. Citation2015).
The desired sample size was calculated using the formula given by Thrusfield (Citation2005). By considering a 95% confidence interval, 5% desired absolute precision, and 50% expected prevalence. Since there was no previous research done in this area, 50% expected prevalence, 95% confidence interval, and 5% precision were used to estimate the sample size. Hence, a minimum of 384 fish was considered in this study.
2.2.2. Laboratory examinations and identification of parasites
A squash preparation of fish eyes was examined to reveal the organisms through microscopic examination. Using a hand lens skin smear of the fish was examined for parasites. The smear was made by the scrapping of the skin and observed under the microscope at ×40 and ×1000 magnification (Paperna Citation1980, Citation1996; Khalil Citation1971; Bichi and Ibrahim Citation2009) for identification. A fish parasitology guide was used to aid the identification of each specimen examined and compared to the plates as described by Barker and Cone (Citation2000). The gills were dissected, removed, and examined under the microscope. Moreover, according to the methods described by Noga (Citation2010), Amare et al. (Citation2014) and Mitiku et al. (Citation2018) each fish was opened dorso-ventrally and its internal organs were fully examined for the presence of internal parasites. The entire digestive system was removed and placed in a Petri dish with physiological saline (0.9% NaCl solution), for parasite recovery and the gut was divided into sections. The muscles, gonads, liver, and heart were examined with the aid of a dissection microscope and a light microscope at 10 and 40 magnifications. Parasites were counted, their location recorded, and preserved in 70% ethanol. Identification of most parasites was made immediately following standard keys in the literature (Klinger and Francis Floyol Citation2002; Pouder et al. Citation2005; Roberts Citation2012). Taxonomic identifications were mostly limited to genus level as the fish harbours mostly larval stages of many nematodes and trematode parasites and could not be distinguished to species level morphologically (Mitiku et al. Citation2018; Hoffman Citation2019). The helminthic parasites were identified morphologically and parasitologically using standard identification keys and pictorial guides (Kabata Citation1985; Yamaguti Citation1985; Yanong Citation2002; Chandra Citation2004; Pouder et al. Citation2005). In addition, an identification key modified from Paperna (Citation1996) was used for the identification of the major taxa of adult and larval parasites of fish.
2.2.3. Measurement of physicochemical parameters
Organisms known as parasites exist at the expense of their hosts, but not to the point where they can kill them. Fisheries, the kind of habitat, fish biology, and climatic circumstances are all factors that affect the severity of parasite assaults on fish (Kennedy Citation1976). Parasitic infection is influenced by environmental conditions such as salinity, temperature, pH, and other water quality elements (Philips Citation1996). The majority of fish health issues are caused by environmental factors, such as poor water quality, overcrowding, nutritional inadequacies, or ‘stress’. Preventive care is the best treatment for any fish health issue.
The physicochemical parameters of the reservoir were measured with common multimetres available in our laboratory. Dissolved oxygen (DO), pH, conductivity, and turbidity were measured in situ from four regions where the seining was performed. Dissolved oxygen was measured using an oxygen metre (HQ 40d multimeter). pH (model No: pH-013), conductivity (model No: SX713), and turbidity (model No:8000-001) were measured with their corresponding electronic metres (Marcogliese Citation2002).
2.3. Data analysis
The data obtained from the laboratory findings were summarized and then analyzed using SPSS version 16 analyzing software. The difference in parasite intensity between sexes was tested using the t-test. The effect of the parasites on the health of their host was determined by calculating Fulton’s condition factor (K), a measure of an individual fish’s health that uses standard weight. Proposed by Fulton (Citation1904), it assumes that the standard weight of a fish is proportional to the cube of its length. K = 100(W/L3), Where W is the whole body wet weight in grams and L is the length in centimetres; factor 100 is used to bring K close to a value of one. Pearson correlation was used to find the correlation between the body conditions of fish with the number of parasites (Mohammed et al. Citation2015).
In addition, prevalence, mean intensity, and abundance concepts as suggested by Margolis et al. (Citation1982) and Mohammed et al. (Citation2015) are used in the present study and the formulas are given below:
Mean intensity is the arithmetic mean of the number of individuals of a particular parasite species per infected host in a sample.
3. Results
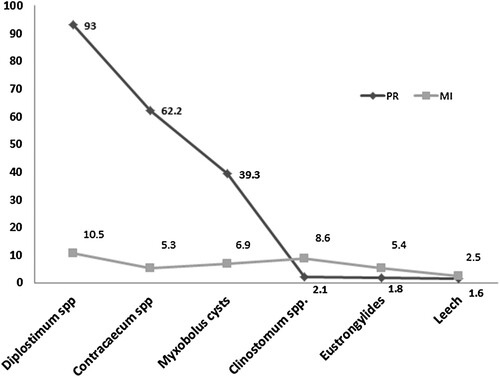
Out of 384 specimens examined, 364(94.8%) were found to be infected. The taxonomic group of the parasites was trematodes, nematodes, protozoa and hurnidea. The number (prevalence) of fishes infected by these taxonomic groups were 365(95.1%), 246(64%), 151(39.3%) and 6(1.6%), respectively. The eyes of the fishes were observed to be more infected by Diplostomum spp. parasites; the common trematodes in fish's eyes commonly known as eye fluke (; ). Contracaecum species were the second most infective parasites () and Myxosporea (Myxobolus cysts) (), Clinostomum spp., Eustrongylides, and leech were the third, fourth, and fifth, respectively.
Figure 2. Diplostomum spp. metacercariae in the vitreous humour of eyes, Contracaecum spp. in the intestine, and Myxosporea (Myxobolus cysts) beneath the scales or on the surface of the skin from fingerlings of O. niloticus.
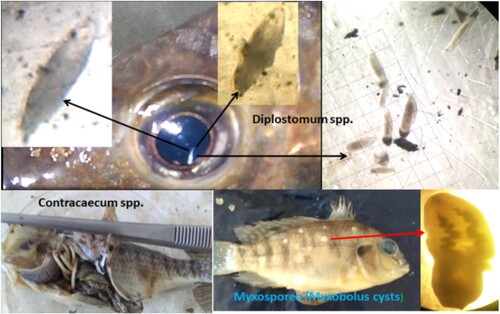
Table 1. The prevalence and mean intensity of Diplostomum spp., Contracaecum spp., Myxosporea, Clinostomum spp., Eustrongylides and Leech.
Table 2. Distribution, locations and number of ecto and intestinal parasites O. niloticus fingerlings recovered in Midmar reservoir.
Table 3. Parasite infestation in O. niloticus fingerlings and their numerical distribution in body parts.
3.1. Prevalence of ecto and endoparasites in Oreochromis niloticus fingerlings in Midmar reservoir
Throughout the study, we found a total of 151 fishes infected with Myxosporea and 6 with leeches. These are ectoparasites with a prevalence (mean intensity) of 39.3% (6.9) and 1.6% (2.5), respectively (). Diplostomum spp. and Clinostomum spp. were among the endo parasite trematodes retained in the study fingerlings. Moreover, endoparasite nematodes such as Eustrongylides, and Contracaecum spp. were recorded. Among these, Diplostomum spp., and Contracaecum spp. were the most prevalent and familiar parasites found infesting most of the Oreochromis niloticus fingerlings in Midmar reservoir. Diplostomum spp. showed the highest prevalence (93%) and mean intensity (10.5). Contracaecum spp. was also found distributed in different locations of the host body mainly inhabiting intestines ( and ).
3.2. Parasites’ diversity, distribution, infection intensity and prevalences
A total of 6197 parasites with Shannon diversity index H’ = 1.167 and species richness S = 6 were isolated from the 364 specimens of O. niloticus thus a mean of 17 parasites per fish. Of these 3853 with a mean of 16.97 were isolated from males and 2344 with a mean of 14.92 from females. The species of parasites isolated from O. niloticus and their distribution in body organs are shown in . Diplpstomum spp. and Contracaecum spp. were the most prevalent parasites. The infection intensity of the digenetic flukes Diplostomum spp. and clinostomum spp. were higher than the other parasites ( and ; ).
3.2.1. The prevalence and intensity change with fish size or age group
In this study majority of the fish were between 10.5 and 14 cm and 96.1% of them were infected with parasites (). The body weights of the fishes ranged from 4 to 83 g and the highest number of parasites recovered in fishes having sizes of 24–33 g ().
3.3. Determine and relate the most parasitized organ to fish health
Based on our observation and survey in the field and laboratory examinations, the most parasitized organ of the fingerlings were their eyes with a prevalence of 91.6% and mean intensity of 10.5. The parasites responsible for this infection are eye fluke; Diplostomum spp. which are under the taxonomic group of trematodes. Metacercariae of Diplostomum spps were found actively moving in the vitreous humour and lens of the sampled fish. This movement was also observed during microscopic examinations. Following this group, Contracaecum spp. of nematodes with a prevalence of 62.2% and mean intensity of 5.3 was found distributed among the organs intestine, pericardium, swim bladder, mouth, and gills (). The numerical distribution of this parasite in each body part is indicated in . The third most infective parasite is Myxosporea; a protozoan group that causes fish skin ulcers. It is covered with a white or yellowish-white cyst known as Myxobolus cyst beneath the scales of Oreochromis niloticus.
The six parasites were distributed among the fish in the reservoir. The total and multiple infections were calculated, hence, out of the 384 specimens examined 364 of them were infected by at least one of the parasites (), and multiple infection accounts for 285 (74%; ). The prevalence of infection by Diplostomum spp. accounts for the highest (93%) and leeches the lowest (1.6%) (). Similarly, Diplostomum spp. had the highest infection intensity followed by Myxosporea (Myxobolus cysts), Contracaecum spp., Clinostomum spp., Eustrongylides and leeches, respectively (; ).
Table 4. The prevalence of parasites in relation to host sex.
3.4. Physico-chemical condition of the dam and suitability for parasite survival and multiplication
The mean temperature of the dam during the study periods was found to be 24.4oC. It fluctuated between a low of 22.8°C and a high of 26.1°C. The dissolved oxygen ranged from 6.26 to 8.61 mg/L with a mean of 7.55 mg/L. shows the mean and standard deviation of the measured physicochemicals of the dam during January and April 2016. The measurements of the water quality parameters were found to be close to satisfactory levels and have no influence on the parasite diversity and species richness. The dam was conducive and suitable for different fish parasites. Because the majority of the specimens examined were infected with parasites ().
3.5. Relationship between fish condition factor and number of parasites
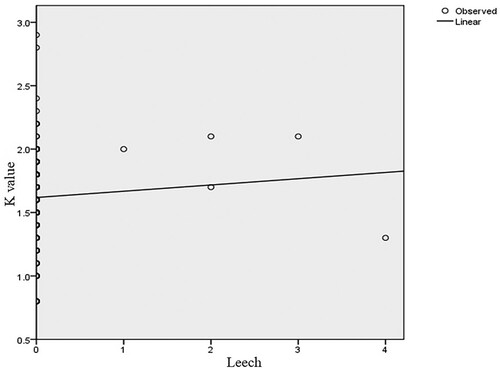
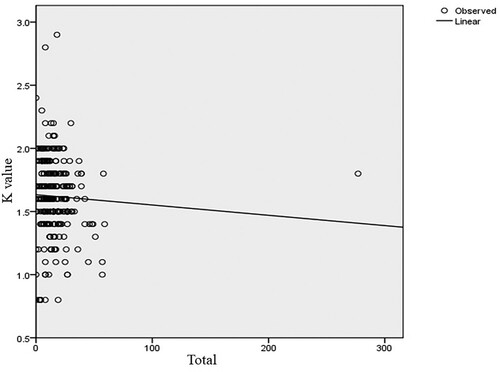
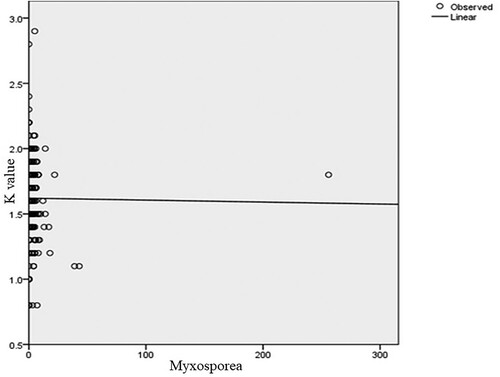
indicate the relationship between the fish condition factor and the number of parasites of four representative and one mixed genera. The health of the fingerlings in the dam is affected by the parasite burden. Most of the fish in the dam are infected by different parasites. The optimum size and weight of a healthy fingerling in the dam are roughly 12 cm and 30.5 g, respectively. However, these measurements are lower in those fingerlings affected by parasites. Overall, males were slightly in better condition (K = 1.62 ±0.27) than females (K = 1.61 ± 0.32). In general, the mean condition factor of the fish varied slightly between sexes. However, there was no significant difference in condition factors between males and females. The two groups show evidence of having similar mean values (t = 0.723, d.f. = 382, p < 0.05). In addition, there was no significant difference between males and females in parasite burden or intensity of infection (t = 1.008, d.f. = 382, p > 0.05) because their mean difference is low.
Figure 4. Relationship between condition factor and the number of Eye flukes (Diplostomum spp.) Parasites (y = −0.004x + 0.014; P = 0.031; df = 1).
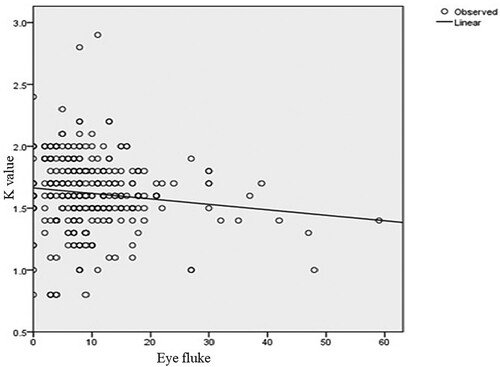
Figure 5. Relationship between condition factor and the number of Contracaecum parasites (Y = 0.003x + 0.002; P = 0.456; df = 1).
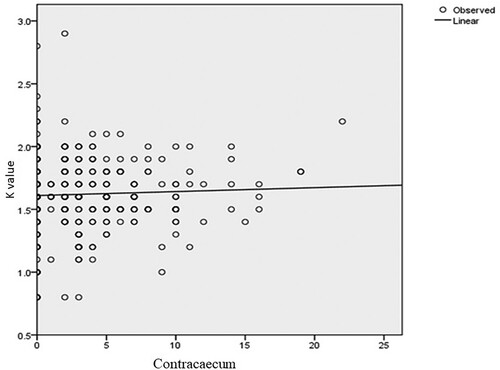
Figure 6. Relationship between condition factor and the number of Myxosporea parasites (y = 0; P = 0.894; df = 1).
4. Discussion
The study reveals six types of parasites and infested O. niloticus fingerlings with an overall prevalence of 94.8% (). Among these two of them were ectoparasites and there was a profound variability in the prevalence and intensity. One is Myxosporea (Myxobolus cysts) which is found and distributed beneath scales or on the surface of the skin and the leeches were other ectoparasites found in gills of the Nile tilapia fingerlings. In the current investigation, the Myxosporea was more prevalent and greater in the intensity of infection than the leeches (). In Africa, in agreement with this study, histozoic myxosporidians (Myxobolus spp.) occur as skin infections in fishes particularly in juvenile cichlids, with cysts formed in the dermis, under the scales, extending to the surface of the head (face, lips) or onto the fins (Paperna Citation1973; Abolarin Citation1974; Fomena et al. Citation1984/Citation1985; Landsberg Citation1986; Paperna Citation1996). Regarding the leeches, a report by Wangatia (Citation2012) from Ziwa Dam (Kenya) indicates that leeches had a prevalence rate of 3% which is twice the results ( and ).
The rest four groups of parasites were endoparasites. The two most common parasite genera encountered in the present study were Diplostomum spp. and Contracaecum spp. The infection prevalence of these parasites was 93% and 62.2%, respectively (). Eye flukes of the species Diplostomum are well known for their strong impact on freshwater fish populations. Diplostomum metacercariae are the major fish parasites with complex three-host lifecycles that involve piscivorous birds, snails, and fish as the definitive host, first intermediate host, and second intermediate host, respectively (Ndeda et al. Citation2013). In the fish, the metacercariae move to the lens, retina, and aqueous humour of fish eyes as well as the brain, spinal cord, and nasal spaces causing substantial losses. They cause diplostomosis (eye fluke disease) which is characterized by severe ocular involvement, lens opacity, and blindness (Ibrahim et al. Citation2016). In Nile tilapia, Diplostomum in beginning periods caused exophthalmia without eye murkiness and in interminable stages caused total eye waterfall and white specks around the eye cornea (Abd El-Monem et al. Citation2005). Mass infections with Diplostomum cercariae have been reported to kill young/small fish or fingerlings (Brassard et al. Citation1982) and by reduction of visual capacity of their second intermediate hosts, the parasite interferes with feeding efficiency and predator avoidance (Crowden and Broom Citation1980; Owen et al. Citation1993; Seppälä et al. Citation2004). In addition, since some fish's physiological activities like swimming, feeding, and mating depend on vision, the presence of this parasite affects its ability to perform and compete well with others. Poor feeding affects fish growth, and consequently the fish farming business. It could also expose the fish to predation increasing mortality. Diplostomum spp. metacercariae have been recovered from eyes at various prevalences. In the study O.niloticus fingerlings specimens we obtained 95.2% prevalence in males and 89% in females (). In a study by Ibrahim et al. (Citation2016) in Oyo state Nigeria, 33.18% of the cat samples had diplostomum parasites in the eye lens with higher occurrence in males (23.5%) than the females (9.7%).
The genus Contracaecum was the second most prevalent parasite affecting both sexes of the study fish species in Midmar dam. Contracaecum spp, which was the most prevalent nematode larvae, was found in the pericardial cavity of the heart coiled in a circular form with a mean of five parasites having different sizes. This result is in agreement with Yimer (Citation2000) from Lake Ziway (Ethiopia) and Wangatia (Citation2012) from Ziwa Dam (Kenya). These coiled parasites were observed in fish specimens both alive and cooled ones. Parasites that were observed from live specimens were moving actively in a wriggling manner (Wangatia Citation2012). There are about nine species of Contracaecum identified from African birds (Canaries and Gardner Citation1967), with at least seven species present in South Africa (Mashego Citation1989). It has not been possible yet to differentiate the species from larvae in fish (Mashego Citation1982).
In the present investigation, the explanation behind the high predominance of trematodes and nematodes pervasion may be because of the low host particularity of the grown-up phases of these parasites that makes these parasites conceivably tainting distinctive fish genera and species. It may likewise be a direct result of the accessibility of the various hosts required for the culmination of the existence cycle of these parasites. These hosts incorporate the piscivorous, savage, and man as definite hosts and gastropod molluscs and fishes as transitional hosts (Yanong Citation2002). The fish may go about as a moderate host in certain parasites while in others it is the last host.
The helminths larval parasites belonging to the genera Clinostomum and Contraecum species are known to occur in most African freshwater fishes (Tedla and Tadesse Citation1979; Paperna Citation1980). Adult stages are found in birds and most of the freshwater lakes in Ethiopia support a large population of aquatic birds (Tedla and Tadesse Citation1979). It is therefore likely that the major aquatic birds in Tigray particularly in Midmar reservoir support the adult stages of these parasites.
Fish samples with weights ranging between 24 and 33 g recorded the highest percentage of infection (36.3%) while fish with weights ranging from 74 to 83 g recorded a very low level of infection (0.5%). The total number of parasites increased with fish length, with the highest number of parasites observed between the sizes of 10.5–14.0 cm. Fish samples within this range showed about 96.1% of infection which is the highest in terms of the total fish examined. Although the number of fish with lengths ranging from 14.5 to 18 cm was less than the other ranges, 100% of them were infected with parasites ( and ). This is in view with Goselle et al. (Citation2008) who reported a low level of infection in larger sizes of fish in the Lamingo reservoir, Jos, Nigeria. Another study in Nigeria by Uneke Bilikis Iyabo (Citation2015) showed an almost similar result to this study.
Table 5. Prevalence of ecto and intestinal parasite in O. niloticus fingerlings collected in relation to their total length in Midmar reservoir.
Table 6. Prevalence of ecto and endoparasite in O. niloticus fingerlings collected by their body weight in Midmar reservoir.
Knowing the physicochemical characteristics of the dam is important to associate with the intensity and prevalence of the parasite. For example, at temperatures above 22°C tilapias rarely show disease signs. However, tilapias are prone to diseases due to stress from low temperature, handling, overcrowding, or poor water quality. At temperatures between 16 and 18°C and in the absence of severe stress, Nile tilapia (O. niloticus) rarely becomes diseased (Cheng Citation1986). The mean dissolved oxygen was 7.55 mg/l, fluctuating between a low of 6.26 mg/l and a high of 8.61 mg/l. Mean Conductivity was 849.5 µs/cm ranging between 730 μs/cm and 1020μs/cm and the mean pH of the dam was 7.5 with the highest at 7.69 and lowest at 7.23 as indicated in . The current physio-chemical parameter of the dam was suitable for parasites’ growth and fish survival. The fact that the parasite in the fish was low despite the favourable condition recorded, suggests the fish may have a high tolerance and/or resistance to parasitic infection (Bichi and Bizi Citation2002 as cited in Bichi and Ibrahim Citation2009).
Table 7. Water quality parameters of Midmar dam during January and April 2016.
The inlet stream channel had emergent semi-marshes, waterweeds (macrophytes) and sparsely grown papyrus reeds. The dam had flocks of birds (water ducks) that lived within the wetland. The birds feed on fish and invertebrates of the dam. The papyrus reeds and macrophytes observed in the stream are a possible habitation of molluscs that act as intermediate hosts for some parasites (Wangatia Citation2012). A lot of grazing activities go on around this dam and the water is used by cattle for drinking. The area around the dam and inlet stream was densely populated by people who grow different crops and irrigational activities that could contribute to eutrophication through fertilizers washed into the dam. Besides this, the surrounding rural dwellers use the water for washing clothes, swimming and drinking.
Water quality plays a vital role in unsettling the stability between the host and the pathogen (Jadhav Citation2009). Poor water quality parameters such as high organic matter, high ammonia, low dissolved oxygen and high bacterial load can create a suboptimal environment that is stressful for the fish leading to a higher incidence of parasitic outbreaks (Komar and Wendover Citation2007). According to, Gupta and Gupta (Citation2006) the preferably appropriate water quality parameters necessary to maintain good growth and healthy conditions in cultured fish such as Tilapia species are greater than 5 ppm of dissolved oxygen, 6.5–8.5 and 25–35°C ranges of pH and temperatures, respectively. The minimum and maximum values of most of the water quality parameters in Midimar dam were within the literatures range as indicated in . Hence, these suitable ranges as required since they are favourable for the survival of the fish and their parasites (DWAF Citation1996).
In Midmar dam there was no previous study to check with this result, but as explained in most literatures, helminths are largely found in freshwater dwelling fishes wherever factors like parasite species and its biology, host and its feeding habitats, physical factors, hygiene of the water body and presence of intermediate hosts contribute to their prevalence and intensity (Chandra Citation2006; Hussen et al. Citation2012).
5. Conclusion and recommendations
The results of the study indicated that the majority of the fish in the study reservoir experienced a serious parasitic infestation. Fingerlings of O. niloticus from Midmar dam were infected with parasites of Diplostomum spp, Contracaecum spp, Myxosporea, Clinostomum spp., Eustrongylides and leeches. The first three parasites showed a high prevalence of infection with Contracaecum spp being the highest. The intensity of infection did not vary between sexes and was not statistically significant in all because the samples were from different sites of the same environment, subject to similar water quality parameters and feeding opportunities. Out of the four taxonomic groups, the females had lower infection intensity than the males. Therefore to overcome the above problems the following recommendations were forwarded. These are (1) the problem of fish infestation by parasites in the dam could be best handled through proper management procedures that will help eliminate the suitable conditions favouring the parasite infestation (2) regular surveillance of the water body for parasites and pollution control will go a long way in controlling parasitic infestation in the dam; this should include control and monitoring of possible sources of parasites that include predators such as fish-eating birds and molluscs to ensure effective control of parasites (3) public awareness creation activities should be conducted on zoonotic nature of fish parasites (4) to protect the dissemination of these parasites to other reservoirs, ponds and other water bodies stocking fingerlings of O. niloticus from this dam should be protected until the reservoir is treated (5) further study is needed to investigate the exact sources of parasites.
Authors' contributions
The corresponding author Mr. Solomon carried out the responsibilities of proposal drafting, data collection and write-up of the manuscript. Dr. Mekonen Teferi and Prof. Dr. Tadesse Djenie participated in write up and commenting of the manuscript. Mr. Tsegaluel Abay and Mr. Girmay Hiluf participated in the field sampling and laboratory analyzes. All authors read and approved the final manuscript.
Consent for publication
It is declared that the information given in the manuscript now can be published by the Publication House and the ‘Journal of Applied Animal Research’.
Availability of data and material
We declare that whatever data have been used in the manuscript will keep remain intact. These data can be made available to anyone who desires to see them from the corresponding author on request.
Acknowledgments
The authors would like to acknowledge the Department of Biology, College of Natural and Computational Sciences, Mekelle University, for laboratory facilities and financial support. We are grateful to Belay Gebreyohannes and to all Aquatic research team members for their help during field sample collection and technical support.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Abd El-Monem SA, Bayoumy SM, Mahdey EA. 2005. Description of five new Myxosporean parasites infecting some sea fishes in Egypt. Egypt J Zool 45:333–348.
- Abolarin MO. 1974. Myxobolus tilapiae sp. nov. (Protozoa: Myxosporidia) from three species of freshwater tilapia in Nigeria. J West Afr Sci Ass. 19:109–114. Retrieved from FAO, Rome, Technical papers.
- Agbeko E, Kwarfo-Apegyah K, Akongyuure DN. 2014. Optimization of dugout fisheries for climate change adaptation in Northern Region, Ghana. J Energy Nat Res Manage. 1:63–68.
- Ahmad I, Kaur H. 2018. Prevalence, site and tissue preference of myxozoan parasites infecting gills of cultured fingerlings of Indian major carps in District Fatehgarh Sahib, Punjab (India). J Parasit Dis. 42:559–569. doi:10.1007/s12639-018-1035-6.
- Aken’ova AF. 2000. Fish mortalities associated with Goezia sp. (Nematoda: in Ascarididae) Central Florida. Proc. 25th Ann. Conf. Southeast. Assoc. Game Fish Comm.; p. 496–497.
- Ali SS. 1990. An introduction to fresh water fishery biology. Islamabad: University Grants Commission; p. 142–145.
- Alune E, Andrew G. 1996. Fishes. London, UK: Cambridge University Press.
- Aly SM. 2013. A review of fish diseases in the Egyptian aquaculture sector. Working report.
- Amare A, Alemayehu A, Aylate A. 2014. Prevalence of internal parasitic helminthes infected Oreochromis niloticus (Nile Tilapia), Clarias gariepinus (African Catfish) and Cyprinus carpio (Common Carp) in Lake Lugo (Hayke), Northeast Ethiopia. J Aquac Res Dev. 5(3):1–5.
- Ambani MM. 2014. Fish parasites (Labeo rohita and Schizothorax niger) of Jammu and Kashmir in India. Adv Aquacult Fish Manage. 2(11):i+-203.
- Anderson DP. 1974. Fish immunology. In: Sneiszko SF, Axelrod HR, editors. Diseases of fishes. Neptune City, NJ: F. H. Publication; p. 239.
- Areda TA, Mitiku MA, Woldearegay YH, Teklu A, Getachew S. 2019. Prevalence of major parasites of Nile tilapia (Oreochromis niloticus) in south west Showa zone selected fish farms, Oromia region, Ethiopia. Int J Fish Aquat Stud. 7(3):165–170.
- Ayalew DW. 2018. Theoretical and empirical review of Ethiopian water resource potentials, challenges and future development opportunities. Int J Waste Resour. 8(4):353.
- Ayotunde EO, Ochang SN, Okey IB. 2007. Parasitological examinations and food composition in the gut of feral African carp, Labeo coubie in the Cross River, Southeastern, Nigeria. Afr J Biotechnol. 6(5):625–630.
- Banerjee S, Bandyopadhyay PK. 2010. Observation on prevalence of ectoparasites in carp fingerlings in two districts of West Bengal. J Parasit Dis. 34(1):44–47. doi:10.1007/s12639-010-0003-6.
- Barker DE, Cone DK. 2000. Occurrence of Ergasilus celestis (Copepoda) and Pseudodactylogryrus anguillae (Monogenea) among wild eels (Anguilla rostrata) in relation to stream flow, pH and temperature and recommendations for controlling their transmission among captive eels. Aquaculture. 187:261–274. doi:10.1016/S0044-8486(00)00324-0.
- Bichi A, Ibrahim A. 2009. A survey of ecto and intestinal parasites of Tilapia Zillii (Gervias) in Tiga lake, Kano, northern Nigeria. Bayero J Pure Appl Sci. 2(1):79–82.
- Bichi AH, Bizi AG. 2002. Survey of ecto and endo parasites of fishes of Challawa George Dam. NISEB Journal. 2(3):219–222.
- Bondad-Reantaso MG, Subasinghe RP, Arthur JR, Ogawa K, Chinabut S, Adlard R, Tan Z, Shariff M. 2005. Disease and health management in Asian aquaculture. Vet Parasitol. 132:249–272. doi:10.1016/j.vetpar.2005.07.005.
- Brassard P, Rau ME, Curtis MA. 1982. Infection dynamics of Diplostomum spathaceum cercariae and parasite-induced mortality of fish hosts. Parasitol. 85:489–493. doi:10.1017/S0031182000056262.
- Canaris AG, Gardner SL. 1967. A guide to helminth species described from African vertebrates. Morgantown, WV: West Virginia University Library.
- Chandra KJ. 2004. Fish parasitology. Mymensingh: Lima Printing Press; p. 176.
- Chandra KJ. 2006. Fish parasitological studies in Bangladesh: a review. J Agric Ext Rural Dev. 4(1):9–18.
- Chappell LH, Hardie LJ, Secombes CJ. 1994. Diplostomiasis: the disease and host parasite interactions. In: Pike AW, Lewis JW, editors. Parasitic diseases of fish. Otley: Samara Publishing Limited; p. 59–86.
- Cheng TC. 1986. General parasitology. Orlando: Academic press; p. 253.
- Cowx IG. 1992. Aquaculture development in Africa, training and reference manual for aquaculture extensionists. Food production and rural development. London: Commonwealth secretariat; p. 246-295.
- Crowden AE, Broom DM. 1980. Effects of the eyefluke, Diplostomum spathaceum, on the behavior of dace (Leuciscus leuciscus). Anim Behav. 28:287–294. doi:10.1016/S0003-3472(80)80031-5.
- Davies OA, Inko-Tariah MB, Amachree D. 2006. Growth response and survival of Heterobranchus longifilis fingerlings fed at different feeding frequencies. Afr J Biotechnol. 5(9):778–780.
- DWAF. 1996. South African water quality guidelines. Volume 7: Aquatic ecosystems. Department Of Water Affairs And Forestry.
- Eskendir AD. 2015. Four Ethiopian Towns Water Supply and Sanitation Improvement Programme: Environmental and Social management framework summary, African Development Bank, Ethiopia, Project Code: P-ET-E00-011, 14pp.
- FAO. 2005. Ethiopia Information on Fisheries management (From IFMC) water report No. 29, Fao, Rome, Italy.
- FAO. 2014. The state of world fisheries and aquaculture. Fisheries and Aquaculture department of food and agriculture organization of the United Nations, Rome, Italy; p. 75–76.
- Florio D, Gustinelli A, Caffara M, Turci F, Quaglio F, Konecny R, Nikowitz T, Wathuta EM, Magana A, Otachi EO, Matolla GK. 2009. Veterinary and public health aspects in tilapia (Oreochromis niloticus niloticus) aquaculture in Kenya, Uganda and Ethiopia. Ittiopatologia, 6: 51–93.
- Fomena A, Bouix G, Birgi E. 1984/1985. Contribution a l"etude des Myxosporidies des poisson d'eau douce du Cameroun. II: Especes nouvelle du genre Myxobolus Butschli, 1882. Bull de L'Inst Fon Afr Noir. 46(ser. A):168–191.
- Fulton TW. 1904. The rate of growth of fishes. In: 22nd Annual Report of the Fishery Board of Scotland 1904 (3); p. 141–241.
- Gopalakrishnan V. 1961. Observations on a new epidemical eye disease affecting the Indian carp Catla catla (Hamilton Buchanan). Indian J Fish. 8(1):222–232.
- Goselle N, Shir GI, Udeh EO, Abelau M, Imandeh GN. 2008. Helminth parasites of Clarias gariepinus and Tilapia zillii at Lamingo dam, Jos, Nigeria. Sci World J. 3(4):23–28. doi:10.4314/swj.v3i4.51823.
- Gulelat Y, Yimer E, Asmare K, Bekele J. 2013. Study on parasitic helminths infecting three fish species from Koka reservoir, Ethiopia. SINET: Ethiop J Sci. 36(2):73–80.
- Gunn A, Pitt SJ. 2022. Parasitology: an integrated approach. New York: John Wiley & Sons.
- Gupta SK, Gupta PC. 2006. General and applied ichthyology (fish and fisheries). New Delhi: S. Chand and Company Ltd; p. 1133.
- Heckman R. 1996. Protozoan parasites of fish, part 1. Aquat. Mag. 22(3):44–57.
- Hoffman GL. 1998. Parasites of North American freshwater fishes. 2nd ed. London: Univ. California Press.
- Hoffman GL. 2019. Parasites of North American freshwater fishes. New York: Cornell University Press.
- Hoogendoorn C. 2020. Study of Diplostomum (Digenea: diplostomoidea) in South Africa: diversity and effect of metacercariae on fish behaviour [doctoral dissertation]. North-West University (South Africa).
- Hossain MD, Hossain MK, Rahaman MH, Akter A, Khanom DA. 2008. Prevalence of ectoparasites of carp fingerlings at Santaher, Bogra. Univ J Zool Rajshahi Univ. 27:17–19. doi:10.3329/ujzru.v27i0.1947.
- Hussen A, Tefera M, Asrate S. 2012. Gastrointestinal helminth parasites of Clarias gariepinus (catfish) in Lake Hawassa Ethiopia. Sci J Anim Sci. 1(4):131–136.
- Ibrahim A, Adetola J, Emmanuel A. 2016. Natural occurrence of diplostomum spp. in farm-raised African catfish (Clarias gariepinus) from Oyo state, Nigeria. Int J Vet Sci Med. 4(2):41–45. doi:10.1016/j.ijvsm.2016.10.006.
- Islam SI, Rodkhum C, Taweethavonsawat P. 2023. An overview of parasitic co-infections in tilapia culture. Aquac Int. 1–29.
- Iyabo UB. 2015. Prevalence of Helminthes Parasites of Oreochromis niloticus in the Mid Cross River Flood System, Southeastern, Nigeria.
- Jadhav U. 2009. Aquaculture technology and environment. New Delhi: PH1 Learning Private Ltd.
- Janko AM. 2014. Fish production, consumption and management in Ethiopia. Int J Econ Manage. 3(3):1–6.
- Jerbe. 2007. Fish diversity in the main drainage system of Ethiopia.
- Jossy B, Daniel H. 2015. Prevalence of internal parasites of Oreochromis niloticus and Clarias gariepinus fish species in Lake Ziway. Ethiopia. Journal of Aquaculture Research and Development. 6(2).
- Kabata Z. 1985. Parasites and diseases of fish cultured in the tropics. London: Taylor and Francis Ltd; p. 318.
- Kaminskas S. 2022. Alien fish ascendancy and native fish extinction: ecological history and observations on the lower Goodradigbee River, Australia. Pac Conserv Biol. 29(1):38–73. doi:10.1071/PC21048.
- Kayis S, Ozcelep T, Capkin E, Altinok I. 2009. Protozoan and metazoan parasites of cultured fish in Turkey and their applied treatments.
- Kennedy CR. 1976. Ecological aspects of parasitology. North-Holland Publishers.
- Khalil LF. 1971. Checklist of the helminth parasites of African freshwater fishes. Commonwealth Institute of Helminthology, St Albans. Technical Communication No. 42.
- Klinger R, Francis Floyol R. 2002. Introduction to freshwater fish parasites. Fisheries and Aquatic Science Department Floride Co-operative extension services.
- Komar DC, Wendover N. 2007. Parasitic diseases of Tilapia - The Fish Site [https://thefishsite.com/] site visited on 21/06/2017.
- Koyuncu CE, Toksen E. 2010. Ectoparasitic diseases in freshwater ornamental fish and their treatments. In: 2nd International symposium on sustainable Development, Sarajevo; p. 683–688.
- Landsberg JH. 1986. Myxosporean parasites of the catfish, Clarias lazera (Valenciennes). Syst Parasitol. 9:73–81. doi:10.1007/BF00009899.
- Machado PM, Takemoto RM, Pavanelli GC. 2005. Diplostomum (Austrodiplostomum) compactum (Lutz, 1928) (Platyhelminthes, Digenea) metacercariae in fish from the floodplain of the Upper Paraná River, Brazil. Parasitol Res. 97:436–444. doi:10.1007/s00436-005-1483-7.
- Marcogliese DJ. 2002. Food webs and the transmission of parasites to marine fish. Parasitol. 124:583–599. doi:10.1017/S003118200200149X.
- Marcogliese DJ. 2004. Parasites: small players with crucial roles in the ecological theater. EcoHealth. 1(2):151–164.
- Margolis L, Esch GW, Holmes JC, Kuris AM, Schad GA. 1982. The use of ecological terms in parasitology (report on an ad-hoc committee of the American society of parasitologists). J Parasitol. 68:131–133. doi:10.2307/3281335.
- Mashego SN. 1982. A seasonal investigation of the helminth parasites of Barbus species in water bodies in Lebowa and Venda, South Africa. Doctoral dissertation, University of the North.
- Mashego SN. 1989. Nematode parasites of Barbus species in Lebowa and Venda, South Africa. S Afr J Wildl Res. 19(1):35–37.
- Meyer FP. 1991. Aquaculture disease and health management. J Anim Sci. 69(10):4201–4208.
- Michel C. 1989. Pathology of tilapias. Aquat Living Resour. 2(2):117–126.
- Mitiku MA, Konecny R, Haile AL. 2018. Parasites of Nile tilapia (Oreochromisniloticus) from selected fish farms and Lake Koftuin central Ethiopia. Ethiop Vet J. 22(2):65–80. doi:10.4314/evj.v22i2.6.
- Mitra AK, Haldar D. 2004. First record of Chilodonella hexasticha (Kiernik, 1909) Kahl, 1931 (Ciliophora: Chilodonellidae) infesting a freshwater fish Nandus nandus (Hamilton) from Gangetic West Bengal, India. Anim Biol 54:111–118. doi:10.1163/1570756041445182.
- MoARD. 2008. Ministry of Agriculture and rural development annual report.
- Mohammed R, Marshet A, Redda YT, Nesibu A, Awot T. 2015. A study of Clinostomum (trematode) and Contracaecum (nematode) parasites affecting Oreochromis niloticus in Small Abaya Lake, Silite Zone, Ethiopia. J Aquacult Res Develop. 6(3):316.
- Ndeda VM, Owiti DO, Aketch BO, Onyango DM. 2013. Genetic relatedness of Diplostomum Species (Digenea :Diplostomidae) Infesting Nile Tilapia (Oreochromis Niloticus L.) in Western Kenya. Open J Appl Sci. 3(December):441–448. doi:10.4236/ojapps.2013.38055.
- NFLARRC. 2002. The total survey report on current National Fisheries and Other Living Aquatic Resources Research Centre, Addis Ababa.
- Niewiadomska K. 1996. The genus Diplostomum – taxonomy, morphology and biology. Acta. Parasitologica. 41:55–66.
- Noga EJ. 2010. Fish disease: diagnosis and treatment. John Wiley and Sons.
- Okaeme AN, Obiekezie AI, Rehmen J, Mark OA. 1986. Infections and diseases of cultured fish of Lake Ka’inji Area, Nigeria. J Fish Biol. 32:479–481. doi:10.1111/j.1095-8649.1988.tb05383.x.
- Owen SF, Barber I, Hart PJB. 1993. Lowlevelinfection by eye fluke, Diplostomum spp., affects the vision of 3-spined sticklebacks, Gasterosteus aculeatus. J Fish Biol. 42:803–806.
- Paperna I. 1973. Occurrence of Cnidospora infections in freshwater fishes in Africa. Bull L’Inst Fond D’Afrique Noire. 35:509–521. Retrieved for FAO, Rome, Technical paper.
- Paperna I. 1980. Parasites, Infections and Diseases of Fish in Africa. CIFA Technical Paper, 7. Rome, FAO. 216 p.
- Paperna I. 1996. Parasites, infections and diseases of fishes in Africa - An update. CIFA Technical Paper. No.31. Rome, FAO. 220p.
- Phillips MJ. 1996. Better health management in the Asia-Pacific through systems management. FAO Fisheries Technical Paper (FAO), (360).
- Pouder DB, Curtis EW, Yanong RPE. 2005. Common freshwater fish parasites pictorial guide. Digenean trematodes (FA112) and nematodes (FA113).
- Roberts RJ. 2012. Fish pathology. Idaho: John Wiley & Sons.
- Seppälä O, Karvonen A, Valtonen ET. 2004. Parasite-induced change in host behaviour and susceptibility to predation in an eye fluke – fish interaction. Anim Behav. 68:257–263. doi:10.1016/j.anbehav.2003.10.021.
- Seppälä O, Karvonen A, Valtonen ET. 2005. Manipulation of fish host by eye flukes in relation to cataract formation and parasite infectivity. Anim Beh. 70:889–894. doi:10.1016/j.anbehav.2005.01.020.
- Seppälä O, Karvonen A, Valtonen ET. 2012. Behavioural mechanisms underlying ‘specific’ host manipulation by a trophically transmitted parasite. Evol Ecol Res. 14:73–81.
- Shinn A, Pratoomyot J, Bron J, Paladini G, Brooker E, Brooker A. 2015. Economic impacts of aquatic parasites on global finfish production. Glob Aquacult Advocate. 2015:58–61.
- Smyly WJP. 1957. The life-history of the bullhead or Miller's thumb (Cottus Gobio L.). Proc Zool Soc London. 128:431–454. doi:10.1111/j.1096-3642.1957.tb00336.x.
- Tedla S, Tadesse GE. 1979. Observations on the parasites of Tilapia nilotica and Clarias mossambicus, lake Awassa, Ethiopia. Ethiop J Agric Sci. 1(2):126–130.
- Tessema W. 2020. Review on parasites of fish and their public health importance. ARC J Anim Vet Sci. 6:23–27.
- Thrusfield M. 2005. Sampling. In: Veterinary epidemiology. London: Black Well Science Ltd; p. 228–246.
- Tigabu Y. 2010. Stocking-based fishery enhancement programmes in Ethiopia. Ecohydrol Hydrobiol. 10(2-4):241–246. doi:10.2478/v10104-011-0012-9.
- Tokşen E. 2006. Argulus foliacesus (Crustacea: Branchiura) infestation on Oscar, Astronotus ocellatus (Cuvier, 1829) and its treatment. Su Ürünleri Dergisi. 23(1):177–179.
- Wangatia MV. 2012. Parasite infection in O. niloticus from Ziwa Dam,Kenya, (Blind Manuscript).docx.
- Yamaguti S. 1985. Systema helminthes of fish. Vol 1 digentic trematodes of the vertebrata part 1 and 2. New York: Interscience.
- Yanong RPE. 2002. Nematode (roundworm) infection in fish 1st edn University of Florida, IFAS Cooperatives Extension 1-10.
- Yimer E. 2000. Preliminary survey of parasites and bacterial pathogens of fish at Lake Ziway. SINET: Ethiop J Sci. 23(1):25–33.