?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This study aimed to explore the therapeutic activity of Artemisia argyi essential oil (AAEO) nasal drops against avian infectious bronchitis and the underlying mechanism in the avian respiratory tract. Overall, 96 14-day-old broilers were randomly divided into 4 groups: control (control), A. argyi essential oil (AAEO) control, infectious bronchitis virus (IBV), and AAEO treatment (IBV + AAEO) groups (n = 24/group). Mortality was 25% (6/24) in the IBV group compared to 4.2% (1/24) in the AAEO treatment group. The clinical symptoms of the treatment group were significantly improved compared with those of the IBV group after 5 days of treatment. The broilers recovered at the end of the experiment. AAEO treatment relieves IBV-induced tracheal mucosal edema and bleeding and inhibits viral replication in the lungs. In addition, it enhanced SIgA secretion in the tracheal mucosa and increased type I interferon release by regulating IRF7 expression. Furthermore, we explored the mechanism underlying the therapeutic effect of AAEO. It maintains TLR7 at a high level in broilers and further upregulates MyD88, IRF7, and IL–10. Thereby enhancing the immune system and anti-inflammation. Our results suggest that AAEO can be used as a therapeutic agent for IBV.
Introduction
Infectious bronchitis (IB) is a disease that has affected poultry worldwide with high morbidity, considerable infectivity, and strong variability. IBV belongs to the Coronavirus family, which mainly causes respiratory symptoms in chicks and adult chickens and can reduce daily gain and feed conversion rate in broilers (Cook et al. Citation2012). Some strains cause renal pathogenicity, which triggers the appearance of urate deposition, resulting in what is called ‘spot kidney’, nephritis, and even death from renal failure (Cavanagh Citation2007). IBV infection can lead to decreased egg production and quality, such as soft-shelled eggs, malformed eggs, and thin egg whites (Zhang et al. Citation2020). After infection, egg production in immunized laying hens usually recovers, but does not return to normal levels. Treating IB in broilers is clinically difficult, and there is no specific treatment for this disease. Antibiotics are often used to treat potential secondary infections; however, they are often ineffective and treat only symptoms rather than the cause. In addition, with the emergence of resistant strains, it is necessary to reduce the use of antibiotics. At present, IB treatment chiefly focuses on immunizing broilers against infectious bronchitis virus (IBV) through vaccine immunization to prevent infection. However, because there are numerous IBV serotypes, little cross-protection exists between the serotypes and different strains circulating in different regions and vaccine immunization is difficult to achieve ideal expectations (Awad et al. Citation2014). Therefore, there is an urgent need to explore other perspectives of economical and effective IB treatment in broilers.
Artemisia argyi (Artemisia argyi Levl. et Vant.) has been widely used to treat respiratory diseases since ancient times. A large number of modern pharmacologic studies have reported that the volatile oil extracted from the leaves of A. argyi possesses various biological functions and plays a vital role in anti-inflammatory, immune-regulatory, and antimicrobial activities (Wenqiang et al. Citation2006; Saddi et al. Citation2007; Ahameethunisa and Hopper Citation2010, Citation2012; Choi et al. Citation2012; Ge et al. Citation2016; Yun et al. Citation2016; Chung Citation2017). Modern pharmacology has demonstrated that AAEO is safe and non-toxic and has abundant biological activity (Li et al. Citation2008; Choi et al. Citation2012; Ng et al. Citation2013; Wan et al. Citation2013; Ge et al. Citation2016; Yun et al. Citation2016; Chen et al. Citation2017). The method of extracting the volatile oil is simple and easy to perform (Wenqiang et al. Citation2006; Li et al. Citation2008; Chen et al. Citation2017; Lu et al. Citation2018). In addition, only a small amount of volatile oil is required for the treatment, which is very suitable for the poultry industry. However, to the best of our knowledge, there are no relevant reports on avian IB. Therefore, we investigated the effect of A. argyi essential oil (AAEO) in treating avian IB.
In this experiment, we first evaluated the therapeutic effect of AAEO nasal drops on IB in broilers based on clinical manifestations, respiratory pathology, and changes in viral titration in the lungs of broilers. In addition, we explored the effect of AAEO on mucosal immunity and a mechanism involving the MyD88-dependent and nuclear factor κB (NF-κB) signalling pathways.
Materials and methods
Broilers and eggs
We acquired both male and female (M: F = 1:1) 1-day-old unimmunized AA broilers from Beijing Arbor Acres Poultry Breeding Co., Ltd (PR China). The broilers were kept under controlled conditions (38°C and 60% humidity) inside the isolator and fed sterilized feed and drinking water without any antibiotics with ad libitum access to feed. Specific pathogen-free (SPF) eggs were purchased from Beijing Meriaweitong Experimental Animal Technology Co., Ltd (PR China). All animal experimental protocols received ethics approval from the Institutional Animal Care and Use Committee of China Agricultural University (No. Aw02603202-2-1). Specific pathogen-free (SPF) eggs were purchased from Beijing Meriaweitong Experimental Animal Technology Co., Ltd (PR China).
Virus
The avian IB virus type QX was provided by the Key Laboratory of Animal Epidemiology and Zoonosis of the Ministry of Agriculture of China Agricultural University.
Rejuvenation
QX-type IBV diluent was injected into 9-day-old chicken embryos and incubated in an incubator for 48 h. After incubation, we sacrificed the chicken embryos at 4°C and disinfected them with iodophor and alcohol. Allantoic fluid was extracted and centrifuged at 3,000 g/min to obtain the supernatant. The supernatant was filtered using a 0.22-μm microporous membrane and stored in a refrigerator at −80°C.
Extraction of AAEO
The A. argyi leaves used in this experiment were purchased from the Beijing Tongrentang Malianwa branch (PR China). We extracted the volatile AAEO via steam distillation (Commission Citation2009). In this experiment, we obtained 1 kg of AAEO using this method, and the oil yield was ∼3.5%. The obtained AAEO was added to distilled water containing 0.5% dimethyl sulfoxide for a final concentration of 0.3%. The active components in AAEO were detected by the Beijing QingXi technology Research Institute (PR China), and shows the components. The results of the preliminary test () revealed that 0.3% AAEO would not cause an obvious stress reaction in broilers; thus, we selected that dosage for use in this study.
Table 1. Components of Artemisia argyi essential oil.
Table 2. Clinical symptoms of broilers after nasal drops of AAEO at different concentrations.
Modelling
Overall, 40 1-day-old SPF chicken embryos were divided into eight 8 with 5 embryos in each group. The concentrations of the virus allantoic fluid of obtained via the above method were diluted to 10−1–10−7. Each concentration was inoculated in one group, and the blank control group was inoculated with 0.2 mL of normal saline. Following inoculation, the chicken embryos were placed into the incubator and their death was observed on time. The chicken embryos that died within 24 h were discarded. Continuous observation was performed for 1 week. We determined and calculated the EID50 of the virus using the Reed–Muench method (Saganuwan Citation2011).
In total, 35 1-day-old nonimmunized AA broilers were randomly divided into 5 groups, in which 4 groups were administered 0.2 mL IBV allantoic fluid at concentrations of 102, 103, 104, and 105 EID50 via nasal drip, respectively. The other groups were administered 0.2 mL normal saline. We observed the flock closely and recorded mortality and clinical symptom scores (). The final modelling dose was 104 EID50/0.2 mL.
Table 3. Clinical symptom rating scale.
Treatment protocols
Overall, 96 1-day-old nonimmunized AA broilers were randomly divided into 4 groups, including a blank control (Control), AAEO control (AAEO), model group (IBV), and AAEO treatment (IBV + AAEO) groups (n = 24/group). After feeding till 14 days of age, the model and AAEO treatment groups were modelled with IBV nasal drops. We carefully observed the state changes of the broilers within 72 h after the challenge. Treatment began 3 days after nasal dripping. The AAEO and AAEO treatment groups were treated intranasally with 0.1 mL of 0.3% AAEO twice a day for 10 days.
All broilers were weighed on days 1, 3, 5, 7, and 10 post-challenge. Eight broilers from each group were weighed and sacrificed on days 3, 7, and 10. Blood was obtained to prepare serum for antibody content. Organ indices were calculated by weighing the spleen and lungs separately. The lungs and trachea were collected for viral titration, pathology, immunohistochemistry, and real-time fluorescence quantitative polymerase chain reaction (RT-qPCR) analysis.
Histology and immunohistochemistry analysis
The trachea samples were fixed in 4% paraformaldehyde and then sectioned into 3 × 3 × 5-mm tissue blocks. The tracheal samples were dehydrated using graded ethanol (50%, 70%, 80%, 90%, 100%, and 100% for 1 h each) in cassettes, cleared with xylene (xylene and alcohol 1:1 for 30 min; xylene I and xylene II for 10 min), immersed in wax, and embedded. The wax blocks were frozen overnight at −20°C and sectioned for hematoxylin–eosin (HE) staining (Yin-Ji et al. Citation2012).
We used SP-9000 kit (Beijing Zhongshan Jinqiao Biotechnology Co., Ltd., China) and the DAB chromogenic kit (Wuhan Boster Bioengineering Co., Ltd., China) to detect the expression of immunoglobulin A (IgA) in the trachea according to the manufacturer’s instructions. IgA+ cells appear as brown or yellow under 40× microscopy.
Enzyme-linked immunosorbent assay analysis
Using the IBV IgA enzyme-linked immunosorbent assay (ELISA) kit purchased from Sichuan Weikeqi Biotechnology Co., Ltd. (PR China), we determined the level of chicken infectious bronchitis antibody IgA (IBV IgA) using the double antigen sandwich method.
RT-qPCR analysis
Each lung was ground at a low temperature, and total RNA was extracted using TransZol Up Plus RNA Kit (Beijing TransGen Biotech Co., LTD, PR China) according to the manufacturer’s instructions. Total RNA was stored at −80°C. 2×EasyTaq® PCR SuperMix and TransScript® First-Strand cDNA Synthesis SuperMix (Beijing TransGen Biotech Co., LTD, PR China) were used to reverse transcribe the extracted total RNA according to the manufacturer’s instructions. shows the primer sequences used in this study, and the primers were synthesized by Beijing Optimus Biotechnology Co., LTD (PR China). The two-step method was adopted, and the reaction procedure was as follows: 94°C initial denaturation for 30 s followed by 45 cycles of 94°C for 5 s and 60°C for 30 s. The original data of this trial were collected via CFX Maestro.
Table 4. Real gene-specific oligonucleotide primer used for RT-qPCR.
Statistical analysis
The experimental data were analyzed using SPSS 25.0 statistic software. Diagrams were created using GraphPad Prism 8.0 software. All data are presented as the mean ± standard error of the mean. Data were obtained using one-way analysis of variance followed by Duncan’s test for multiple comparisons and log-rank (Mantel–Cox) test for survival curve analysis. For all studies, a P-value of <0.05 was considered statistically significant and P < 0.01 was considered extremely significant.
Results
Treatment effect of AAEO
The IBV and AAEO treatment groups were challenged with infectious dose 104 EID50 via nasal drip, and all broilers exhibited clinical symptoms within 36 h. shows the mortality statistics. The score of the treatment group decreased gradually as the treatment progressed, whereas the score of the IBV group remained at a high level (). The infected broilers that were crowded together but not sensitive to the external environment exhibited depression, disordered hair, anorexia, and frequent head flicking, occasionally breathing with their mouths open and mucus flowing out of the nasal cavity. The control group demonstrated no abnormal performance, and the broilers with a strong appetite that liked to play were lively. The broilers in the AAEO group exhibited no obvious abnormal performance except for scratching their nose occasionally after the AAEO was gently dropped onto their nose several times over a short time period. The IBV group demonstrated some obvious clinical symptoms after 3 days of challenge, such as a few broilers dying and others exhibiting depression, loss of appetite, disordered hair, unstable standing, eyes closed, head flicking, open-mouth breathing, increased mouth and nose secretions, and loose feces with yellow granular. With the symptoms gradually worsening over the next few days, the number of dead broilers also gradually increased. After 10 days of challenge, the condition of the surviving broilers gradually stabilized, and the condition of individual broilers significantly improved; however, mental appetite remained poor, and the body was visibly thinner. Typical clinical symptoms caused by IBV infection also appeared in the treatment group at the initial stage of treatment, but their clinical symptom scores were not significantly different (P > 0.05) from those of the challenge control group (). However, from the third day of treatment, the scores of the treatment group were significantly (P < 0.01) lower than those of the IBV group, and the clinical symptoms of the treatment group also gradually improved during treatment. After 7 days of treatment, the frequency of head flicking, amount of mucus in the mouth and nose, and spiritual appetite of the treatment group were significantly reduced, and some broilers even returned to normal. A total of six broilers in the IBV group and one broiler in the treatment group died 10 days after the treatment. The mortality rate in the treatment group was only 4.2%.
Figure 1. Effect of AAEO on clinical parameters in broilers infected with IBV. The IBV and IBV + AAEO groups were infected with 104 EID50/0.2 mL IBV. After 3 days, the IBV + AAEO group received 0.3% AAEO (0.1 mL, nasal drop administration) per day for 7 consecutive days. Broilers in the AAEO group were given only 0.3% AAEO (0.1 mL, nasal drop administration), and the control group was not infected with a virus and was not given AAEO. The body weight of the broilers was measured, and the clinical symptom scores were evaluated and recorded. A: Average weight of the broilers between days 1 and 10 post-challenge (dead broilers weighing 0). B: Clinical symptom score of broilers. #P < 0.05, ##P < 0.01 versus the control group; *P < 0.05, **P < 0.01 versus the IBV group.
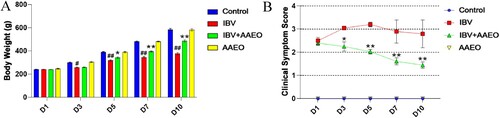
Table 5. Mortality statistics for each group during the study period.
Lung index and lung viral titration
After calculation, on day 3 post-challenge, the index of the IBV and treatment groups was significantly higher than that of the other two groups (P < 0.05). On days 7 and 10 post-challenge, the index of the IBV group was significantly higher than that of the control group (P < 0.01) and showed a rising trend. The index of the treatment group was also higher than that of the control group (P < 0.01), but there was no gradual upward trend ().
Figure 2. Effect of AAEO on the lungs following IBV infection. On days 3, 7, and 10 post-challenge, the lungs were collected from eight broilers per group. The lung and body weights were measured, and the lung indices were calculated. IBV titers in the lung were determined via RT-qPCR. #P < 0.05, ##P < 0.01 versus the control group; *P < 0.05, **P < 0.01 versus the IBV group.
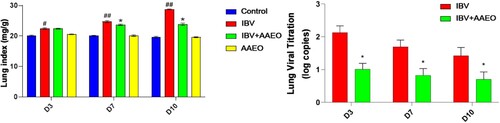
From the third day of treatment, IBV viral titration in the lungs of all the groups demonstrated a downward trend. shows that the viral titration of the treatment group was significantly lower than that of the IBV group during the entire experiment (P < 0.05).
Histopathological examination
After 10 days of the challenge, HE staining was performed on the tracheal and lung tissue of each group for histopathology. The tracheal structure of both the control and AAEO groups was complete (). However, in the IBV group, epithelial cells were exfoliated and necrotic mucosal edema was significant, and a number of red cells infiltrated. In the treatment group, the structure of epithelial cells was relatively intact, the mucosa exhibited mild edema, and no inflammatory cell infiltration was observed.
Figure 3. Histological and gross pathological effects of AAEO on the lungs and trachea were reflected by dissection and pathological sections via hematoxylin–eosin (HE) staining. Each group of broilers was randomly selected and sacrificed on day 7 post-challenge, and the lungs and trachea were dissected and fixed with formaldehyde and embedded in paraffin to create pathological sections. A: Pulmonary necropsy of each group after 10 days of the challenge. The length and width of the IBV-infected lungs were ∼2 mm shorter than those of normal broilers. Bleeding in the AAEO treatment group was reduced compared with that in the IBV group. B: Autopsy of the trachea in each group after 7 days of the challenge. There was severe bleeding with abundant purulent exudation in the IBV group. Bleeding was not apparent in the treatment group, but there was also substantial purulent exudation. C: Pulmonary pathological changes in each group, HE 100×. The lung tissue of the IBV group exhibited significant red blood cell spillage into the alveolar space (black arrows), whereas the alveolar morphology and structure of the other three groups were intact and no significant hemorrhage was observed. Hyperemia was more severe in the treatment group than in the control and AAEO groups. D: Pathological changes in the trachea in each group, HE 100×. In the IBV group, the mucosal layer was thickened with massive red blood cell spillage, and the submucosal layer was edematous. The mucosal layer was significantly thicker in the treatment group than in the control and AAEO groups.
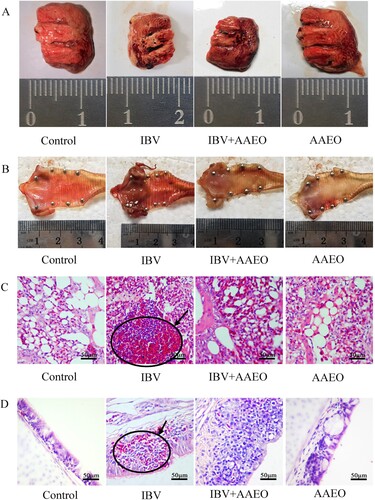
In the lung tissue, the alveoli of the control and AAEO groups contained no red blood cells. The alveolar structure of the IBV group was not clear, and there were many red blood cells in the visual field. Compared with the control and AAEO groups, the treatment group exhibited more red blood cells; however, clear alveolar tissue could still be observed.
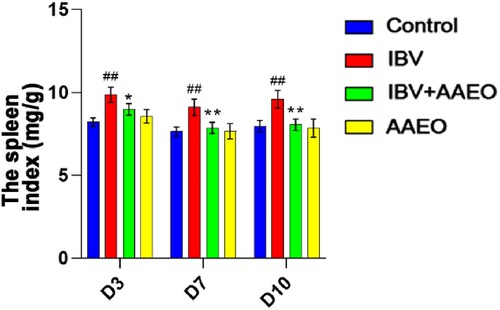
Spleen index
After calculation, the spleen index of the IBV group was significantly (P < 0.05) higher than that of the other groups around the whole treatment cycle. However, there was no statistically significant difference between the other groups (P > 0.05; ).
IgA level in the trachea and serum
We used immunohistochemistry to determine the proportion of IgA+ cells in the trachea (A). The area of positive cells in the treatment group was significantly (P < 0.05) higher than that in the control, AAEO, and IBV groups. The proportion of IgA+ cells in the IBV group was significantly (P < 0.05) lower than that in the control group and that in the AAEO group was significantly (P < 0.05) higher than that in the control group (B).
Figure 5. Effect of AAEO on the immune status of broilers infected with IBV. Tracheal sections were obtained from broilers on day 7. Tracheal wash fluid and serum were collected on days 3, 7, and 10, respectively. Tracheal sections were examined via immunohistochemistry, and antibody levels were detected via ELISA. A and B: Distribution and percentage of IgA+ cells in the trachea of broilers between the groups. IgA+ cells (red arrows) stained with yellow–brown. C: Antibody levels of broilers in different treatment groups. #P < 0.05, ##P < 0.01, ###P < 0.001 versus the control group; *P < 0.05, **P < 0.01, ***P < 0.001 versus the IBV group.
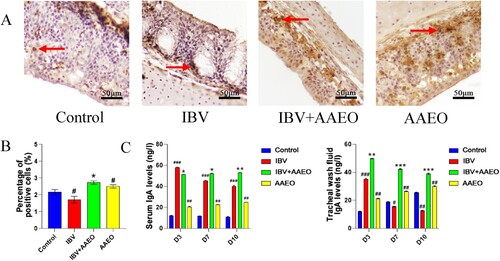
To observe the regulatory function of AAEO nasal drops on the antibody, we used ELISA to detect the content of IBV-specific antibody IgA in the serum and tracheal lavage fluid (Hu and McDougald Citation2003). C shows the change in the IgA level in the serum and tracheal lavage fluid of each group. On day 3, the serum IgA level of the IBV group was significantly (P < 0.05) higher than that in the treatment group, whereas on the 7th and 10th days of treatment, the serum IgA level of the IBV group was significantly (P < 0.05) lower than that in the treatment group. The IgA level in the IBV group exhibited a downward trend, whereas the that in the treatment and AAEO groups demonstrated a slow upward trend. The IBV group exhibited significantly (P < 0.001) higher levels of IgA in the tracheal flush solution than in the control group on day 3 but showed a decreasing trend and was lower than that in the control group on day 7. There was also a gradual downward trend in the treatment group, but this group was always able to maintain a high level. The expression level of the AAEO group increased gradually and was always significantly (P < 0.01) higher than that of the control group.
Expression levels of inflammation-related genes and IRF7 in trachea and lung
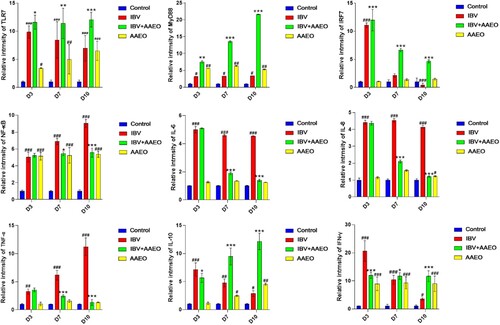
The mRNA levels of TLR7, MyD88 and IRF7 in the treatment group were significantly higher than those in the IBV group during the 3–10 days post-challenge (P < 0.01). The mRNA levels of IL-10 and IFN-γ in the IBV group gradually decreased, but the treatment group gradually increased and became significantly higher than the IBV group after 7 days post-challenge (P < 0.01). The mRNA levels of proinflammatory cytokines IL-8, IL-6, NF-κB and TNF-α in the IBV group were significantly higher than those in the treatment group from day 7 post-challenge (P < 0.05 or P < 0.01). Compared with the control group, the mRNA levels of TLR7, MyD88, NF-κB, IL-10, and IFN-γ were significantly increased in the AAEO group (P < 0.05), while there was no significant difference in the mRNA levels of IL-6, IL-8, TNF-α and IRF-7 (P > 0.05) ().
Figure 6. Effect of AAEO on cytokines in the trachea and lungs infected with IBV was examined using RT-qPCR. The trachea and lungs obtained on days 3, 7, and 10 were ground and lysed under frozen conditions for RNA extraction. #P < 0.05, ##P < 0.01, ###P < 0.001 versus the control group; *P < 0.05, **P < 0.01, ***P < 0.001 versus the IBV group.
Discussion
It can be seen from the clinical symptom score and mortality rate that AAEO nasal drops exert a significant therapeutic effect on IB. At the initial stage of treatment, there was no statistically significant difference between the clinical symptom score of the treatment and IBV groups, and both the groups exhibited obvious clinical symptoms. However, after 3 days of treatment, the symptoms of the treatment group gradually began to reduce, spiritual appetite gradually recovered, and clinical symptom score gradually decreased, which was significantly (P < 0.01) lower than that of the IBV group. In addition, the mortality rate in the treatment group was only 4.2%, which was 20.8% lower than the 25% in the IBV group. The autopsy results revealed that the organs treated with AAEO exhibited significantly reduced bleeding, inflammation, and other symptoms. According to the results of HE staining, AAEO nasal drip exerts no adverse effect on the tracheal epithelium and alveoli of uninfected broilers and demonstrates a certain protective effect on the respiratory structure of the IB broilers, which can reduce inflammatory reactions and protect the integrity of epithelial and alveolar cells. Viral titration measurements also revealed that the viral expression was reduced in the treated broilers. At present, many studies have reported that whether in vivo or in vitro, AAEO can kill and inhibit various viruses and bacteria (Saddi et al. Citation2007; Meneses et al. Citation2009; Ahameethunisa and Hopper Citation2010, Citation2012; Chung Citation2017; Xuelin et al. Citation2021). Simultaneously, it can resist inflammation and antioxidant stress and subsequently play a therapeutic and preventive effect on infectious diseases, consistent with the results of this experiment.
The spleen plays a central role in avian immune function. During treatment, the spleen index of the IBV group was always higher than that of the other groups and was different from the decline of the spleen index caused by infection with IBV, as reported in other studies (Awad et al. Citation2019; Amanollahi et al. Citation2020). Combined with the autopsy, the body weight of the broilers in the model group was lower than that in the other groups; however, the weight of the spleen did not considerably change, which might be the reason for increased spleen index in the model group. However, more research data are needed for further discussion.
Secretory IgA (SIgA) is an important part of mucosal immunity and plays a vital role in mucosal resistance to the invasion of foreign microorganisms (Chou et al. Citation2016). The immunohistochemical results revealed that AAEO increased the level of SIgA in the tracheal mucosa following nasal drip, whether infected with IBV or not, and the level was significantly higher (P < 0.05) than that in the IBV and control groups. Given the histopathological results, it is suggested that AAEO can maintain the integrity of the mucosal structure and enhance and regulate the immune function of the tracheal mucosa.
The ELISA results revealed that the body produced a large amount of IgA to resist IBV invasion following IBV infection, and the addition of AAEO could appropriately inhibit the excessive expression of IgA in the serum in the early stage of IBV infection and then remained at a higher and more stable level for some time afterward. The IgA level in the tracheal lavage fluid also increased significantly 3 days after IBV infection; however, untreated broilers showed significantly decreased expression of IgA in the trachea, which was lower than that of normal broilers in the short term, consistent with the content of the positive cells in each group in the immunohistochemical section. However, the AAEO-treated broilers maintained a higher level of antibody, and this level decreased more gradually. The results indicated that AAEO nasal drip could improve the immune status of infected broilers and increase and maintain the content of IBV-specific antibodies in the serum and tracheal lavage fluid, thereby exerting a certain antiviral effect. In addition, using AAEO in normal broilers can enhance immune function.
TLR7 is an important pattern-recognition receptor for avian virus recognition (Annamalai et al. Citation2015). The TLR7 receptor activates immune cells through the MyD88-dependent signalling pathway and plays an immunomodulatory role. In this experiment, all factors in the body of broilers infected with the virus increased to varying degrees and were significantly higher than those in the control group, indicating the presence of an inflammatory reaction in the body of broilers infected with the virus. Compared with the IBV group, the treatment group maintained a high level of TLR7 expression, and the expression of MyD88 gradually increased during treatment. Studies have shown that MyD88 can enhance the expression of IRF7 and IL-10, consistent with the results of this experiment (Ernst et al. Citation2019; Mishima et al. Citation2019). IRF7, which regulates the IFN-I expression, is a key transcription factor and plays an important antiviral role (Zhou et al. Citation2015; Benson et al. Citation2022; Tang et al. Citation2022; Xiang et al. Citation2022). The IRF7 results revealed that IBV infection results in the expression of a large amount of IFN-I in the broilers for a nonspecific antiviral response at the early stage of infection, and the addition of AAEO could enhance this response. The immune function of IBV-infected broilers was impaired in the later stage of the infection, and they could not express IFN-I normally, whereas the treated broilers still exerted a strong expression ability. This suggests that AAEO nasal drops can enhance the ability of broilers to secrete IFN-I. NF-κB is a transcriptional regulatory factor that induces the activation of many factors, including IL-6, IL-8, and TNF-α, which play important roles in the immune response (Cheng et al. Citation2017; Wang et al. Citation2018; Peng et al. Citation2019; Shi et al. Citation2020; Yiming et al. Citation2020). We speculated that the high levels of IL-6 and IL-8 were maintained and that TNF-α gradually increased and was significantly higher in the IBV group than in the other groups, which was related to the gradual rise of NF-κB p65, consistent with the clinical symptoms of tracheal and lung bleeding and inflammation of broilers.
Over the course of the AAEO treatment, the expression of NF-κB p65 remained relatively stable, and the levels of IL-6, IL-8, and TNF-α also decreased with increasing IL-10 anti-inflammatory factor. IFN-γ exerts antiviral, antitumour, and immunomodulatory functions, which can improve the efficiency of antigen presentation, participate in the maturation and differentiation of lymphocytes, enhance the vitality of natural killer cells, and regulate the secretion of antibodies by B cells. Many factors affect the expression of IFN-γ (Fenimore and A Citation2016; Kak et al. Citation2018). According to the experimental results, AAEO maintains the IFN-γ level in the body at a relatively stable state to ensure that it can continue to play a role in immune regulation. However, the specific mechanism requires further exploration. We hypothesize that the administration of AAEO nasal drops to normal broilers can moderately increase TLR7, MyD88, NF-κB, IFN-γ, and IL-10 levels, thus enhancing the immunity of the broilers. Confirmation through additional experiments is warranted.
Conclusion
Our data suggest that the intranasal administration of AAEO attenuates the inflammatory response mainly by reducing the expression of key factors in the NF-κB signalling pathway and increases the levels of antibodies and IFNs by enhancing the expression of TLR7 in the MyD88-dependent signalling pathway. Eventually, these effects can neutralize the virus, treat organ damage, and reduce mortality. Our results also indicate that AAEO nasal drops can increase the levels of the above factors in broilers without IBV infection, thus enhancing immunity. Further studies regarding these effects on the composition and mechanism of AAEO are warranted.
Abbreviations
AAEO, Artemisia argyi essential oil; IB, infectious bronchitis; IBV, infectious bronchitis virus; EID50, median infective dose; DMSO, dimethyl sulfoxide; RT-qPCR, real-time fluorescence quantitative polymerase chain reaction; SPF, specific pathogen free; DAB, diaminobenzidine; OD, optical density; NK, natural killer.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ahameethunisa AR, Hopper W. 2010. Antibacterial activity of Artemisia nilagirica leaf extracts against clinical and phytopathogenic bacteria. BMC Complement Altern Med. 10:6. doi:10.1186/1472-6882-10-6.
- Ahameethunisa AR, Hopper W. 2012. In vitro antimicrobial activity on clinical microbial strains and antioxidant properties of Artemisia parviflora. Ann Clin Microbiol Antimicrob. 11:30. doi:10.1186/1476-0711-11-30.
- Amanollahi R, Asasi K, Abdi-Hachesoo B. 2020. Effect of Newcastle disease and infectious bronchitis live vaccines on the immune system and production parameters of experimentally infected broiler chickens with H9N2 avian influenza. Comp Immunol Microbiol Infect Dis. 71:101492. doi:10.1016/j.cimid.2020.101492.
- Annamalai A, Ramakrishnan S, Sachan S, Sharma BK, Anand KB, Kumar V, Badasara SK, Kumar A, Saravanan BC, Krishnaswamy N. 2015. Administration of TLR7 agonist, resiquimod, in different types of chicken induces a mixed Th1 and Th2 response in the peripheral blood mononuclear cells. Res Vet Sci. 100:105–108. doi:10.1016/j.rvsc.2015.04.007.
- Awad F, Baylis M, Ganapathy K. 2019. Detection of variant infectious bronchitis viruses in broiler flocks in Libya. Int J Vet Sci Med. 2:78–82. doi:10.1016/j.ijvsm.2014.01.001.
- Awad F, Chhabra R, Baylis M, Ganapathy K. 2014. An overview of infectious bronchitis virus in chickens. Worlds Poult Sci J. 70:375–384. doi:10.1017/S0043933914000385.
- Benson LN, Liu Y, Deck K, Mora C, Mu S. 2022. IFN-γ contributes to the immune mechanisms of hypertension. Kidney360. 3(3):2164–2173. doi:10.34067/KID.0001292022.
- Cavanagh D. 2007. Coronavirus avian infectious bronchitis virus. Vet Res. 38:281–297. doi:10.1051/vetres:2006055.
- Chen LL, Zhang HJ, Chao J, Liu JF. 2017. Essential oil of Artemisia argyi suppresses inflammatory responses by inhibiting JAK/STATs activation. J Ethnopharmacol. 204:107–117. doi:10.1016/j.jep.2017.04.017.
- Cheng P, Wang T, Li W, Muhammad I, Wang H, Sun X, Yang Y, Li J, Xiao T, Zhang X. 2017. Baicalin alleviates lipopolysaccharide-induced liver inflammation in chicken by suppressing TLR4-mediated NF-κB pathway. Front Pharmacol. 8:547. doi:10.3389/fphar.2017.00547.
- Choi YG, Yeo S, Kim SH, Lim S. 2012. Anti-inflammatory changes of gene expression by Artemisia iwayomogi in the LPS-stimulated human gingival fibroblast: microarray analysis. Arch Pharmacal Res. 35:549–563. doi:10.1007/s12272-012-0319-0.
- Chou W, Chen C, Vuong CN, Abi-Ghanem D, Waghela SD, Mwangi W, Bielke LR, Hargis BM, Berghman LR. 2016. Significant mucosal sIgA production after a single oral or parenteral administration using in vivo CD40 targeting in the chicken. Res Vet Sci. 108:112–115. doi:10.1016/j.rvsc.2016.08.013.
- Chung MS. 2017. Antiviral activities of Artemisia princeps var. orientalis essential oil and its α-thujone against norovirus surrogates. Food Sci Biotechnol. 26:1457–1461. doi:10.1007/s10068-017-0158-3.
- Commission CP. 2009. Pharmacopoeia of People’s Republic of China 2005 edition. Beijing, People’s Republic of China: Chemical Industry Press.
- Cook JK, Jackwood M, Jones RC. 2012. The long view: 40 years of infectious bronchitis research. Avian Pathol. 41:239–250. doi:10.1080/03079457.2012.680432.
- Ernst O, Glucksam-Galnoy Y, Athamna M, Ben-Dror I, Ben-Arosh H, Levy-Rimler G, Fraser I, Zor T. 2019. The cAMP pathway amplifies early MyD88-dependent and type I interferon-independent LPS-induced interleukin-10 expression in mouse macrophages. Mediat Inflamm. 2019:3451461. doi:10.1155/2019/3451461.
- Fenimore J, A YH. 2016. Regulation of IFN-gamma expression. Adv Exp Med Biol. 941:1–19. doi:10.1007/978-94-024-0921-5_1.
- Ge Y, Wang Z, Xiong Y, Huang X, Mei Z, Hong Z. 2016. Anti-inflammatory and blood stasis activities of essential oil extracted from Artemisia argyi leaf in animals. J Nat Med. 70:531–538. doi:10.1007/s11418-016-0972-6.
- Hu J, McDougald LR. 2003. Direct lateral transmission of Histomonas meleagridis in turkeys. Avian Dis. 47:489–492. doi:10.1637/0005-2086(2003)047[0489:DLTOHM]2.0.CO;2.
- Kak G, Raza M, Tiwari BK. 2018. Interferon-gamma (IFN-γ): exploring its implications in infectious diseases. Biomol Concepts. 9:64–79. doi:10.1515/bmc-2018-0007.
- Li N, Mao Y, Deng C, Zhang X. 2008. Separation and identification of volatile constituents in Artemisia argyi flowers by GC-MS with SPME and steam distillation. J Chromatogr Sci. 46:401–405. doi:10.1093/chromsci/46.5.401.
- Lu XF, Zhou Y, Zhang J, Ren YP. 2018. Determination of fluoroquinolones in cattle manure-based biogas residue by ultrasonic-enhanced microwave-assisted extraction followed by online solid phase extraction-ultra-high performance liquid chromatography-tandem mass spectrometry. J Chromatogr B. 1086:166–175. doi:10.1016/j.jchromb.2018.01.029.
- Meneses R, Ocazionez RE, Martinez JR, Stashenko EE. 2009. Inhibitory effect of essential oils obtained from plants grown in Colombia on yellow fever virus replication in vitro. Ann Clin Microbiol Antimicrob. 8:8. doi:10.1186/1476-0711-8-8.
- Mishima Y, Oka A, Liu B, Herzog JW, Eun CS, Fan TJ, Bulik-Sullivan E, Carroll IM, Hansen JJ, Chen L, et al. 2019. Microbiota maintain colonic homeostasis by activating TLR2/MyD88/PI3K signaling in IL-10-producing regulatory B cells. J Clin Invest. 129:3702–3716. doi:10.1172/JCI93820.
- Ng SC, Lam YT, Tsoi KK, Chan FK, Sung JJ, Wu JC. 2013. Systematic review: the efficacy of herbal therapy in inflammatory bowel disease. Aliment Pharmacol Ther. 38:854–863. doi:10.1111/apt.12464.
- Peng LY, Yuan M, Song K, Yu JL, Li JH, Huang JN, Yi PF, Fu BD, Shen HQ. 2019. Baicalin alleviated APEC-induced acute lung injury in chicken by inhibiting NF-κB pathway activation. Int Immunopharmacol. 72:467–472. doi:10.1016/j.intimp.2019.04.046.
- Saddi M, Sanna A, Cottiglia F, Chisu L, Casu L, Bonsignore L, De Logu A. 2007. Antiherpevirus activity of Artemisia arborescens essential oil and inhibition of lateral diffusion in Vero cells. Ann Clin Microbiol Antimicrob. 6:10. doi:10.1186/1476-0711-6-10.
- Saganuwan A. 2011. A modified arithmetical method of Reed and Muench for determination of a relatively ideal median lethal dose (LD50). Afr J Pharm Pharmacol. 5, 1543–1546. doi:10.5897/AJPP11.393.
- Shi X, Wang W, Zheng S, Zhang Q, Xu S. 2020. Selenomethionine relieves inflammation in the chicken trachea caused by LPS though inhibiting the NF-κB pathway. Biol Trace Elem Res. 194:525–535. doi:10.1007/s12011-019-01789-1.
- Tang X, Qi J, Sun L, Zhao J, Zhang G, Zhao Y. 2022. Pathological effect of different avian infectious bronchitis virus strains on the bursa of Fabricius of chickens. Avian Pathol. 51:339–348. doi:10.1080/03079457.2022.2063710.
- Wan J, Guo Q, Fu J. 2013. Study on chronic toxicity in rats by atomize inhalation of volatile oil from Artemisia Argyi (in Chinese). Asia-Pacific Tradit Med. 9:15–18.
- Wang W, Chen M, Jin X, Li X, Yang Z, Lin H, Xu S. 2018. H2s induces Th1/Th2 imbalance with triggered NF-κB pathway to exacerbate LPS-induce chicken pneumonia response. Chemosphere. 208:241–246. doi:10.1016/j.chemosphere.2018.05.152.
- Wenqiang G, Shufen L, Ruixiang Y, Yanfeng H. 2006. Comparison of composition and antifungal activity ofArtemisia argyi lévl. et vantinflorescence essential oil extracted by hydrodistillation and supercritical carbon dioxide. Nat Prod Res. 20:992–998. doi:10.1080/14786410600921599.
- Xiang C, Yang Z, Xiong T, Wang T, Yang J, Huang M, Liu D, Chen R. 2022. Avian IRF1 and IRF7 play overlapping and distinct roles in regulating IFN-dependent and -independent antiviral responses to duck tembusu virus infection. Viruses-Basel. 14:1506. doi: 10.3390/v14071506.
- Xuelin Z, Xinwang C, Yiming W. 2021. Research progress on chemical constituents and pharmacological activities of volatile oil of Aiye(Artemisia argyi)(in Chinese). Chinese Arch Tradit Chin Med. 39:111–118.
- Yiming Z, Qingqing L, Hang Y, Yahong M, Shu L. 2020. Selenium deficiency causes immune damage by activating the DUSP1/NF-κB pathway and endoplasmic reticulum stress in chicken spleen. Food Funct. 11:6467–6475. doi:10.1039/D0FO00394H.
- Yin-Ji J, Li-Ben C, Xiao-Qing L, Xiao-Yu F. 2012. A novel rapid paraffin section technique for pathological analyses. Prog Vet Med. 33:211–213. doi: 10.16437/j.cnki.1007-5038.2012.12.050.
- Yun C, Jung Y, Chun W, Yang B, Ryu J, Lim C, Kim JH, Kim H, Cho SI. 2016. Anti-inflammatory effects of artemisia leaf extract in mice with contact dermatitis in vitro and in vivo. Mediat Inflamm. 2016:8027537.
- Zhang X, Liao K, Chen S, Yan K, Du X, Zhang C, Guo M, Wu Y. 2020. Evaluation of the reproductive system development and egg-laying performance of hens infected with TW I-type infectious bronchitis virus. Vet Res. 51:95. doi:10.1186/s13567-020-00819-4.
- Zhou Q, Lavorgna A, Bowman M, Hiscott J, Harhaj EW. 2015. Aryl hydrocarbon receptor interacting protein targets IRF7 to suppress antiviral signaling and the induction of type I interferon. J Biol Chem. 290:14729–14739. doi:10.1074/jbc.M114.633065.