ABSTRACT
Anaesthetics in aquaculture serve as a stress avoidance mechanism, mitigating stress-related impacts during fish handling and transportation. This study assessed the efficacy of sodium bicarbonate and clove stem powder as anaesthetic agents for Nile Tilapia juveniles. Four hundred and twenty uniform O. niloticus juveniles (5 ± 0.5 g, 74 ± 5 mm) were exposed to varying concentrations of sodium bicarbonate (10, 15, 20, 25, 30, 35, 40 g/L) and clove stem powder (0, 1, 3, 5, 7, 10, 15 g/L) in 20 L transparent plastic tanks. The results showed an inverse relationship between anaesthetic concentration and induction time, a direct relationship with recovery time, and an inverse correlation between induction and recovery times for both agents (P < 0.001). The Appropriate concentrations were determined as 25 g/L for sodium bicarbonate and 10 g/L for clove stem powder. At these levels, induction and recovery times met ideal criteria (3-5 min induction, 5-10 min recovery), with fish exhibiting normal behavior and 100% survival after a week of monitoring.
1. Introduction
Aquaculture, recognized as a crucial food production system globally, has garnered considerable attention for its role in addressing food nutrition and security. In Africa, the contribution to aquaculture remains comparatively low, notwithstanding its substantial potential for economic development, enhanced food security, and decreased unemployment (Langi et al. Citation2024). In southern Africa countries, noteworthy growth in the sector has been observed in countries including Zambia, Malawi, South Africa and Mozambique (Hasimuna et al. Citation2019; Adeleke et al. Citation2021; Muhala et al. Citation2021; Hasimuna et al. Citation2023; Mphande et al. Citation2023). Throughout aquaculture practices, routine activities are conducted, leading to fish handling, and consequently, anesthesia is frequently employed as a means to alleviate stress and promote animal welfare (Hasimuna et al. Citation2021).
Anaesthesia is usually understood to be a biological state brought on by an external substance that, depending on how it is used, causes a loss of sensation by depressing the nervous system (Ackerman et al. Citation2006; Javahery et al. Citation2012). Anaesthetic agents may consist of either chemical or natural ingredients that can result in a temporary loss of an organism’s awareness or perception. The use of anaesthesia is common in the aquaculture sector and is an inevitable aspect because it helps in lowering fish stress during transportation, weighing, artificial reproduction, tagging, counting, surgical procedures, and vaccination among others (Hasimuna et al. Citation2020a; Siavwapa et al. Citation2022; Mphande et al. Citation2023). These routine handling activities can cause stress to the fish and physical injuries, which can significantly negatively affect fish stock (Hasimuna et al. Citation2020b; Mphande et al. Citation2023). They can cause a reduction in the egg size and number, a reduction in the sperm count, reduced fish spawning, a low survival rate of the offspring and low immunity making the fish more susceptible to pathogens that could infest them and cause disease outbreaks (Matin et al. Citation2010; Hasimuna et al. Citation2020b). When the stress levels are high, it can lead to fish mortality resulting in a reduction in the degree of success of an aquaculture enterprise (Sneddon Citation2012; Opiyo et al. Citation2013; Githukia et al. Citation2016; Hasimuna et al. Citation2020a).
Anaesthetic substances can be administered to the aquatic organism through an injection or immersion. However, the most common route of anaesthetic delivery to fish is by immersion in water that contains the desired concentration of the anaesthesia (Skår et al. Citation2017; Schroeder et al. Citation2021). The drug will enter the fish through the gills and circulate to the brain through the arterial blood and the substance or its metabolites are eliminated through the skin and the gills immediately after the fish is submerged in freshwater (Ross and Ross Citation2008). An effective anaesthetic should cause loss of sensation as soon as possible and with little hyperactivity or tension. It should be simple to administer and keep the animal in the ideal condition and recovery should occur quickly once the animal is taken out of the anaesthesia.
To ensure a large margin of safety, the anaesthetic should be effective at low dosages and the hazardous dose should substantially exceed the effective amount (Coyle et al. Citation2004; Dheeran et al., Citation2022). Stetter (Citation2001), Akinrotimi et al. (Citation2015) and Hasimuna et al. (Citation2021) describe ideal dosages for anaesthetic agents to be able to instigate a rapid induction into anaesthesia in 3–5 min and with a corresponding recovery time of 5–10 min. Several anaesthetics are readily available and permitted for use in modern-day aquaculture. These include clove oil and powder, sodium bicarbonate, carbon dioxide (CO2), metomidate, benzocaine, tricaine methanesulphonate (MS-222), 2-phenoxyethanol, and quinaldine (Bowser Citation2001; Palic´ et al. Citation2006; Gabriel et al. Citation2020; Hasimuna et al. Citation2020a; Rairat et al. Citation2021; Liu et al. Citation2022; Siavwapa et al. Citation2022).
Sodium bicarbonate (NaHCO3) is soluble in water and slowly releases carbon dioxide (CO2) which is one of the anaesthetics for fish (Bowser Citation2001; Coyle et al. Citation2004; Altun et al. Citation2009; Opiyo et al. Citation2013; Hasimuna et al. Citation2021). NaHCO3 has been used successfully as an anaesthetic in juvenile African catfish (Clarias gariepinus), common carp (Cyprinus carpio), Rainbow trout (Oncorhynchus mykiss), Mozambique tilapia (Oreochromis mossambicus) and Greenhead tilapia juveniles (Oreochromis macrochir) both in warm and cold-water conditions (Booke et al. Citation1978; Keene et al. Citation1998; Altun et al. Citation2009; Gabriel et al. Citation2020; Hasimuna et al. Citation2021). Opiyo et al. (Citation2013) assert that NaHCO3 is inexpensive, easily accessible, and is classified as a low regulatory priority substance by the Food and Drug Administration (FDA). Its recommended dose is dependent on species and the required final product. Moreover, it is considered safe with necessary precautions just like any other anaesthetic.
On the other hand, clove powder is derived from the dry flower buds, stalks and stems of the clove plant (Eugenia caryophyllata). It is partially soluble in water and clove products are found in herbal supermarkets and a few local markets. They are typically used to flavour food (as a spice) and as a topical therapy for toothaches, the common cold, coughing, and inflammation of the mouth and throat (Okey et al. Citation2018). Clove extracts have become more widely used as fish anaesthetics in recent years and their effectiveness has been tested on the common Iranian fish roach (Rutilus rutilus) (Akinrotimi et al. Citation2015; Okey et al. Citation2018). Some researchers have worked on plant extracts as natural anaesthetics because they are inexpensive, safer and more effective at lower concentrations when compared with synthetic anaesthetics and clove powder is not an exception (Akinrotimi et al. Citation2015; Okey et al., Citation2022).
Despite several studies reporting the anaesthetic effects of Sodium bicarbonate and Clove powder on different fish species, there are no published studies on their anaesthetic effect on Oreochromis niloticus juveniles. For this reason, the study's main objective was to: (a). compare the anaesthetic effectiveness of NaHCO3 and clove powder on O. niloticus juveniles; (b). assess the juvenile fish's subsequent induction and recovery time; and, (c) determine the survival rate of the juvenile O. niloticus.
2. Materials and methods
2.1. Study site
This study was conducted at Kriskon Farms and General Dealers in Chongwe District in Lusaka Province, Zambia. Kriskon Farms is located at farm 87A subdivision 20D, Great East Road. Kriskon Farms is an emerging Zambian company engaged in fingerling and grow-out fish production .
2.2. Experimental fish
A total of four hundred and twenty (420) sex-reversed juveniles of O. niloticus with an average weight of 5 ± 0.50 g were obtained from hapas at Kriskon Farms and General Dealers in Chongwe, Zambia. A scoop net made of a 2 × 2 mm mesh size sieve supported by a metal frame mounted on a plastic handle was used to transfer fish into a plastic bucket (100 L). The same scoop net was used to transfer the fish from the 100 L plastic bucket to the anaesthetic chambers (20 L plastic buckets). Ten juveniles of O. niloticus were randomly assigned to each anaesthetic chamber. Before undertaking the experiment, the sample fish were fasted for 24 h to allow for gut evacuation.
2.3. Anaesthetic agents
The efficacy of two anaesthetics – Sodium bicarbonate and Clove powder was assessed in this study. Sodium bicarbonate used in this study is a white powder commonly called ‘Chapa Mandashi’ baking powder. It contains wheat cereal, sodium phosphate, and bicarbonate of soda and is manufactured by Kapa Oil Refinery. The clove powder used was made from the stems of the clove plant (Syzygium aromaticum) and it was ground and packaged by Spice Land CC. Clove powder contains phenolic compounds with eugenol as the primary bioactive compound. Both anaesthetics were purchased from a local market in their powdered forms.
2.4. Water analysis
Water quality parameters such as temperature, pH, dissolved oxygen, and ammonia were determined using a water testing kit (SUNPU TEST ZL 2010 2 0681385. X – BEIJING SUNPU BIOCHEM. TECH. CO., LTD.). The parameters were taken at the beginning of the study to confirm that the parameters were in the acceptable range for fish. The water was collected in sampling bottles from each tank and the reagents for the different parameters were added to the bottles as recommended in the water testing kit manual.
2.5. Experimental designs
A total of twenty-one (21) transparent plastic tanks were set up in a complete randomized design (CRD) for each anaesthetic agent. Seven sodium bicarbonate and clove treatments were replicated three times having ten (10) juvenile fish of similar weights (5 ± 0.50 g) in each replicate tank. The treatment concentrations of Sodium bicarbonate were 10, 15, 20, 25, 30, 35 and 40 g/L while that of clove powder was 0, 1, 3, 5, 7, 10, and 15 g/ L. The concentrations dissolved were adapted from previous studies tested in other tilapia species (Opiyo et al. Citation2013; Avillanosa and Caipang Citation2019; Hasimuna et al. Citation2020a). Upon full anaesthesia, fish were transferred into clean water for the recovery trial.
2.6. Anaesthetic stages
Induction and recovery times were recorded using a stopwatch at three anaesthetic stages. Descriptions of the three anaesthetic stages are presented in .
Table 1. Different stages of anaesthesia for Nile Tilapia (O. niloticus).
2.7. Data analysis
One-way Analysis of Variance (ANOVA) was used to test mean differences in induction and recovery times for each anaesthetic agent’s treatment concentrations. The Fisher’s Least Significant Difference (LSD) method was used for multiple comparisons to ascertain where the differences were. The statistical significance level for all tests was set at 5%. The entire analysis was conducted using R Statistical Software version 4.1.2 (R Core Team Citation2021).
4. Results and discussion
4.1. Sodium bicarbonate
Nile Tilapia (Oreochromis niloticus) is the most farmed fish species globally due to the species’ fast growth, high resistance to stressful conditions and disease, capability to reproduce in captivity, trophic plasticity, and ability to tolerate various environmental conditions traits (Maulu et al. Citation2021; El-Sayed and Fitzsimmons Citation2023). In Zambia, O. niloticus is found in some hatcheries and is the main fish species farmed in cages on Lake Kariba (Hasimuna et al., Citation2019; Hasimuna et al. Citation2023; Mphande et al. Citation2023). Therefore, this study used different concentrations of Sodium Bicarbonate (Baking Soda) and Clove Stem Powder (Syzygium aromaticum) to unravel their anaesthetic efficacy on O. niloticus Juveniles.
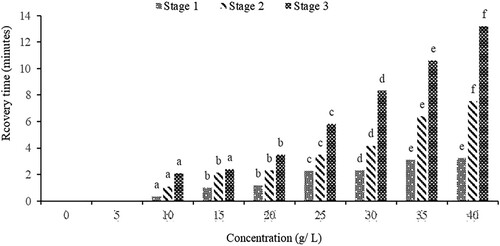
When sedating O. niloticus Juveniles using sodium bicarbonate, a decreasing trend with increasing concentration was observed across all stages between induction time and concentration as indicated in . This can be credited to a faster reduction in the amount of dissolved oxygen in the water and subsequently blood due to the carbon dioxide produced by the sodium bicarbonate as the quantities increase (Siavwapa et al. Citation2022).
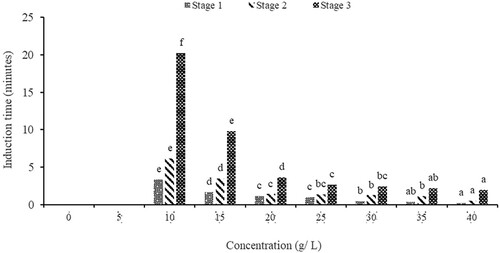
Figure 2. Anaesthetic effects of baking soda on the induction time of O. niloticus (different letters on the same anaesthetic stage show significant differences in induction time).
Sodium bicarbonate leads to an increase in the concentration of carbonic acid (H2CO3) by releasing carbon dioxide, which subsequently raises the water's acidity and lowers its pH (Altun et al. Citation2009; Avillanosa and Caipang Citation2019). This increase in concentration of carbon dioxide disturbs the biological and energy metabolism in fish, resulting in immobilization (Mota et al. Citation2019). However, no mortalities were recorded during and a week after the experiments. These findings corroborate with previous studies on tilapia species conducted in developing countries (Altun et al. Citation2009; Avillanosa and Caipang Citation2019; Githukia et al. Citation2016; Hasimuna et al. Citation2020a, Citation2021; Siavwapa et al. Citation2022). On the other hand, the longer time taken for induction to occur in lower concentrations is due to less carbon dioxide released into the water and picked up by the gills into the bloodstream than at higher concentrations (Opiyo et al. Citation2013).
On the effect of sodium bicarbonate on recovery time (), there was a positive relationship between concentration and recovery time. In other words, the increase in concentration causes an increase in recovery time taken by O. niloticus Juveniles when put in water without the anaesthetic substance. This phenomenon can be attributed to the increased accumulation of carbon dioxide in the blood of the O. niloticus Juveniles with increasing concentration necessitating an increase in time for carbon dioxide to be fully removed from the blood when put in water without sodium carbonate. Furthermore, the transportation of blood with higher levels of carbon dioxide to different organs of the fish requires more time for this gas to be eliminated from these organs and bring the fish to normalcy, hence, more time is needed for recovery to occur with increasing concentration. This is consistent with the findings of other earlier studies (Olufayo and Ojo Citation2018; Avillanosa and Caipang Citation2019; Gabriel et al. Citation2020; Hasimuna et al. Citation2021; Siavwapa et al. Citation2022). Therefore, this study has shown that higher exposure to sodium bicarbonate leads to a higher concentration of carbon dioxide in the fish's body, which takes longer to be fully replaced by dissolved oxygen once placed in water without the anaesthetic agent. Based on the findings of the current study, it can be suggested that the appropriate concentration range for handling O. niloticus Juveniles is between 20 and 30 g/L while the most optimal concentration is 25 g/L because this causes the induction within 4 min and recovery of less than 6 min. This contrasts with the recommendation of 45 g/L by Opiyo et al. (Citation2013) Nile tilapia fingerlings within the size ranges of 2–27 g compared to 5 ± 0.5 g used in this study. According to Anju et al. (Citation2015), Metin et al. (Citation2015) and Priborsky and Velisek (Citation2018) indicated fish of different size reacts differently to anaesthetic effect i.e. small size fish require smaller concentration for induction and recovery time and the opposite is true. This relates to the differences in metabolic rates, body surface area, and respiratory rates between small and large fish. Smaller fish typically have higher metabolic rates and respiratory rates relative to their body size, which could lead to faster uptake and elimination of the anaesthetic agent (Rubio-Gracia et al. Citation2020). In addition, this study does not agree with the optimal concentration of sodium bicarbonate suggested by Hasimuna et al. (Citation2021) of 20 g/L, and the variance is attributed to the differences in species investigated. In the other study, anaesthetic effects were investigated on O. macrochir juveniles compared to O. niloticus Juveniles used in this study. This supports the literature that different species will respond differently to anaesthetic substances (Okey et al. Citation2018; Siavwapa et al. Citation2022; Gajutos and Gajutos Citation2023).
4.2. Clove powder
In the present study, accessibility to clove stem powder was a challenge and the price was relatively higher than sodium bicarbonate. This can be attributed to its use in sectors like food processing where it is used as a spice making its availability challenging (Mphande et al. Citation2023). However, regarding the induction of O. niloticus Juveniles using Clove stem powder, the induction time reduced with increasing concentration showing a clear inverse relationship (). In this study, a concentration of 10 g/L is recommended for use because it causes a quick induction of 5.07 and a timely recovery of 10.30 min making this concentration optimal for handling O. niloticus fingerlings which is consistent with the recommendation made by Hasimuna et al. (Citation2021).
Figure 4. Anaesthetic effects of clove powder on induction times of O. niloticus (different letters on the same anaesthetic stage show significant differences in induction time).
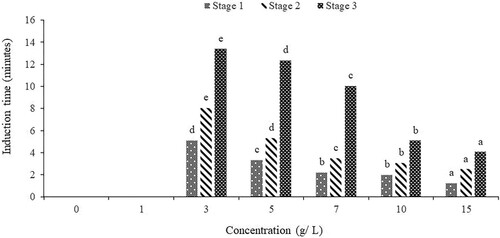
The observed anaesthetic effect of clove powder in this study can be attributed to the effect of its chemical constituents on the species, mainly Eugenol its bioactive compound (Neveu et al. Citation2010; Cortés-Rojas et al. Citation2014). When fish were exposed to higher concentrations of clove powder, they received a greater dosage of eugenol, leading to a more pronounced and rapid anaesthetic effect (De Oliveira et al. Citation2019). It interferes with the fish's nervous system and disrupts neural transmission causing the anaesthetic effect (Summerfelt Citation1990; Mylonas et al. Citation2005; Okey et al. Citation2018). Eugenol acts as a neurotoxin, targeting the fish's sensory neurons and causing a depression of nerve impulses (Okey et al. Citation2018; Akbar et al. Citation2021). As a result, the fish experience sedation, and a loss of consciousness, becoming less responsive to external stimuli (Okey et al. Citation2018).
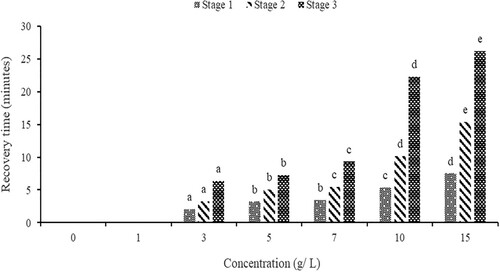
The fish are exposed to a higher concentration of eugenol at higher concentrations of clove stem powder, which leads to a faster and more potent anaesthetic effect than at lower concentrations. The increased dosage of eugenol accelerates the depression of nerve impulses, causing the fish to enter a state of anaesthesia more rapidly than at lower concentrations (Mylonas et al. Citation2005; Hasimuna et al. Citation2021). The significant effect of clove concentration indicates that concentration plays a key role in the time to induct and recover. This corroborates with Simões et al. (Citation2011) and Yostawonkul et al. (Citation2019) who reported similar results on Nile tilapia. In addition, similar results were reported by other studies (e.g. Sudagara et al. Citation2009; Bownik Citation2015; Okey et al. Citation2018; De Oliveira et al. Citation2019; Caipang et al. Citation2021; Hasimuna et al. Citation2021; Jia et al. Citation2022) in different aquaculture species. In contrast, clove powder was found not to induce fish to complete anaesthesia faster on Clarias gariepinus fingerlings, with only partial central nervous system (CNS) anaesthetic behaviour expressed by catfish, such as accelerated opercular movements, partial loss of reaction to external stimuli, and loss of equilibrium (Okey et al. Citation2018). This can be partly attributed to what was observed during the execution of the current study where, unlike sodium bicarbonate, clove stem powder failed to completely dissolve in water as particles settled at the bottom of the anaesthetic chambers. This may have increased the induction time by failing to effectively and timely cause sedation. Furthermore, it was observed that clove stem powder was sticking to the gills of the fish due to its inability to completely dissolve thereby affecting the recovery time. The slow recovery time may be due to the continued release of the active agent of clove stem powder stuck on the gills. In this study, a concentration of 10 g/L is recommended for use because it causes a quick induction of 5.07 and a timely recovery of 10.30 min making this concentration optimal for handling O. niloticus fingerlings. The longer recovery time () was recorded in this study when O. niloticus was exposed to clove stem powder sticking to the gills of the fish.
Figure 5. Anaesthetic effects of clove powder on the recovery time of O. niloticus (different letters on the same anaesthetic stage show significant differences in recovery time).
This study has revealed that both anaesthetics are effective anaesthetic agents for O. niloticus Juveniles because they were able to sedate fish and no mortality was recorded during and after one-week post-experiment. To add on, they meet the criteria of quick induction and longer recovery time to facilitate desired manipulation (Priborsky and Velisek Citation2018; Hasimuna et al., Citation2020a, Citation2021). Even though these two anaesthetics are part of human food ingredients, they are environmentally friendly safe to both consumers and require no withdrawal period to consumers. Additionally, our findings are consistent with the findings of other studies (i.e. Opiyo et al. Citation2013; Akinrotimi et al. Citation2015; Hassan Citation2016; Hasimuna et al. Citation2021) who reported that clove and sodium bicarbonate can be used in aquaculture as anaesthetic substance, especially in developing countries where conventional anaesthetics are not readily available and expensive. The major drawback of clove powder especially the stem is its ability to turn the water brownish which may affect the normal observation of the various behavioural changes occurring in the fish thereby affecting the recording of the induction time.
5. Conclusion and recommendations
The efficacy of sodium bicarbonate and clove stem powder on O. niloticus juveniles has been investigated and this study revealed that these two agents are and can be used during routine handling procedures in fish farms. Additionally, 10 g/L and 25/L for clove stem powder and sodium bicarbonate have been established to be the appropriate concentrations for O. niloticus juveniles. The concentrations were able to bring the juveniles to induction and recovery and no mortalities were recorded during and after the experiment. In this study we recommend sodium bicarbonate more as a preferable anesthetic, considering its widespread availability and cost-effectiveness, particularly in many developing countries, including Zambia. We also recommend that further studies investigate the effect of temperature and pH on the efficacy of sodium bicarbonate and clove stem power on O. niloticus juveniles. Additionally, the effect of these anaesthetic agents on the haematological and biochemical properties of O. niloticus broodstock must be investigated.
Authors’ contribution
OJH: Conceptualization; OJH, OM, JM & KN: Supervision; MC & OJH: Resources and Funding acquisition; GM: Investigation; GM, HB, RCC, and JM: Methodology and Writing the original draft manuscript; MM: Data Curation; IM: Software and Formal Analysis; OJH & JM: Visualization; KN: Project administration; CJP, KS, VM, MC & OM: Validation and Review editing. All authors read the final manuscript and approved its submission to the journal for publication.
Acknowledgements
The authors would also like to thank the management of Kriskon Farms and General Dealers for providing support before, during, and after the experiment. We are indebted to the facilities and human resources provided to us during the study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data used to support the findings and conclusions of this study are contained in this article.
References
- Ackerman PA, Morgan JD, Iwama GD. 2006. Anaesthetics. https://ccac.ca/Documents/Standards/Guidelines/Add_PDFs/Fish_Anesthetics.pdf
- Adeleke B, Robertson-Andersson D, Moodley G, Taylor S. 2021. Aquaculture in Africa: A Comparative review of Egypt, Nigeria, and Uganda vis-à-vis South Africa. Rev Fish Sci Aquac. 29(2):167–197. doi:10.1080/23308249.2020.1795615.
- Akbar L, Juliandi B, Boediono A, Batubara I, Subangkit M. 2021. Effects of eugenol on memory performance, neurogenesis, and dendritic complexity of neurons in mice analyzed by behavioral tests and Golgi staining of brain tissue. J Stem Cells Regen Med. 17(1):35. doi:10.46582/jsrm.1701005.
- Akinrotimi OA, Gabriel UU, Edun OM. 2015. The efficacy of clove seed extracts as an anaesthetic agent and its effect on haematological parameters of African Catfish (Clarias Gariepinus). Int J Aquac Fish Sci. 1(2):042–047. doi:10.17352/2455-8400.000008.
- Altun T, Bilgin R, Danabaş D. 2009. Effects of sodium bicarbonate on anaesthesia of common carp (Cyprinus carpio L., 1758) juveniles. Turk J Fish Aquat Sci. 9(1). doi:10.4194/trjfas.2009.005.
- Anju TD, Solomon SG, Cheikyula JO. 2015. Effects of aqueous leaf extract of Tephropsia vogeli as a tranquillizer on the African catfish Heterobranchus longifilis val. (Pisces 1840). Am J Res Communicat. 3(6):45–59.
- Avillanosa AL, Caipang CMA. 2019. Use of sodium bicarbonate as an inexpensive general anesthetic for juvenile red tilapia hybrids. Int Aquat Re. 11(3):287–294. doi:10.1007/s40071-019-00235-1.
- Booke HE, Hollender B, Lutterbie G. 1978. Sodium bicarbonate, an inexpensive fish anaesthetic for field use. Prog Fish-Cult. doi:10.1577/1548-8659(1978)40[11:SBAIFA]2.0.CO;2.
- Bownik A. 2015. Clove essential oil from Eugenia caryophyllus induces anesthesia, alters swimming performance, heart functioning and decreases survival rate during recovery of Daphnia magna. Turk J Fish Aquat Sci. 15(1):157–166. doi:10.4194/1303-2712-v15_1_17.
- Bowser PR 2001. Anaesthetic options for fish. In Gleed RD and Ludders JW, editors. Recent advances in veterinary anaesthesia and analgesia: Companion animals. Ithaca, New York, USA: International Veterinary Information Service (www.ivis.org). http://www.ivis.org/
- Caipang CMA, Deocampo JE, Pakingking RV, Suharman I, Fenol JT, Onayan FB. 2021, November. Utilization of sodium bicarbonate as an anesthetic during routine husbandry activities in ornamental fish. In IOP Conference Series: Earth and Environmental Science (Vol. 934, No. 1, p. 012001). IOP Publishing. doi:10.1088/1755-1315/934/1/012001.
- Cortés-Rojas DF, de Souza CRF, Oliveira WP. 2014. Clove (Syzygium aromaticum): a precious spice. Asian Pac J Trop Biomed. 4(2):90–96. doi:10.1016/S2221-1691(14)60215-X.
- Coyle SD, Durborow RM, Tidwell JH. 2004. Anesthetics in aquaculture. Vol. 3900. Stoneville, TX: Southern Regional Aquaculture Center.
- De Oliveira CPB, Da Paixão Lemos CH, Vidal LVO, Couto RD, Pereira DSP, Copatti CE. 2019. Anaesthesia with eugenol in hybrid Amazon catfish (Pseudoplatystoma reticulatum× Leiarius marmoratus) handling: Biochemical and haematological responses. Aquaculture. 501:255–259. doi:10.1016/j.aquaculture.2018.11.046.
- Dheeran P, Nivas SN, Kumara R, Bhargavi SS, Chithira P, Seenivasan P, Roy U. 2022. Anaesthetics-efficacy to mitigate stress in aquaculture. J Exp Zool India. 26(1). doi:10.51470/jez.2023.26.1.891.
- El-Sayed AFM, Fitzsimmons K. 2023. From Africa to the world—The journey of Nile tilapia. Rev Aquac. 15:6–21. doi:10.1111/raq.12738.
- Gabriel NN, Erasmus VN, Namwoonde A. 2020. Effects of different fish sizes, temperatures, and concentration levels of sodium bicarbonate on anaesthesia in Mozambique tilapia (Oreochromis mossambicus). Aquaculture. 529:735716. doi:10.1016/j.aquaculture.2020.735716.
- Gajutos LJB, Gajutos AB. 2023. Anaesthetic effects of different concentrations of sodium bicarbonate on common carp (Cyprinus carpio). J Fish. 11(1):111206–111206. doi:10.17017/j.fish.425.
- Githukia CM, Kembenya EM, Opiyo MA. 2016. Anaesthetic effects of sodium bicarbonate at different concentrations on African Catfish (Clarias gariepinus) juveniles. J Aquacult Eng Fish Res. 2(3):151–158. doi:10.3153/JAEFR16017.
- Hasimuna OJ, Maulu S, Monde C, Mweemba M. 2019. Cage aquaculture production in Zambia: Assessment of opportunities and challenges on Lake Kariba, Siavonga district. Egypt J Aquat Res. 45(3):281–285. doi:10.1016/j.ejar.2019.06.007.
- Hasimuna OJ, Maulu S, Mphande J. 2020b. Aquaculture health management practices in Zambia: status, challenges and proposed biosecurity measures. J Aquacult Res Dev. 11(3):1–6.
- Hasimuna OJ, Maulu S, Nawanzi K, Lundu B, Mphande J, Phiri CJ, Kikamba E, Siankwilimba E, Siavwapa S, Chibesa M. 2023. Integrated agriculture-aquaculture as an alternative to improving small-scale fish production in Zambia. Front Sustain Food Syst. 7. doi:10.3389/fsufs.2023.1161121.
- Hasimuna OJ, Monde C, Bbole I, Maulu S, Chibesa M. 2021. The efficacy of sodium bicarbonate as an anaesthetic agent in Oreochromis macrochir juveniles. Sci African. 11:e00668. doi:10.1016/j.sciaf.2020.e00668.
- Hasimuna OJ, Monde C, Mweemba M, Nsonga A. 2020a. The anaesthetic effects of sodium bicarbonate (baking soda) on greenhead tilapia (Oreochromis macrochir, Boulenger 1912) broodstock. Egypt J Aquat Res. 46:195–199. doi:10.1016/j.ejar.2019.12.004.
- Hassan BR. 2016. Impact of clove and mustard as anesthetics on small common carp (Cyprinus carpio L.) (Doctoral dissertation, Council of the Faculty of Agricultural Sciences at the University of Sulaimani in Partial Fulfillment of the Requirements for the Degree of Master’s in Animal Production Fish Health Management by Bakhan Rafiq Hassan B. Sc. Animal production (2012), University of Sulaimani).
- Javahery S, Nekaubin H, Moradlu AH. 2012. Effect of anesthesia with clove oil if fish (review). Fish Physiol Biochem. 38:1545–1552. doi:10.1007/s10695-012-9682-5.
- Jia Y, Xie T, Gao Y, Qin H, Guan C. 2022. Anesthetics efficacy and physiological response of MS222 and clove oil in spotted knifejaw Oplegnathus punctatus. Aquacult Rep. 25:101201. doi:10.1016/j.aqrep.2022.101201.
- Keene JK, Noakes DLG, Moccia RD, Soto CD. 1998. The efficacy of clove oil as an anaesthetic for rainbow trout, Oncorhynchus mykiss (Walbaum). Aquac Res. 29:89–101. doi:10.1111/j.1365-2109.1998.tb01113.x.
- Langi S, Maulu S, Hasimuna OJ, Kapula VK, Tjipute M. 2024. Nutritional requirements and effect of culture conditions on the performance of the African catfish (Clarias gariepinus): a review. Cogent Food Agric. 10(1):2302642. doi:10.1080/23311932.2024.2302642.
- Liu Y, Zhou X-W, Ding H-T, Dong X-J, Zhang J-J, Zheng Y-C, Chen X-N, Cheng H-L, Ding Z-J, Xu J-H. 2022. Effects of Tricaine methanesulfonate (MS-222) on sedation and responses of yellow catfish (Pelteobagrus fulvidraco) subjected to simulated transportation stress. Aquaculture. 549:737789. doi:10.1016/j.aquaculture.2021.737789.
- Matin SMA, Hossain MA, Hashim MA. 2010. Clove oil anaesthesia in Singhi (Heteropneuses fossilis) and Lata (Channa punctatus) fish. Bangladesh Vet. 26(2):68–73. doi:10.3329/bvet.v26i2.4953.
- Maulu S, Hasimuna OJ, Mphande J, Munang’andu HM. 2021. Prevention and control of streptococcosis in tilapia culture: a systematic review. J Aquat Anim Health. 33(3):162–177. doi:10.1002/aah.10132.
- Metin S, Didinen IB, Kubilay A, Pala M, Aker I. 2015. Determination of Anesthetic Effects of Some Medicinal Plants on Rainbow Trout (Oncorhynchus mykiss Walbaum, 1792). J Limnol Freshw Fisheries Res. doi:10.17216/LimnoFish-5000099756.
- Mota VC, Nilsen TO, Gerwins J, Gallo M, Ytteborg E, Baeverfjord G, Kolarevic J, Summerfelt ST, Terjesen BF. 2019. The effects of carbon dioxide on growth performance, welfare, and health of Atlantic salmon post-smolt (Salmo salar) in recirculating aquaculture systems. Aquaculture. 498:578–586. doi:10.1016/j.aquaculture.2018.08.075.
- Mphande J, Hasimuna OJ, Kikamba E, Maulu S, Nawanzi K, Phiri D, Chibesa M, Siankwilimba E, Phiri CJ, Hampuwo BM, et al. 2023. Application of anaesthetics in fish hatcheries to promote broodstock and fish seed welfare in Zambia. Cogent Food Agricult. 9(1):2211845. doi:10.1080/23311932.2023.2211845.
- Muhala V, Rumieque A, Hasimuna OJ. 2021. Aquaculture production in Mozambique: approaches and practices by farmers in Gaza Province. Egypt J Aquat Res. 47(1):87–92. doi:10.1016/j.ejar.2020.11.004.
- Mylonas CC, Cardinaletti G, Sigelaki I, Polzonetti-Magni A. 2005. Comparative efficacy of clove oil and 2-phenoxyethanol as anesthetics in the aquaculture of European sea bass (Dicentrarchus labrax) and gilthead sea bream (Sparus aurata) at different temperatures. Aquaculture. 246(1-4):467–481. doi:10.1016/j.aquaculture.2005.02.046.
- Neveu V, Perez-Jiménez J, Vos F, Crespy V, du Chaffaut L, Mennen L, Knox C, Eisner R, Cruz J, Wishart D, Scalbert A. 2010. Phenol-Explorer: an online comprehensive database on polyphenol contents in foods. Database. 2010:bap024. doi:10.1093/database/bap024.
- Okey IB, Igiri MR, Ekpenyong JJ, Inya FU. 2022. Haematological and biochemical responses in African catfish, (Clarias gariepinus) juveniles immobilized with clove basil, (Ocimum gratissimum) powder. Anaesthetic. J Aquat Sci Oceanogr. 3(1):102.
- Okey IB, Keremah RI, Gabriel UU. 2018. The efficacy of clove (Eugenia caryophyllata) powder as anaesthesia on African catfishes (Clarias gariepinus and Heterobranchus bidorsalis) fingerlings. J Aquacult Marine Biol. 7(4):182–188. doi:10.15406/jamb.2018.07.00206.
- Olufayo MO, Ojo OM. 2018. Anaesthetic effects and haematological responses of heterobranchus bidorsalis juveniles exposed to clove oil. Int J Fish Aquat Stud. 10(9):116–121. doi:10.5897/IJFA2016.0590.
- Opiyo MA, Ogello EO, Charo-Karisa H. 2013. Effectiveness of Sodium Bicarbonate as an Anaesthetic for different sizes of Nile tilapia (Oreochromis niloticus L., 1758) Juveniles. Int J Aquat Sci. 4(2):14–22.
- Palic´ D, Herolt DM, Andreason CB, Menzel BW, Roth JW. 2006. Anaesthetic efficacy of tricaine methane sulfonate, metomidate and eugenol: Effects on plasma cortisol concentration and neutrophil function in fathead minnows, Pimephales promelas. Aquaculture. 254:675–685. doi:10.1016/j.aquaculture.2005.11.004.
- Priborsky J, Velisek J. 2018. A review of three commonly used fish anesthetics. Rev Fish Sci Aquacult. 26(4):417–442. doi:10.1080/23308249.2018.1442812.
- Rairat T, Chi Y, Chang S-K, Hsieh C-Y, Chuchird N, Chou C-C. 2021. Differential effects of aquatic anaesthetics on the pharmacokinetics of antibiotics: examples using florfenicol in Nile tilapia (Orechromis niloticus). J Fish Dis. 44(10):1579–1586. doi:10.1111/jfd.13480.
- R Core Team. 2021. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. https://www.R-project.org/.
- Ross L, Ross B. 2008. Anaesthetic and sedative techniques for aquatic animals, ISBN-13: 978- 1405149389, 240.
- Rubio-Gracia F, García-Berthou E, Guasch H, Zamora L, Vila-Gispert A. 2020. Size-related effects and the influence of metabolic traits and morphology on swimming performance in fish. Curr Zool. 66(5):493–503. doi:10.1093/cz/zoaa013.
- Schroeder P, Lloyd R, Mckimm R, Metslaar M, Navarro J, O`farrel M, Readman GD, Speilberg L, Mocho J-P. 2021. Anaesthesia of laboratory, aquaculture and ornamental fish: Proceedings of the first LASA-FVS Symposium. Lab Anim. 55(4):317–328. doi:10.1177/0023677221998403.
- Siavwapa S, Hasimuna OJ, Maulu S, Monde C. 2022. A comparative analysis of the anaesthetic effect of sodium bicarbonate (NaHCO3) on male and female three spotted tilapia (Oreochromis andersonii). J Appl Anim Res. 50(1):269–274. doi:10.1080/09712119.2022.2064478.
- Simões LN, Lombardi DC, Gomide A, Gomes LC. 2011. Efficacy of clove oil as an anesthetic in handling and transportation of Nile tilapia, Oreochromis niloticus (Actinopterygii: Cichlidae) juveniles. Zoologia (curitiba). 28:285–290. doi:10.1590/S1984-46702011000300001.
- Skår MW, Haugland GT, Powell MD, Wergeland HI, Samuelsen OB. 2017. Development of anaesthetic protocols for lumpfish (Cyclopterus lumpus L.): Effect of anaesthetic concentrations, sea water temperature and body weight. PLoS One. 12(7):e0179344. doi:10.1371/journal.pone.0179344.
- Sneddon LU. 2012. Clinical anesthesia and analgesia in fish. J Exot Pet. 21(1):32–43. doi:10.1053/j.jepm.2011.11.009.
- Stetter MD. 2001. Fish and amphibian anesthesia. Vet Clin N Am Small Anim Pract. 4(1):69–82. doi:10.1016/S1094-9194(17)30052-X.
- Sudagara M, Mohammadizarejabada A, Mazandarania R, Pooralimotlagha S. 2009. The efficacy of clove powder as an anesthetic and its effects on hematological parameters on roach (Rutilus rutilus). J Aquacult Feed Sci Nut. 1(1):1–5.
- Summerfelt RC. 1990. Anesthesia, surgery, and related techniques. Meth Fish Biol.
- Yostawonkul J, Kitiyodom S, Kaewmalun S, Suktham K, Nittayasut N, Khongkow M, Namdee K, Ruktanonchai UR, Rodkhum C, Pirarat N, Surassmo S. 2019. Bifunctional clove oil nanoparticles for anesthesia and anti-bacterial activity in Nile tilapia (Oreochromis niloticus). Aquaculture. 503:589–595. doi:10.1016/j.aquaculture.2018.12.058.