 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This study investigated the effect of clove essential oil used prior to stunning by cranial percussion and hypothermia on Nile tilapia (Oreochromis niloticus) welfare and fillet quality. A total of 100 tilapias were divided into four groups in a 2 × 2 factorial design, exploring two stunning methods with and without clove essential oil. Behavioral responses, glucose, lactate, pH, water holding capacity, color parameters, lipid and protein oxidation, and oxidative stress were analyzed. The application of clove essential oil resulted in 100% numbing in swimming and stimulus response behaviors, and 96% in balance and eye vestibule reflex variables. Significant reductions in glucose (12.12 mg dL−1) for hypothermia group (p = 0.001) and lactate (3.36 mmol L−1) for cranial percussion (p = 0.004), 0.028 nmol lipid oxidation in hypothermia treatment (p = 0.009), and 0.134 nmol carbonyls/mg protein (p = 0.001) were observed. There was a reduction in luminosity (p = 0.010) and red intensity (p = 0.001) in the group of fish fillet that received cranial percussion, however, there was no difference in the group stunned by hypothermia. Clove essential oil demonstrates effectiveness in pre-slaughter stress mitigation, influencing the antioxidant system positively. Its usage in pre-slaughter protocols could contribute to improved fillet quality, presenting a promising alternative for enhancing tilapia welfare during slaughter.
1. Introduction
Once fish are sentient beings, capable of feeling fear, pain, and suffering (Brown Citation2015), the moment leading up to fish slaughter is gaining attention from all parties, being it scientists, farmers, retailers, consumers, and even NGOs (Saraiva and Arechavala-Lopez Citation2019). Consequently, this stance generates attitudes that change the traditional production model (Branson Citation2008). Thus, the period before slaughter should be carried out with due care, decreasing stress caused to the animals. Besides the ethical concern, stress causes physiological and biochemical reactions promoting imbalance that results in poorer quality meat (Daskalova et al. Citation2016).
The consideration of slaughter timing and its relation to stress in fish is intrinsically connected to the complex physiological responses elicited by stressful events. These responses begin with primary reactions, such as elevated catecholamines and cortisol secretion, leading to secondary metabolic reactions, particularly changes in plasma glucose and lactate levels. Understanding this sequence is necessary for implementing humane stunning methods that aim to reduce stress and thereby maintain meat quality (Barton Citation2002; Gomes et al. Citation2003).
To be considered ideal, within the precepts of animal welfare, fish should be stunned before slaughter, avoiding any suffering and pain (EFSA Citation2004; OIE Citation2022). The OIE (World Organization for Animal Health), in chapter 7.3, recommends electronarcosis and mechanical stunning as methods of stunning that guarantee the welfare of animals during slaughter (OIE Citation2022). Despite the existence of recommendations with humane methods, unfortunately most fish are still stunned by protocols that do not cause immediate insensitivity, such as hypothermia, the method most used in fish farming (Filho et al. Citation2015), this occurs mainly due to the practicality of application in large-scale productions (EFSA Citation2009).
The use of anesthetics can become an alternative to induce sedation at the time before the slaughter of fish, because they are commonly used to reduce the consequences caused by pain and stress during management in fish farming (Rucinque et al. Citation2021). This procedure aims to lightly sedate aquatic animals, in a reversible manner, by depressing their central and peripheral nervous systems (Javahery et al. Citation2012). The use of plant extracts as alternatives to chemical anesthetics has demonstrated beneficial effects, notably in minimizing adverse outcomes like mucus loss and irritation to gills and eyes. Additionally, these extracts are generally more cost-effective, environmentally friendly due to faster degradation, and produce lower levels of waste, underscoring their relevance (Inoue et al. Citation2003).
Clove oil, derived from Syzygium aromaticum, is a notable sedative for such purposes. Its primary active component, eugenol (4-allyl-2-methoxyphenol), constitutes 83.75% of its makeup, followed by β-caryophyllene (10.98%) and α-humulene (1.26%), with other compounds present in smaller quantities (Scherer et al. Citation2009). Eugenol, a readily available natural extract, possesses antibacterial, anticancer, antifungal, insecticidal, and anti-inflammatory properties (Cortés-Rojas et al. Citation2014). Research by Zahran et al. (Citation2021) on Nile tilapia demonstrated that both propofol and eugenol significantly reduced glutathione reductase and catalase activity, highlighting their antioxidant effects and indicating no lasting changes in stress biomarkers or antioxidant enzymes due to their exposure.
Furthermore, clove oil's natural anesthetic is recognized by the US Food and Drug Administration (FDA) (Ross et al. Citation2009). A study by Kildea et al. (Citation2004) on silver perch (Bidyanus bidyanus) revealed that the application of this extract resulted in residual values below the limits permissible for human consumption, negating the need for a purification period. This underscores its effectiveness as a stunning agent that does not produce detectable residues in the fillet.
Eugenol can also be used in the food industry as a preservative, mainly due to its antioxidant property (Kamatou et al. Citation2012). Since oxidation results in toxic effects and changes in the normal redox state, associated with cell damage, lipid and protein peroxidation (Hematyar et al. Citation2019). Fish are rich in n-3 polyunsaturated fatty acids (PUFA), thus the risk of quality loss due to lipid oxidation is magnified when compared to other species produced as human food (Medina et al. Citation2009). Besides lipid oxidation, which acts directly on the taste and shelf life of the filet, protein oxidation can also cause changes in product quality. And the progress of the initial oxidative reactions in food is enhanced by the interactions between proteins and lipids due to the similarity of oxidation reactions (Hematyar et al. Citation2019).
In an attempt to minimize the oxidative cascade, antioxidants play a vital role in inhibiting lipid, protein peroxidation and/or protecting against free radicals (Dargel Citation1992). Eugenol acts by sequestering free radicals in the DPPH (2,2-Diphenyl-1-picrylhydrazyl) assay as well as inhibiting reactive oxygen species (ROS) (Pérez-Rosés et al. Citation2016). Thus, the inclusion of this compound at the moment before stunning can help in the management of tilapia acting as a sedative, causing less stress to the animals, besides acting as an antioxidant, consequently reducing oxidative stress, thus preserving the quality characteristics of the filet.
The objective of this study was to assess the effectiveness of clove essential oil as a pre-stunning agent in Nile tilapia (Oreochromis niloticus), employing cranial percussion and hypothermia. This research aimed at simultaneously enhancing animal welfare and preserving the quality of the fish fillet, offering potential advancements in aquaculture practices.
2. Material and methods
2.1. Tilapias and holding conditions
Fish were grown in an excavated tank (115 m × 35 m/4.000 L water blade). About 28.000 juveniles all males, sex reversed (±15 g) were placed in this tank, where they remained for 11 months (April to February), being fed 3 times a day with commercial feed containing 32% crude protein and 3.000 Kcal/kg digestible energy, until they reached the weight for slaughter (±900 g). The diet provided to the fish was Supra®, with the biomass percentage for feeding maintained at 4%, in accordance with the manufacturer’s recommendations.
Before slaughter, feeding was stopped to empty the digestive tract, totaling 18 hours of fasting until the moment of slaughter. The emptying of the tank started 12 hours before the experiment, slowly, leaving 40% of the total water capacity in the tank at the time of harvest. The animals were randomly removed from the pond with the aid of a collecting net, weighed with a portable scale, and measured with a tape measure, then placed in transport boxes. The transport boxes were 1.000 L fiberglass, equipped with diffusers and oxygen cylinders. Temperature and oxygen were measured at the beginning of transport and during the resting period of the fish in the depuration tank using a portable instrument (YSI 550A Dissolved Oxygen Meter). At the beginning of transport, the temperature was = 28.5°C and the dissolved oxygen = 8.1 mg L−1.
The fish were transported by local roads to the commercial Refrigerator, located on the same property (140 m away). Upon arrival, the fish were held in the purification tank for a period of 6 hours before the slaughter process. The depuration tank was 2.5 m × 4 m and had a volume of 8.000 liters of water, pumped by a water injection pump (1.5 hp single phase MBI-1 I1-26 SCHINEIDER) with a renewal capacity of 21.000 liters/hour. Temperature and dissolved oxygen were monitored every one hour during the entire resting period, where the average water temperature was = 27.8 ± 1.16°C and dissolved oxygen = 7.8 ± 2.2 mg L.
Following the depuration phase, animals were individually extracted from the purification tank using a collection net to commence the stunning process. The stunning protocol involved four distinct treatments. The first animal was administered clove essential oil prior to hypothermia-induced stunning. The second fish was subjected to hypothermia-induced stunning. The third animal received clove essential oil before undergoing percussive stunning, and the fourth fish was exposed only to percussive stunning. This protocol was systematically applied to a total of 100 animals.
All personnel involved in the handling of animals were adequately trained and experienced in minimizing stress during the pre-stunning period. Procedures were standardized across all groups to ensure methodological consistency, thereby reducing potential variables and enabling an accurate comparison between the different stunning methods. The variation in treatments was confined solely to the method of stunning (percussive vs. hypothermic, with and without clove essential oil), while other experimental parameters such as harvesting, loading, transportation, and unloading were uniformly controlled.
2.2. Experimental design
A total of 100 tilapias (Oreochromis niloticus) premium® strain, with an average weight of 900 ± 100 g and an average length of 35 ± 5 cm, were used for this study. The fish were divided into two groups according to stunning methods, i.e. cranial percussion or hypothermia. Between these two groups of 50 fish, 25 were treated with clove essential oil and 25 without before stunning. The experimental design was completely randomized in a 2 × 2 factorial scheme correspond to 4 treatments (25 fish/treatment) totaling 100 fish.
2.3. Clove essential oil treatment
Pure clove essential oil was obtained from Acs Cientifica®. A solution was produced by diluting 1.5 g of clove oil in 15 mL of absolute ethanol, forming an alcoholic solution with a concentration of 100 mg/mL, in order to allow the dissolution of this essential oil in water, as described by da Silva et al. (Citation2021). In a box containing 30 liters of water, 100 mg L−1 of the solution was added, and each fish was kept in it for 3 min (Delbon and Paiva Citation2012). For each stunning treatment, 25 tilapias were subjected to the clove essential oil-induced anesthetic process prior to the stunning procedure.
2.4. Stunning methods
2.4.1. Cranial percussion
Previously an anatomical study was performed through desiccation to identify the position of the tilapia brain. Thus, the impact in the correct place would cause concussion and acceleration of the brain inside the skull, interrupting its normal electrical activity, followed by a sudden drop of pressure, and consequently leading to stunning. Then it was directed to the employee responsible for the blow that for tilapia of this average weight and size, the ideal position would be 5.5 cm from the upper lip towards the dorsal fin as shown in .
Figure 1. Indication of the position for cranial percussion blow. In image a, the ideal distance for applying the blow is observed. Image b, operator. Source: Author.
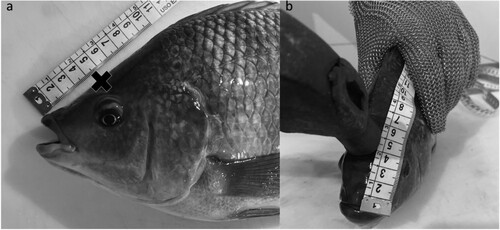
The animals were individually restrained, where they were quickly removed from the scrubbing tank. The employee held the animal by the back with one hand, supporting it on the bench, leaving the animal in an upright position, and with the other hand, with the help of an iron hammer, performed the blow in the indicated place (). The hammer weighed 1.020 kg, was 45 cm long, and the point where it hit the fish’s head was cylindrical with a 2.5 cm diameter. The same procedure happened in the animals previously stunned with clove essential oil.
2.4.2. Hypothermia
The method was performed according to Oliveira Filho et al. (Citation2017), with minor modifications. The animals were placed individually in a box containing 15 L of water and 15 kg of ice, maintaining an average temperature of 0.8 °C, for 20 minutes.
2.5. Evaluation of the efficacy of stunning methods
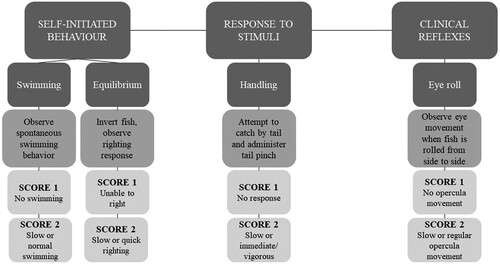
Immediately after stunning, each fish was transferred to a plastic box containing 30 L of water remaining fully immersed. Their behavioral parameters were then evaluated, according to the ‘Protocol for brain evaluation in fish and the efficacy of methods used for stunning and killing’ by (Kestin et al. Citation2002), with minor modifications. The observed variables were divided into the following three categories: (1) self-initiated behavior (swimming and balance), (2) response to stimulus (needle prick on tail), and (3) clinical reflex (vestibulo-ocular reflex).
The first three variables, swimming, balance, and response to needle prick, were tested in water. To test swimming ability, the fish were placed in the water in the normal position to see if they could swim normally. To determine balance, the fish were positioned inversely to their normal position to check their ability to return to their normal position. Finally, they were subjected to a pinprick on their tail to induce escape behavior. Subsequently, the fish were removed from the water and subjected to rotational movements to check the functioning of the vestibulo-ocular reflex. For each attribute, a score was stipulated, where 1 = stunned and 2 = not stunned ().
Figure 2. Protocol for observing self-initiated behavior, stimulus-response, and clinical reflex post-stunning. Source: Adapted from Kestin et al. (Citation2002).
2.6. Blood plasma evaluation
Immediately after assessing the effects of stunning, individually fish were placed on a bench for blood collection. About four milliliters of blood from each fish was collected by caudal puncture with 5 mL disposable syringes using a 0.8 × 30 mm needle. After collection, the blood was transferred to 4 mL FirstLab® vacuum tubes containing fluoride and ethylenediaminetetraacetic acid (EDTA).
Blood samples were centrifuged in a Centurion Scientific K3 Series centrifuge at 3.000 rpm for 10 min at 18 °C. Subsequently, only plasma was collected and transferred to 2 mL Eppendorf tubes, frozen and stored at – 20 °C, for glucose and lactate analyses.
Plasma samples were evaluated by the Siemens/Dimension Gluc Ver Flex and Dimension Lactic Acid methods for glucose and lactate, respectively. All of these analyses were performed by the Siemens Dimension Xpand Plus device (Siemens Healthcare Diagnostics Inc., U.S.A.).
2.7. Fillet quality
The animals were slaughtered by cutting the gill arches and filleted. The quality indicators of the fillets were evaluated by hydrogen potential (pH), water holding capacity (WHC) and color, which were performed on the left fillet of each animal 45 min after slaughter. All analyses were performed in duplicate.
2.7.1. Hydrogen potential (pH)
The pH analysis was performed using a portable potentiometer with Testo insertion electrode, model 205.
2.7.2. Water holding capacity (WHC)
The water holding capacity was estimated by weighing two grams of sample on a BEL engineering® M214Ai analytical balance. After weighing, the sample was placed on Whatmann n°1 filter paper of area 10 × 10 cm², weight 80 g/m2 between 15 × 15 cm2 acrylic plates. A uniform pressure was then applied using a ten kilogram weight for five minutes. After this time the samples were weighed again, and by obtaining the difference in sample weight, the percentage of water loss by pressure was calculated. The WHC was determined based on the technique described by Hamm (Citation1960).
To calculate the WHC the following formula was used:
where WHC = water holding capacity, Pi = initial weight, Pf = final weight.
2.7.3. Color
Color was measured on the dorsal surface of the fillet using a portable colorimeter (Minolta CR-10™, Konica Minolta, INC., Osaka, Japan) with D65 illuminant, 10° standard observer and 8.0 mm aperture, by the CIE system L* (brightness), a* (red-green component), b* (yellow–blue component) (Minolta Citation1998). The color determination was performed in duplicate and from the L*, a* and b* values.
2.8. Lipid and protein oxidation
For the lipid and protein oxidation analyses, tissue samples were collected from the dorsal region of the right filet of each animal. These samples were placed in 2 mL Eppendorf tubes and stored in −80 °C freezer for further analysis. All analyses were performed in duplicate.
2.8.1. Thiobarbituric acid reactive substances (TBARS)
Lipoperoxidation (LPO) was measured by quantifying thiobarbituric acid reactive substances (TBARS) according to Camejo et al. (Citation1998). TBARS analysis was measured by mg protein−1, where protein analysis was performed according to the protocol of Bradford (Citation1976).
2.8.2. Tryptophan concentration
It was measured by fluorescence spectroscopy according to the methodology described by Estévez et al. (Citation2008a), with some modifications. We homogenized (1:10 w/v) the samples in sodium phosphate buffer (pH 6.0 with 8 M urea) using the ultraturray. Then, two mL of 20 mM sodium phosphate buffer (pH 6.5 with uHCl 6) was added to a 0.5 mL aliquot of the homogenate and stirred on the vortex. After dilution (1: 1000 v / v), the samples were transferred to a 4 mL flat-walled quartz cuvette (101-QS 10 × 10 mm, Analytics Hellma). The spectrum was recorded between 300 and 400 nm wavelength, and the excitation was set at 283 nm (Perkin-Elmer LS 55 luminescence spectrometer, Beaconsfield, UK). The excitation and emission slit widths were fixed at 10 nm and data were collected at 500 nm per minute. Tryptophan content was calculated based on a five-point standard curve of N-acetyl-L- tryptophan amide (NATA) ranging from 0.1 to 0.5 μM. The results were expressed as mg NATA equivalent per 100 g sample.
2.8.3. Schiff's bases
It was performed according to Estévez et al. (Citation2008a), with minor modifications. The samples were homogenized similarly to the tryptophan analysis procedure. Then a dilution was performed (1:20 v/v) and transferred to a 4 mL quartz cuvette (101-QS 10 × 10 mm, Analytics Hellma). The emission spectrum was recorded between 400 nm and 500 nm wavelength and excitation was set at 350 nm (Perkin-Elmer LS 55 luminescence spectrometer, Beaconsfield, UK). Excitation and emission widths were set to 10 nm and data were collected at 500 nm per minute. The results were expressed as units of fluorescence intensity emitted by Schiff base structures at 450 nm. A correction was performed according to the protein concentration of each sample, where Pt = average total amount of protein of all samples and Pp = protein content in each sample type.
2.8.4. Protein carbonyls
Protein oxidation was measured by total carbonyl content (DNHP) according to Ganhão et al. (Citation2011), with minor modifications. One gram sample was homogenized 1:10 (w/v) in 20 mM sodium phosphate buffer containing 0.6 M NaCl (pH 6.5) using an ultraturray. Two equal aliquots of 0.15 mL were taken from the homogenized sample and dispensed into 2 mL eppendorf tubes. Proteins were precipitated using cold 10% trichloro acetic acid (TCA) (1 mL) and subsequently centrifuged for 3 min at 4500 g. One pellet was treated with 1 mL of 2 N HCl (measurement of protein concentration) and the other with an equal volume of 0.2% (w/v) DNPH in 2 N HCl (measurement of carbonyl concentration). Both samples were incubated for 1 h at room temperature. The samples were precipitated with 10% TCA (1 mL) and washed twice with 1 mL ethanol: ethyl acetate (1: 1, v / v). Then the sediments were dissolved in 1.5 mL of 20 mM sodium phosphate buffer containing 6 M guanidine HCl (pH 6.5) and centrifuged for 2 min at 2000g. Bovine serum albumin (BSA) was used as a standard and the protein concentration was calculated from the absorbance at 280 nm. The amount of carbonyls was expressed as nmol carbonyls per mg protein (nmol carbonyls / mg protein) using an absorption coefficient of 21.0 nM -1 cm -1 at 370 nm for the protein hydrazones.
2.9. Antioxidant defenses
For the antioxidant defenses analyses, tissue samples were collected from the dorsal region of the right fillet of each animal. These samples were placed in 2 mL Eppendorf tubes and stored in −80 °C freezer for further analysis. All analyses were performed in duplicate.
2.9.1. Protein
Antioxidant defenses were measured per mg of protein, where protein analysis was performed according to the Bradford protocol, (Citation1976).
2.9.2. Glutathione (GSH) reduction activity
The amount of GSH in the tissue sample was determined according to the method described by Beutler et al. (Citation1963) in which the reaction between GSH and its substrate 5,5'-dithiobis-2-nitrobenzoic acid (DTNB) to form thiolate (TNB) is quantified at 412 nm and expressed as μg of GSH mg of protein-1.
2.9.3. Catalase activity (CAT)
Catalase (CAT) activity was determined by the rate of H2O2 breakdown in the tissue samples. The decrease in absorbance was determined at 240 nm according to a protocol described by Beutler (Citation1975). CAT activity was expressed as μmoL of H2O2 min−1 mg protein−1.
2.10. Statistical analysis
2.10.1. Fisher's exact test
Fisher's Exact Test analysis was performed to compare the results between the groups for the evaluation of the effectiveness of the stunning methods, analyzing the variables swimming, balance, response to stimulus and VOR. R 4.0.2 software was used and statistical significance was set at p < 0.05.
2.10.2. Factorial
Data were checked for normality of errors and homogeneity of variances using the Shapiro–Wilk (p < 0.05) and Bartlett (p < 0.05) tests, respectively. Subsequently, these were subjected to analysis of variance (two-way ANOVA), and means were compared using Tukey's test (p < 0.05). Statistical analyses were processed using R 4.0.2 software with the ExpDes package (R Core Team 2020).
3. Results
There was an interaction between stunning method and clove essential oil for the variables glucose, lactate, TBARS, tryptophan, L* (brightness) and a* (red/green hue). For the other variables that showed no interaction between the factors, the factors were evaluated individually.
3.1. Evaluation of stunning method
The group stunned by cranial percussion using clove essential oil beforehand, showed a greater number of stunned animals, reducing behavioral responses. For swimming behavior and response to stimulus the use of clove essential oil caused stunning in 100% of the animals, while for the balance and vestibular eye reflex (VOR) variables it was 96%. Without the use of clove oil extract the variables swimming, balance, response to stimulus and VOR had a reduction in the number of animals stunned, being 72, 72, 68 and 64% respectively. Fish stunned by hypothermia showed no difference (p ≤ 0.05) between the use or not of the anesthetic and both were effective to stunned tilapia ().
Table 1. Percent (%) of stunned tilapia evaluating swimming behaviors, balance, stimulus response, and vestibulo-ocular reflex (VOR) with respect to the interaction between stunning methods (cranial percussion and hypothermia) and the use or non-use of clove essential oil.
3.2. Blood profile: glucose and lactate
Blood glucose was reduced by 12.12 mg dL−1 (p ≤ 0.05) with the use of clove essential oil for hypothermia treatment. However, tilapia that were stunning by cranial percussion, did not differ using clove essential oil or not (). We observed that fish that were not in contact with clove essential oil in the cranial percussion treatment showed higher levels of lactate. There was a reduction in 3.36 mmol L−1 when clove essential oil was used for this method. There was no difference for the hypothermia treatment, and the values were lower when compared to the group without and with clove essential oil in the cranial percussion treatment ().
Table 2. Mean values and standard error of glucose and lactate variables, in relation to the interaction between stunning method and treatment with clove essential oil in tilapia.
Table 3. Mean values and standard error of pH, water holding capacity (WHC), yellow/blue hue (b*), Schiff bases, protein carbonylation (DNPH), glutathione reductase (GSH) and catalase activity (CAT) of tilapia stunned by two stunning methods (cranial percussion and hypothermia), using and not using clove essential oil.
3.3. Meat quality
There was a difference in pH between stunning methods, where the lower value was found for the cranial percussion method. There was no difference between the use or not of clove essential oil for this parameter (). The lowest WHC value was found for the cranial percussion method. There was no difference between the use or not of clove essential oil ().
Higher luminosity value was found for the animals that were stunned by cranial percussion without clove essential oil. When submitted to clove essential oil, a reduction in this variable was observed for the same treatment. There was no difference for hypothermia treatment using or not using clove essential oil ().
Table 4. Mean values and standard error of L* (Luminosity), a* (red/green tonality) variables, in relation to the interaction between stunning method and treatment with clove essential oil in tilapia.
Tilapias submitted to without clove essential oil cranial percussion treatment showed higher red (a*) intensity (p ≤ 0.05). There was no difference between the group without and with clove essential oil for the hypothermia method, however they showed lower red (a*) intensity (Image 4). Fish in the cranial percussion treatment and without clove essential oil showed higher yellow (b*) intensity ().
3.4. Oxidation and its defenses
In the present study, animals subjected to hypothermia without clove essential oil, showed higher value of lipid peroxidation. A significant reduction of 0.028 nmol TBARS/nmol mg protein−1 was observed when these animals were submitted to the use of the anesthetic (). There was no significant difference between the group without and those using clove essential oil for cranial percussion treatment, being 0.0154 and 0.0095 nmol TBARS/nmol mg protein−1 respectively ().
Table 5. Mean values and standard error of TBARS (thiobarbituric acid reactive substances) and tryptophan variables, in relation to the interaction between stunning method and treatment with clove essential oil in tilapia.
Protein oxidation was evaluated using three methods, these being: depletion of protein components (tryptophan) and formation of protein oxidation products (Schiff bases and carbonyls). Interestingly hypothermia treatment with prior use of clove essential oil led to significant tryptophan depletion, 7.70 mg NATA/g protein. Higher tryptophan concentrations were found in the cranial percussion treatments without and with clove essential oil and hypothermia without clove essential oil, which did not differ ().
The group of fish subjected to cranial percussion and without clove essential oil showed higher Schiff bases formation and higher protein carbonylation (DNPH). There was a reduction of 68.55 (fluorescence intensity) for Schiff Bases and 0.134 nmol carbonyls/mg protein for DNPH when using clove essential oil ().
Fish sensitized by hypothermia showed higher glutathione reductase concentration, 7.31 µg of GSH mg protein-1, and catalase activity, 0.35 µmoL H2O2 min−1 protein−1. For GSH there was no difference between the group without and that with clove essential oil (). However, the use of clove essential oil anesthetic decreased 0.05 µmoL H2O2 min−1 protein−1 of CAT ().
4. Discussion
4.1. Evaluation of stunning method
Like mammals and birds, fish should be stunned before slaughter, avoiding pain and suffering and ensuring their welfare. Visual indicators of sensitivity are a method used to evaluate the pre-slaughter stunning of fish (Kestin et al. Citation2002).
According to Becker et al. (Citation2012), the use of anesthetics and/or sedatives in water helps reduce the stress that occurs due to any stimulus. Anesthesia is usually accomplished by immersing the fish in a bath containing an appropriate concentration of an anesthetic that is absorbed through the gills and rapidly enters the bloodstream. The compound initially inhibits the cerebral cortex, causing tactile loss in the animal, followed by excitation of the basal ganglia and cerebellum, and finally reaches the spinal cord, where anesthesia sets in temporarily (Coyle et al. Citation2004). In other words, the decrease in respiratory frequencies caused by the anesthetic is based on the inhibition of the respiratory center in the medulla oblongata in relation to the depression of the central nervous system (Hikasa et al. Citation1986). Simões et al. (Citation2011), stated that the use of the oil had a sedative effect during fish transport, avoiding post-transport impact on fish physiology and behavior.
According to the OIE (Citation2022), cranial percussion is classified in the precepts of animal welfare. However, the recommendations for the use of this technique are mainly for species of medium to large size, where the accuracy of the blow becomes greater. For tilapia, cranial percussion may be difficult at the time of slaughter, since it is difficult to restrain the animal, impairing the accuracy of the blow in the proper place, a result observed in our study for the group that did not use clove essential oil ().
Hypothermia is a widely used method, especially for tropical fish species, where cooling acts to stop fish movement (Lines and Spence Citation2012). Thus, the reduction of movements due to thermal shock, paralyzes them, in addition to removing the sensitivity indicators, a fact observed in our study (). Despite being the most widely used, this is not an indicated method (OIE Citation2022). According to Acerete et al. (Citation2009) hypothermia is not considered a humane method, due to its slow action, where the animal takes time to fully lose consciousness.
The findings of this study indicate that the cranial percussion method, augmented by the pre-application of clove essential oil, is highly effective in stunning Nile tilapia. This combination significantly increased the percentage of effectively stunned fish and reduced adverse behavioral responses. On the other hand, while hypothermia proved effective in immobilizing fish, its slower action rendered it less favorable in terms of the criteria for humane treatment as outlined by the OIE (World Organisation for Animal Health). Consequently, the cranial percussion method, when enhanced with clove essential oil, presents as a superior alternative for the humane stunning of tilapia, contributing to improved animal welfare during the slaughter process.
4.2. Blood profile: glucose and lactate
The study observed a significant interaction between the stunning methods and the levels of glucose and lactate in tilapia. Specifically, the application of clove essential oil markedly influenced these metabolic parameters. The results demonstrated that clove oil effectively reduced the levels of glucose and lactate (). This aligns with existing scientific literature, such as Rotllant et al. (Citation2001), which posits that anesthetic application can positively affect stress management by reducing the secondary metabolic responses of glucose and lactate. These outcomes support the proposition that clove essential oil, particularly when combined with the cranial percussion method, offers a more advantageous approach for the humane stunning of tilapia, aiming to enhance animal welfare.
Petersen and Gleeson (Citation2011), state that anaerobic energy production has a strong relationship with temperature and can reach values up to ten times higher at higher temperatures (20–30°C) compared to lower temperatures. This may explain lower lactate levels in the group of tilapias stunned by hypothermia (). Acerete et al. (Citation2009) when evaluating three stunning methods (CO2, asphyxiation and asphyxiation on ice) in European sea bass (Dicentrarchus labrax), obtained lower plasma lactate levels for the group of animals stunned by asphyxiation on ice and CO2.
4.3. Meat quality
There exists a correlation among pH, water retention, and color in meat. A decrease in pH leads to reduced water retention, causing water to shift from intracellular to extracellular spaces, resulting in increased luminosity (L*), giving the meat a paler appearance (den Hertog-Meischke et al. Citation2011). These findings align with our study, where a pH decrease of 0.09 corresponded to a 3.1% decline in WHC () and a 4.4 increase in luminosity () for animals treated with cranial percussion.
We observed an interaction in the parameters of luminosity and red intensity (a*). Animals without essential oil treatment before cranial percussion had higher luminosity and greater red intensity (a*) (). Moreover, cranial percussion-stunned animals without clove essential oil exhibited higher yellow intensity (b*) (). As per Digre et al. (Citation2011), higher a* values, indicating intensified redness, might be linked to stress during stunning, causing blood from the intestine to concentrate in muscles, increasing fight-or-flight responses. Yellow intensity doesn't impact fillet quality but might seem ‘abnormal’ to consumers (Truong et al. Citation2016).
Color is a primary attribute evaluated by consumers during purchase, as highlighted by Skjervold et al. (Citation2001). Consumer preference tends towards lighter meat in freshwater fish, coupled with a lower red intensity (a*) as noted by Knowles et al. (Citation2008). In this context, employing strategies like the use of clove essential oil, which can minimize red intensity, aligns with consumer expectations for organoleptic quality at the point of purchase.
4.4. Oxidation and its defenses
The rate and extent of oxidation in meats are influenced by pre-slaughtering events such as handling stress, and post-slaughtering events such as slaughter techniques, post-mortem pH drop and carcass temperature (Sant’Ana and Mancini-Filho Citation2000). Lipid oxidation is the most relevant chemical alteration in fish (Nunes et al. Citation2007), being the primary deterioration process of tilapia fillet, due to its high protein and moisture content, thus manifesting changes in smell, color, texture, nutritional value, and possible production of toxic compounds (Fernandes et al. Citation2015).
Generally, the degree of oxidative rancidity is measured through the peroxide index and the Thiobarbituric Acid Reactive Substances (TBARS) test. Tilapia is considered a lean fish, where its maximum body fat percentage does not exceed 2.6% (Simões et al. Citation2007). Thus, it is expected that the lipid peroxidation value will be below the values stipulated by Senapati et al. (Citation2017) as precursors of rancid taste and odor, i.e. below 2 MDA/kg sample. The study identified an interaction effect in the evaluated parameter, demonstrating that clove essential oil application reduced peroxidation levels during hypothermia stunning. Conversely, no significant difference was observed between the treatments involving cranial percussion stunning, with or without the use of clove oil. Notably, despite variations in treatment, all groups exhibited peroxidation values below the established reference threshold, as shown in .
Foods are incessantly exposed to reactive oxygen species and this will not only cause lipid oxidation, but also protein oxidation (di Bernardini et al. Citation2011). The kinetics of lipid and protein oxidation with respect to the formation of hydroperoxides and carbonyls is similar, however, the diversity of protein oxidation products is more complex due to more reactive targets in proteins (Hematyar et al. Citation2019).
Protein oxidation negatively affects the water-holding capacity, as shown by the results of this study, where the cranial percussion method showed higher WHC, Schiff's bases, and DNPH values (). Because polar groups have very high accessibility to pro-oxidants present in fish muscle, they are more prone to oxidation reactions (Standal et al. Citation2018). Protein carbonylation results in the loss of amino groups, which in turn leads to changes in the distribution of electrical charges and the overall arrangement of myofibrillar proteins. Apparently, the result of the strong modification of oxidized proteins is a change in the isoelectric point of the protein. As a result, groups with opposite charges are more likely to attract each other, reducing the amount of water the protein contains. Also, due to the isoelectric point, the repulsive force of the myofibrillar protein structure is reduced, so the protein structure can be more compact with less water holding capacity (Huff-Lonergan and Lonergan Citation2005).
It is also believed that effects of low pH, such as aggregation, denaturation, and reduced solubility of muscle proteins, can alter their susceptibility to oxidation (Estévez Citation2011). In addition, protein oxidation can be caused directly by ROS and reactive nitrogen species or indirectly because of reactions with lipid oxidation products with reducing sugars or carbohydrates (Lund et al. Citation2011).
Since the functions of protein are very specific, oxidative modifications can cause numerous functional consequences and lead to changes in the texture, WHC, digestibility, and succulence of foods (Baron et al. Citation2007). Wang et al. (Citation2009), when evaluating pre-slaughter stress in chickens, observed reduced pH, and in turn increased vulnerability to protein carbonylation and denaturation, and reduced solubility.
According to Halliwell and Gutteridge (Citation1985), the higher the concentration of GSH and the activity of CAT, the worse the cellular redox state is, thus indicating a highly stressed condition of the fish, which leads to a greater need for these enzymes to act against stressors. In the present study, hypothermia treatment for both GSH and CAT activity showed worse redox status, however, there was a reduction in catalase activity when clove essential oil was used ().
Reactive oxygen species are responsible for initiating the oxidation reaction in foods, and react with lipids, proteins, carbohydrates, and vitamins producing undesirable volatile compounds, destroying essential fatty acids, amino acids, and vitamins, consequently decreasing the nutritional value and physicochemical quality of the food during storage, and providing rejection and decreasing consumer acceptability (Choe and Min Citation2007). The adverse effects of ROS in food challenge meat technologist researchers to develop successful antioxidant strategies. Most studies confirm the growing interest in natural antioxidants, especially in plant materials containing high levels of phenolic compounds, as oxidation inhibitors (Estévez et al. Citation2008b). Clove essential oil may become an alternative, since the compound has been used in the food industry as a preservative mainly due to its antioxidant characteristic (Kamatou et al. Citation2012).
Antioxidants are defense elements against the effects of free radicals in the body. They not only eliminate reactive oxygen species, but also regulate the cellular redox status. They also inhibit the initiation and propagation steps, causing the reaction to stop and delaying the oxidation process. However, antioxidants can develop free radical scavenging mechanisms (Gülçin Citation2006; Gülçin et al. Citation2010). The present study corroborates this statement, where in addition to decreasing antioxidant defenses (CAT) (), the use of clove essential oil decreased lipid (TBARS) () and protein (Schiff's bases and DNPH) oxidation ().
This research highlights the efficacy of clove essential oil in improving animal welfare during the slaughter of tilapia, particularly when used in conjunction with cranial percussion. This method resulted in a higher percentage of effectively stunned animals and reduced adverse behavioral responses. In contrast, while hypothermia was capable of immobilizing fish, it did not fully meet the humane criteria as defined by the OIE. Beyond its impact on stunning, clove oil was observed to decrease levels of glucose, lactate, and oxidative markers in lipids and proteins. These findings position clove oil as a viable option for maintaining meat quality, aligning with consumer preferences. From an ethical standpoint in slaughter practices, the combination of cranial percussion and clove oil is proposed as a more effective strategy, ensuring both animal welfare and the quality of the final product in the food industry.
5. Conclusions
Clove essential oil reduced the stress of the animals and acted as an antioxidant by decreasing the levels of antioxidant defenses, reducing lipid and protein oxidation. Consequently, it aided in fillet quality by reducing brightness and redness intensity. Therefore, clove essential oil proves its efficacy in the pre-slaughter period and as an antioxidant and can be used as a pre-insertion protocol and improve the quality of the fillets.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Additional information
Funding
References
- Acerete L, Reig L, Alvarez D, Flos R, Tort L. 2009. Comparison of two stunning/slaughtering methods on stress response and quality indicators of European sea bass (Dicentrarchus labrax). Aquaculture. 287(1–2):139–144. doi:10.1016/j.aquaculture.2008.10.012.
- Baron CP, Kjærsgård IVH, Jessen F, Jacobsen C. 2007. Protein and lipid oxidation during frozen storage of rainbow trout (Oncorhynchus mykiss). J Agric Food Chem. 55(20):8118–8125. doi:10.1021/jf070686f.
- Barton BA. 2002. Stress in fishes: a diversity of responses with particular reference to changes in circulating corticosteroids 1. 42, 517–525. https://academic.oup.com/icb/article/42/3/517/723932.
- Becker AG, Parodi T, Heldwein CG, Zeppenfeld CC, Heinzmann BM, Baldisserotto B. 2012. Transportation of silver catfish, Rhamdia quelen, in water with eugenol and the essential oil of Lippia alba. Fish Physiol Biochem. 38(3):789–796. doi:10.1007/s10695-011-9562-4.
- Beutler E. 1975. The preparation of red cells for assay. In: Beutler E, editor. Red cell metabolism: a manual of biochemical methods. New York: Grune & Straton; p. 8–18.
- Beutler E, Duron O, Kelly BM. 1963. Improved method for the determination of blood glutathione. J Lab Clin Med. 61:882–888. PMID: 13967893.
- Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 72(1–2):248–254. doi:10.1016/0003-2697(76)90527-3.
- Branson EJ. 2008. Fish welfare (E. J. Branson, Ed.). Blackwell Publishing Ltd. https://books.google.com.br/books?hl=pt-BR&lr=&id=-NNlWxyXPIYC&oi=fnd&pg=PR5&dq=Branson,+EJ+(Ed.)+2008&ots=edankM7u-c&sig=1bDlTYRy3OjWZ7hZKjeicxUOBdk#v=onepage&q=Branson%2C%20EJ%20(Ed.)%202008&f=false.
- Brown C. 2015. Fish intelligence, sentience and ethics. Anim Cogn. 18:1–17. doi:10.1007/s10071-014-0761-0.
- Camejo G, Wallin B, Enojärvi M. 1998. Analysis of oxidation and antioxidants using microtiter plates. Methods Mol Biol. 108:377–387. doi:10.1385/0-89603-472-0:377/COVER.
- Choe E, Min DB. 2007. Chemistry and reactions of reactive oxygen species in foods. Crit Rev Food Sci Nutr. 46(1):1–22. doi:10.1080/10408390500455474.
- Cortés-Rojas DF, de Souza CRF, Oliveira WP. 2014. Clove (Syzygium aromaticum): 544 a precious spice. Asian Pac J Trop Biomed. 4:90–96. doi:10.1016/S2221-1691(14)60215-X.
- Coyle SD, Durborow RM, Tidwell JH. 2004. Anesthetics in aquaculture. South Reg Aquacult Center. 3900:1–6. https://fisheries.tamu.edu/files/2013/09/SRAC-Publication-No.−3900-Anesthetics-in-Aquaculture.pdf.
- Dargel R. 1992. Lipid peroxidation – a common pathogenetic mechanism? Exp Toxicol Pathol. 44(4):169–181. doi:10.1016/S0940-2993(11)80202-2.
- da Silva DR, Arvigo AL, Giaquinto PC, Delicio HC, Barcellos LJG, Barreto RE. 2021. Effects of clove oil on behavioral reactivity and motivation in Nile tilapia. Aquaculture. 532:736045. doi:10.1016/j.aquaculture.2020.736045.
- Daskalova A, Pavlov A, Kyuchukova R, Daskalov H. 2016. Humane slaughter of carp – a comparison between three stunning procedures. Turk J Fish Aquatic Sci. 16(4):753–758. doi:10.4194/1303-2712-v16_4_01.
- Delbon MCE, Paiva MJTR. 2012. Eugenol em juvenis de tilápia do Nilo: Concentrações e administrações sucessivas. Bolet Inst Pesca. 38(1):43–52.
- den Hertog-Meischke MJA, van Laack RJLM, Smulders FJM. 2011. The water-holding capacity of fresh meat. Vet Q. 19:175–181. https://www.tandfonline.com/doi/pdf/10.108001652176.1997.9694767?needAccess=true.
- di Bernardini R, Harnedy P, Bolton D, Kerry J, O’Neill E, Mullen AM, Hayes M. 2011. Antioxidant and antimicrobial peptidic hydrolysates from muscle protein sources and by-products. Food Chem. 124(4):1296–1307. doi:10.1016/j.foodchem.2010.07.004.
- Digre H, Erikson U, Skaret J, Lea P, Gallart-Jornet L, Misimi E. 2011. Biochemical, physical and sensory quality of ice-stored Atlantic cod (Gadus morhua) as affected by pre-slaughter stress, percussion stunning and AQUI-S TM anaesthesia. Eur Food Res Technol. 233:447–456. doi:10.1007/s00217-011-1531-8.
- EFSA. European Food Safety. 2004. Welfare aspects of animal stunning and killing methods. Eur Food Safety Author. 23(June):1–4.
- EFSA. European Food Safety. 2009. General approach to fish welfare and to the concept of sentience in fish. EFSA J. 7:2. doi:10.2903/J.EFSA.2009.954.
- Estévez M. 2011. Protein carbonyls in meat systems: a review. Meat Sci. 89(3):259–279. doi:10.1016/j.meatsci.2011.04.025.
- Estévez M, Kylli P, Puolanne E, Kivikari R, Heinonen M. 2008a. Oxidation of skeletal muscle myofibrillar proteins in oil-in-water emulsions: interaction with lipids and effect of selected phenolic compounds. J Agric Food Chem. 56(22):10933–10940. doi:10.1021/jf801784h.
- Estévez M, Kylli P, Puolanne E, Kivikari R, Heinonen M. 2008b. Fluorescence spectroscopy as a novel approach for the assessment of myofibrillar protein oxidation in oil-in-water emulsions. Meat Sci. 80(4):1290–1296. doi:10.1016/j.meatsci.2008.06.004.
- Fernandes AFA, Silva MA, Alvarenga ER, Teixeira EA, Silva Junior AF, Alves GFO, Salles SCM, Manduca LG, Turra EM. 2015. Morphometric traits as selection criteria for carcass yield and body weight in Nile tilapia (Oreochromis niloticus L.) at five ages. Aquaculture. 446:303–309. doi:10.1016/j.aquaculture.2015.05.009.
- Filho PRCO, Oliveira CAF, Sobral PJA, Balieiro JCC, Natori MM, Viegas EMM, Oliveira Filho PRC. 2015. How stunning methods affect the quality of Nile tilapia meat. CyTA J Food. 13(1):56–62. doi:10.1080/19476337.2014.911211.
- Ganhão R, Estévez M, Morcuende D. 2011. Suitability of the TBA method for assessing lipid oxidation in a meat system with added phenolic-rich materials. Food Chem. 126(2):772–778. doi:10.1016/j.foodchem.2010.11.064.
- Gomes LC, Araujo-Lima CARM, Roubach R, Chippari-Gomes AR, Lopes NP, Urbinati EC. 2003. Effect of fish density during transportation on stress and mortality of juvenile Tambaqui Colossoma macropomum. J World Aquacult Soc. 34(1):76–84. doi:10.1111/j.1749-7345.2003.tb00041.x.
- Gülçin I. 2006. Antioxidant and antiradical activities of L-carnitine. Life Sci. 78(8):803–811. doi:10.1016/j.lfs.2005.05.103.
- Gülçin I, Bursal E, Şehitoĝlu MH, Bilsel M, Gören AC. 2010. Polyphenol contents and antioxidant activity of lyophilized aqueous extract of propolis from Erzurum, Turkey. Food Chem Toxicol. 48(8–9):2227–2238. doi:10.1016/j.fct.2010.05.053.
- Halliwell B, Gutteridge JMC. 1985. Free radicals in biology and medicine. In: Acta crystallographica section D: structural biology. Michigan: Oxford University Press; p. 331–334.
- Hamm R. 1960. Biochemistry of meat hydration. Adv Food Res. 10(C):355–463. doi:10.1016/S0065-2628(08)60141-X.
- Hematyar N, Rustad T, Sampels S, Kastrup Dalsgaard T. 2019. Relationship between lipid and protein oxidation in fish. Aquac Res. 50(5):1393–1403. doi:10.1111/are.14012.
- Hikasa Y, Takase K, Ogasawara T, Ogasawara S. 1986. Anesthesia and recovery with tricaine methanesulfonate, eugenol and thiopental sodium in the carp, Cyprinus carpio. Jap J Vet Sci. 48(2):341–351. doi:10.1292/JVMS1939.48.341.
- Huff-Lonergan E, Lonergan SM. 2005. Mechanisms of water-holding capacity of meat: the role of postmortem biochemical and structural changes. Meat Sci. 71(1):194–204. doi:10.1016/j.meatsci.2005.04.022.
- Inoue LAKA, Neto CS, Moraes G. 2003. Clove oil as anaesthetic for juveniles of matrinxã Brycon cephalus (Gunther, 1869). Ciência Rural. 33(5):943–947. doi:10.1590/S0103-84782003000500023.
- Javahery S, Nekoubin H, Abdolmajid MH. 2012. Effect of anaesthesia with clove oil in fish (review). Phys Physiol Biochem. 38:1545–1552. doi:10.1007/s10695-012-9682-5.
- Kamatou GP, Vermaak I, Viljoen AM. 2012. Molecules eugenol-from the remote Maluku islands to the international market place: a review of a remarkable and versatile molecule. Molecules. 17:6953–6981. doi:10.3390/molecules17066953.
- Kestin SC, van de Vis JW, Robb DHF. 2002. Protocol for assessing brain function in fish and the effectiveness of methods used to stun and kill them. Vet Rec. 150(10):302–307. doi:10.1136/vr.150.10.302.
- Kildea MA, Allan GL, Kearney RE. 2004. Accumulation and clearance of the anesthetics clove oil and AQUI-STM from the edible tissue of silver perch (Bidyanus bidyanus). Aquaculture. 232:265–277. doi:10.1016/S0044-8486(03)00483-6.
- Knowles TG, Brown SN, Warriss PD, Lines J, Tinarwo A, Sendon M. 2008. Effect of electrical stunning at slaughter on the quality of farmed turbot (Psetta maxima). Aquac Res. 39(16):1731–1738. doi:10.1111/j.1365-2109.2008.02049.x.
- Lines JA, Spence J. 2012. Safeguarding the welfare of farmed fish at harvest. Fish Physiol Biochem. 38:153–162. doi:10.1007/s10695-011-9561-5.
- Lund MN, Heinonen M, Baron CP, Estévez M. 2011. Protein oxidation in muscle foods: a review. Mol Nutr Food Res. 55(1):83–95. doi:10.1002/mnfr.201000453.
- Medina I, Gallardo JM, Aubourg SP. 2009. Quality preservation in chilled and frozen fish products by employment of slurry ice and natural antioxidants. Int J Food Sci Technol. 44(8):1467–1479. doi:10.1111/j.1365-2621.2009.02016.x.
- Minolta. 1998. Precise color communication – color control from perception to instrumentation. Japan: MinoltaCo., Ltd..
- Nunes ML, Batista I, Cardoso C. 2007. Aplicação do índice de Qualidade (QIM) na Avaliação da frescura do Pescado (Franscisco Ruano, Aida Campos, Fátima Cardador, Irineu Batista, Manuela Falcão, Maria José Brogueira, Maria Manuel Martins, & Rogélia Martins, Eds.; Vol. 15). IPIMAR. https://comum.rcaap.pt/bitstream/10400.26/33873/1/Aplica%C3%A7%C3%A3o%20do%20%C3%ADndice%20de%20qualidade%20%28QIM%29%20na%20avalia%C3%A7%C3%A3o%20da%20frescura%20do%20pescado.pdf.Acess: jan 25 2023.
- OIE, W. O. for A. H. 2022. Aquatic animal health code. In Welfare aspects of stunning and killing of farmed fish for human consumption. https://www.woah.org/fileadmin/Home/eng/Health_standards/aahc/current/chapitre_welfare_stunning_killing.pdf.
- Oliveira Filho PRC, Sobral PJA, Baleiro JCC, Viegas EMM. 2017. Comparison of stunning methods on the physicochemical properties of Frozen Nile Tilapia (Oreochromis niloticus) Fillets. J Aquat Food Prod Technol. 26(3):325–334. doi:10.1080/10498850.2016.1184205.
- Pérez-Rosés R, Risco E, Vila R, Peñalver P, Cañigueral S. 2016. Biological and nonbiological antioxidant activity of some essential oils. J Agric Food Chem. 64(23):4716–4724. doi:10.1021/acs.jafc.6b00986.
- Petersen AM, Gleeson TT. 2011. Acclimation temperature affects the metabolic response of amphibian skeletal muscle to insulin. Comp Biochem Physiol A Mol Integr Physiol. 160(1):72–80. doi:10.1016/j.cbpa.2011.05.005.
- Ross LG, Ross B, Ross B. 2009. Anaesthetic and sedative techniques for aquatic animals: third edition. Wiley. doi:10.1002/9781444302264.
- Rotllant J, Balm PHM, Pérez-Sánchez J, Wendelaar-Bonga SE, Tort L. 2001. Pituitary and interrenal function in Gilthead sea bream (Sparus aurata L., Teleostei) after handling and confinement stress. Gen Comp Endocrinol. 121(3):333–342. doi:10.1006/gcen.2001.7604.
- Rucinque DS, Ferreira PF, Leme PRP, Lapa-Guimarães J, Viegas EM. 2021. Ocimum americanum and Lippia alba essential oils as anaesthetics for Nile tilapia: induction, recovery of apparent unconsciousness and sensory analysis of fillets. Aquaculture. 531:735902. doi:10.1016/j.aquaculture.2020.735902.
- Sant’Ana LS, Mancini-Filho J. 2000. Influence of the addition of antioxidants in vivo on the fatty acid composition of fish fillets. Food Chem. 68(2):175–178. doi:10.1016/S0308-8146(99)00172-7.
- Saraiva JL, Arechavala-Lopez P. 2019. Welfare of fish – no longer the elephant in the room. Fishes. 4(3):39. doi:10.3390/fishes4030039.
- Scherer R, Wagner R, Duarte MCT, Godoy HT. 2009. Composição e atividades antioxidante e antimicrobiana dos óleos essenciais de cravo-da-índia, citronela e palmarosa. Rev Bras Plantas Med. 11(4):442–449. doi:10.1590/S1516-05722009000400013.
- Senapati SR, Kumar GP, Singh CB, Xavier KAM, Chouksey MK, Nayak BB, Balange AK. 2017. Melanosis and quality attributes of chill stored farm raised whiteleg shrimp (Litopenaeus vannamei). Journal of Applied and Natural Science. 9(1):626–631. doi:10.31018/jans.v9i1.1242.
- Simões LN, Lombardi DC, Gomide ATM, Gomes LC. 2011. Efficacy of clove oil as anesthetic in handling and transportation of Nile tilapia, Oreochromis niloticus (Actinopterygii: Cichlidae) juveniles. Zoologia (Curitiba, Impresso). 28(3):285–290. doi:10.1590/S1984-46702011000300001.
- Simões MR, Ribeiro CDFA, Ribeiro SDCA, Park KJ, Murr FEX. 2007. Physicochemical and microbiological composition and yield of thai-style tilapia fillets (Oreochromis niloticus). Food Sci Technol. 27(3):608–613. doi:10.1590/S0101-20612007000300028.
- Skjervold PO, Rora AMB, Fjæra SO, Vegusdal A, Vorre A, Einen O. 2001. Effects of pre-, in-, or post-rigor filleting of live chilled Atlantic salmon. Aquaculture. 194:315–326. doi:10.1016/S0044-8486(00)00531-7.
- Standal IG, Mozuraityte R, Rustad T, Alinasabhematabadi L, Carlsson NG, Undeland I. 2018. Quality of filleted Atlantic Mackerel (Scomber scombrus) during chilled and frozen storage: changes in lipids, vitamin D, proteins, and small metabolites, including biogenic amines. J Aquat Food Prod Technol. 27(3):338–357. doi:10.1080/10498850.2018.1436107.
- Truong BQ, Buckow R, Nguyen MH, Stathopoulos CE. 2016. High pressure processing of barramundi (Lates calcarifer) muscle before freezing: the effects on selected physicochemical properties during frozen storage. J Food Eng. 169:72–78. doi:10.1016/j.jfoodeng.2015.08.020.
- Wang RR, Pan XJ, Peng ZQ. 2009. Effects of heat exposure on muscle oxidation and protein functionalities of pectoralis majors in broilers. Poult Sci. 88(5):1078–1084. doi:10.3382/ps.2008-00094.
- Zahran E, Risha E, Rizk A. 2021. Comparison propofol and eugenol anesthetics efficacy and effects on general health in Nile Tilapia. Aquaculture. 534:736251. doi:10.1016/j.aquaculture.2020.736251.
