ABSTRACT
The objective of the present study was to investigate the potential role of immunization against INH on testicular development, serum reproductive hormone (FSH, LH and T) concentrations, testicular biochemical indexes (GGT, ALP, ACP, LDH, α-neutral glucosidase and carnitine) and testicular cell apoptosis in rats. Forty healthy male rats were randomly divided into four groups (n = 10 per group), and respectively immunized with 0.5 mL of C500/pVAX-asd-IS-L (108 CFU/ mL), C500/pVAX-asd-IS-M (109 CFU/ mL), C500/pVAX-asd-IS-H (1010 CFU/ mL) or C500 (1010 CFU/ mL) (thrice with an interval of 2 weeks). P/N values increased after vaccination and differed (p < 0.05) at 1 week and sharply increased at 2 weeks following the booster vaccination (p < 0.01); P/N values indicated that the immune response was induced and production of anti-INH antibodies in rats; Immunization of INH vaccines enhanced serum concentrations of T and FSH, while reduced LH concentrations (p < 0.05); The testis weight, volume, testis coefficient, epididymal weight, sperm count in the epididymis, and the sperm deformity results indicated that INH gene vaccine immunization could promote testicular development and enhance sperm production rate in rats. After immunization of INH gene vaccines, GGT activity was markedly increased, but ALP, LDH and α-neutral glucosidase activities were observably reduced (p < 0.05). Furthermore, flow cytometry results demonstrated that the apoptosis rate of the testicular cells was extremely increased in the C500/pVAX-asd-IS-M group (p < 0.01). These findings illustrate a functional cross-link between FSH, T, LH and spermatogenic markers via inhibin to maintain spermatogenesis. INH immunization may have a positive effect on spermatogenesis and testicular development in male rats.
1. Introduction
Inhibin (INH) plays a vital role in the hypothalamus-pituitary gonadal (HPG) axis, which is secreted by Sertoli cells of testis in males and granulosa cells of ovarian follicles in females (Ying Citation1988), (Rehman et al. Citation2021). As a member of the transforming growth factor (TGF-β) superfamily, INH down-regulates the production of follicle-stimulating hormone (FSH) by the gonadotropic cells of the anterior pituitary in males (Meachem et al. Citation2001). INH has been proposed as an autocrine/paracrine factor that regulates follicular atresia, growth, steroidogenesis and gonadotropin responsiveness (Knight Citation1996; Akhtar et al. Citation2021). A variety of molecular forms of INH are present in circulation, while INH B is the only detectable one in adult males of most species (Li et al. Citation2018). Serum INH-B concentrations were positively correlated with spermatogenesis, testicular volume and total sperm counts, which appeared to be a reliable marker for human male fecundity (Bohring and Krause Citation1999; Yalti et al. Citation2002; Ball et al. Citation2019).
Many studies have indicated that neutralization of endogenous INH through active immunization could improve reproductive efficiency, spermatogenesis and testicular development in males (Bame et al. Citation1999; Lovell et al. Citation2000). Active immunization against INH α-subunit has been used as a method to check the physiology of INH in bulls and rams (Schanbacher Citation1991). Immunization against INH increases plasma FSH without affecting LH or testosterone concentrations (Akhtar et al. Citation2019). Immunization against INH enhances daily sperm production in the urine of prepubertal rams (Al-Obaidi et al. Citation1987) and spermatid numbers in the testes of bulls (Martin et al. Citation1991). Thus, these findings suggest that INH is a positive regulatory factor of spermatogenesis. However, a report suggests that immunizing adult rams with the INH vaccine increase serum FSH without altering sperm production (McKeown et al. Citation1997). Therefore, DNA vaccine immunization is considered to be safe and effective which has been widely investigated (Dan et al. Citation2016).
In the previous study, an attenuated Salmonella enterica serovar Choleraesuis C500 strain (asd and crp genes were deleted) was used as a delivery system for foreign antigens using the asd- balanced lethal chromosome–plasmid system (Zhao et al. Citation2009). A recombinant INH eukaryotic expression plasmid pVAX-asd-IS (INH fusion gene included) was constructed using attenuated Salmonella as a vector (Han et al. Citation2008) and used in this study. The present study was designed to investigate the potential role of gene immunization against INH on testicular development (testes coefficient), serum reproductive hormone (FSH, LH and testosterone) concentrations, testicular biochemical indexes and testicular cell apoptosis in rats. Our findings will provide a scientific basis for fully exploring the reproductive potential of animals and be of great significance for promoting the popularization of gene vaccine immunization technology.
2. Materials and methods
2.1. Recombinant plasmid
The attenuated asd /crp- S. choleraesuis C500 strain (C500) was stored in our laboratory. The kanamycin sequence in the expression vector pVAX1 (Invitrogen, Carlsbad, U.S.A.) was replaced by asd DNA sequence of S. typhimurium (GenBank AE008863), named pVAX-asd. The porcine inhibin α (1–32) and HBsAg-S recombinant gene fragment obtained from the pCIS plasmid (Invitrogen, Carlsbad, U.S.A.) were inserted into the NdeI and HindIII sites of the expression vector pVAX-asd, named pVAX-asd-IS, and then transfected the reconstructed vector pVAX-asd-IS into attenuated asd /crp- S. choleraesuis C500 strain (C500/pVAX-asd-IS) and stored at ̶ 80℃.
2.2. Identification and large-scale preparation of INH DNA vaccines
C500 and C500/pVAX-asd-IS were recovered in ice and separately cultured at 37℃ overnight a single colony was randomly picked and the plasmids of C500/pVAX-asd-IS and C500 were extracted by Endotoxin-free plasmid extraction Kit (CWBIO, China). The plasmids were digested with EcoRI and HindIII (Ferments, China) for 1.5–2 hours, target fragment was detected using 1.5% agarose gel, and sequenced by Shenzhen BGI Technology Co. LTD.
C500 and C500/pVAX-asd-IS strains were inoculated in LB using large bottles at 37℃ and shaken overnight for large-scale preparation of DNA vaccines, respectively. C500 and C500/pVAX-asd-IS in the bacterial solution were assessed by determining the number of colony-forming units (CFU) in triplicate and the concentration was expressed as CFU/mL.
2.3. Animals and vaccination
All animal experimental procedures were performed following the National Institutes of Health (NIH) Guidelines and the guide of the Chinese Association for Laboratory Animal Science. Forty healthy male SpragueDawley (SD) rats (6-week-olds with similar weights) were purchased from Hunan Silaikejingda Laboratory Animal Co. LTD. Under SPF conditions, all rats had free access to food and water and were fed under a 12-hour light/dark cycle at 25 ± 2℃ with a relative humidity of 50 ± 5%. The rats randomly were divided into four groups (n = 10 per group), and respectively immunized with 0.5 mL of C500/pVAX-asd-IS-L (108 CFU/ mL), C500/pVAX-asd-IS-M (109 CFU/ mL), C500/pVAX-asd-IS-H (1010 CFU/ mL) or C500 (1010CFU/ mL). The rats were administered through a deep intramuscular injection into the medial hind leg of rats and immunized thrice (same as primary immunization) with an interval of 2 weeks.
2.4. Sample collection and measurements at decapitation
Blood samples were collected from the caudal vein on weeks 0, 1, 2, 3, 4, 5 and 6 after primary immunization and centrifuged at 3000 rpm for 15 min at 4℃, and then the serum was separated and stored at −80℃ for further analysis.
After 6 weeks from primary immunization, all rats were weighed and anaesthetized with phenobarbital sodium and then decapitated. Both testes were removed and weighed to calculate the organ coefficients. The left testes were used for biochemical analysis and the right testes were used to detect cell apoptosis by flow cytometry (Thermo Fisher Scientific, U.S.A.).
2.5. Detection of antibodies against INH in serum
Specific IgG antibodies of serum were determined using an indirect ELISA Kit (Jianglai, China) with synthetic INHα (1–32) antigens (Sigma, U.S.A.) as standard antigens on weeks 0, 1, 2, 3, 4, 5 and 6 after primary immunization according to the manufacturer’s instructions. ELISA results were analysed in terms of P/N ratios.
2.6. Hormone determination in serum
Serum concentrations of testosterone, LH and FSH were measured using an Iodine (125I) rat-specific Radioimmunoas-Say Kit (North Institute, China) according to the manufacturer’s instructions. The radioactivity count (CPM) of each hormone was detected by a radio-immunogamma counter (Zhongcheng Electromechanical Technology, China). All samples were measured thrice.
2.7. Biochemical assays
The capsule and main blood vessels of the left testis were removed and analysed for glutamide transpeptidase (GGT), alkaline phosphatase (ALP), acid phosphatase (ACP), lactate dehydrogenase (LDH), α-neutral glucosidase and carnitine. All biochemical indexes were determined as per the instructions provided with the kits (R&D Systems, U.S.A.).
2.8. Cell apoptosis analysis
The testicular cell apoptosis was evaluated using dual-dye staining annexinV-PE and PI kit (R&D Systems, U.S.A.) according to the manufacturer’s instructions. The right testis was placed in a small dish with sterile pre-chilled PBS and the adipose tissue, albuginea and main blood vessels around the testis were carefully removed with ophthalmic forceps. The tissue volume of collagenase IV (0.25 mg/mL) was added ten times to the small dish, blowing with a straw and placed in an incubator with 5% CO2 at 37℃ for 30 min, filtered with a 200-mesh nylon mesh, then transferred to a 1.5 mL centrifuge tube. It was gently blown 1–2 times and centrifuged for 10 min at 800r/min. The cell pellets were washed three times with pre-chilled PBS and resuspended with D-Hanks solution, filtered through 200-mesh nylon mesh, centrifuged again at 800r/min for 10 min, the supernatant was discarded and 1 mL PBS containing 0.5% BSA was added. The cell apoptosis analysis was performed on the BD LSRII Flow Cytometer System with FACS Diva Software within 1 h of staining.
2.9. Statistical analysis
All data were presented as means ± SEM and analysed using SPSS Statistics 24.0 software (SPSS Software, Inc., Chicago, IL). Data was analysed using a one-way analysis of variance (ANOVA) test followed by Dunnett’s multiple comparison test and a significant difference was inferred for p < 0.05 and extremely significant difference p < 0.01.
3. Results
3.1. Identification of vaccines
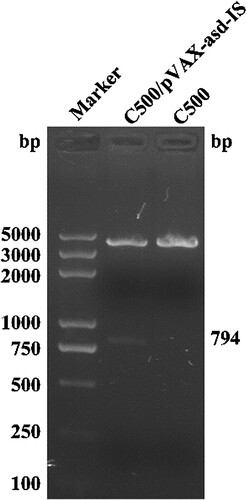
To verify the integrity of the DNA vaccines (C500/pVAX-asd-IS and C500), the plasmids were extracted and digested with EcoRI and HindIII endonucleases, the target fragment of C500/pVAX-asd-IS plasmids (794 bp) was displayed on 1.5% agarose gel and the plasmids of C500 were not shown (). Meanwhile, sequence analysis showed that the C500/pVAX-asd-IS plasmids were intact.
3.2. Detection of antibody in serum
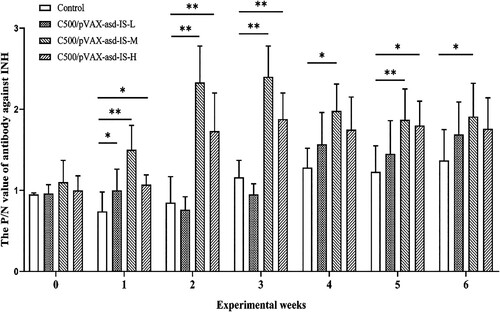
The P/N ratios indicate that anti-INH antibodies in all immunized rats at 0 weeks and the control group were not detectable throughout the experiment. The rats immunized with C500/pVAX-asd-IS induced antibodies against INH at 1 week after primary immunization. Following the booster vaccination, the P/N values were sharply increased in C500/pVAX-asd-IS-M and C500/pVAX-asd-IS-H groups (p <0.01). Rats in the C500/pVAX-asd-IS-M group had P/N ratios of 2.33 and 2.40, respectively, 2 and 3 weeks post-primary vaccination. The results indicated that immunization of INH vaccines induced the immune response and production of anti-INH antibodies in rats. ()
3.3. Serum hormone concentrations in serum
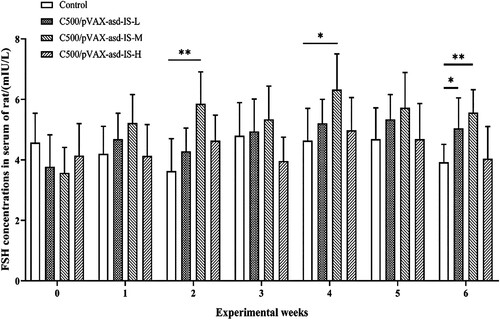
No significant differences were observed in serum concentrations of T, INH B, LH and FSH between rats vaccinated C500/pVAX-asd-IS and C500 at 0 weeks. At 2, 4 and 6 weeks after primary immunization, the FSH levels in C500/pVAX-asd-IS-M group were significantly higher than that in the control group (p < 0.05 or p < 0.01). The FSH levels in C500/pVAX-asd-IS-L group were significantly higher than that in the control group at 6 weeks after primary immunization (p < 0.05). ()
Figure 3. FSH concentrations in the serum of rats. Results were compared with the control group at each week and data are presented as the mean ± SEM (n = 10), * and ** indicate significant differences p <0.05 and p <0.01, respectively among groups.
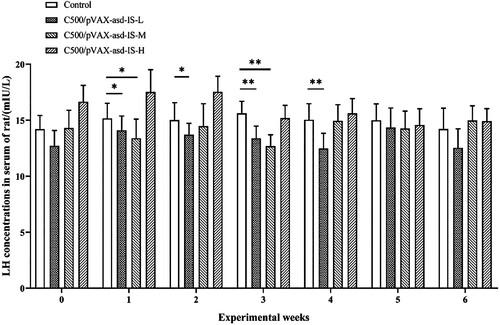
At 1 week after primary immunization, the LH concentrations in serum of C500/pVAX-asd-IS-L and C500/PVAX-asd-IS-M groups were significantly lower than that of the control group (p < 0.05), and at 3 weeks after primary immunization, the LH contents in serum of C500/PVAX-asd-IS-M and C500/PVAX-asd-IS-L groups were significantly lower than that of the control group (p < 0.05). 4 weeks post-immunization LH levels of C500/PVAX-asd-IS-L reached their peak in serum. ()
Figure 4. LH concentrations in the serum of rats. Results were compared with the control group at each week and data are presented as the mean ± SEM (n = 10), * and ** indicate significant differences p <0.05 and p <0.01, respectively among groups.
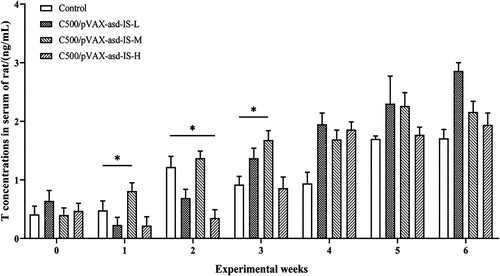
T concentrations in serum of the C500/pVAX-asd-IS-M group were significantly greater than that of the control group at 1 and 3 weeks post-primary immunization (p < 0.05). At 2 weeks post-primary immunization, the T levels of C500/PVAX-asd-IS-H were significantly lower than that of the control group in serum (p < 0.05). ()
3.4. Reproductive traits
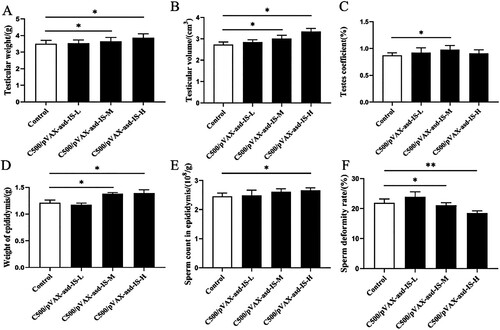
For the period between weeks 0 and 6, INH gene vaccine immunization could promote testicular development in rats (). Testis weight and volume in C500/pVAX-asd-IS-M and C500/pVAX-asd-IS-H groups were increased compared with the control group, respectively (p <0.05). The testis coefficient in the C500/pVAX-asd-IS-M group was greater than that of the control group (p <0.05). Furthermore, the epididymal weight in C500/pVAX-asd-IS-M and C500/pVAX-asd-IS-H groups was raised (p <0.05), the sperm count in epididymis was greater than that of control group (p <0.05) and the sperm deformity rate in epididymis was reduced in C500/pVAX-asd-IS-M and C500/pVAX-asd-IS-H groups compared with the control group (p < 0.05 or p < 0.01).
Figure 6. Effects of INH immunization on testicular weight (A), testicular volume (B), testis coefficient (C), epididymal weight (D), sperm count in the epididymis (E) and sperm deformity rate in the epididymis (F) of rats (%). Results were compared with the control group and data are presented as the mean ± SEM (n = 10), * and ** indicate significant differences p <0.05 and p <0.01, respectively among groups.
3.5. Biochemical indexes of testes
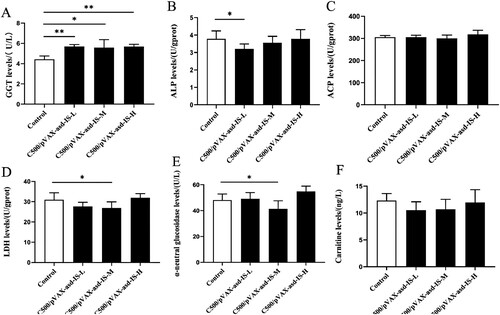
Compared with the control group,the GGT levels were markedly increased in the C500/pVAX-asd-IS groups (p < 0.05 or p < 0.01). The C500/pVAX-asd-IS-L group displayed a significant decrease in the levels of ALP in comparison with the control group. In addition, a significant reduction in LDH and α-neutral glucosidase (p < 0.05) in the C500/pVAX-asd-IS-M group, was observed compared with the control group. ()
Figure 7. Effects of INH gene immunization on GGT (A), ALP (B), ACP (C), LDH (D), α-neutral glucosidase (E) and carnitine (F) levels of rat testes. Results were compared with the control group and data are presented as the mean ± SEM (n = 10), * and ** indicate significant differences p <0.05 and p <0.01, respectively among groups.
3.6. Cell apoptosis analysis of testes
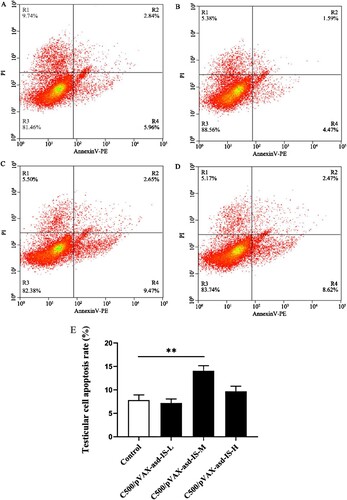
To elucidate whether INH gene vaccines affect testicular cell apoptosis in rats, cell apoptosis was measured by flow cytometry. Results demonstrated that the apoptosis rate of the testicular cells was extremely increased in the C500/pVAX-asd-IS-M group (p < 0.01) ().
Figure 8. Percentage of testicular apoptotic cells in rats immunized with C500 (A), C500/pVAX-asd-IS-L (B), C500/pVAX-asd-IS-M (C) and C500/pVAX-asd-IS-H (D), which were analysed by flow-cytometry using Annexin V-PE/PI double staining. Quantitative analysis of apoptotic cells by Annexin V-PE/PI (E). Results were compared with the control group and data are presented as the mean ± SEM (n = 10), * and ** indicate significant differences p <0.05 and p <0.01, respectively among groups.
4. Discussion
Previous studies have demonstrated that some DNA vaccines expressing the inhibin gene have been successfully constructed and these vaccines elicited better immune responses (Wang et al. Citation2012). In mice, the pVAX-asd-IS vector, containing recombinant inhibin α subunit (1–32) fragment, could be used as a potent immunogenicity enhancer and integration studies confirmed its safety as a DNA vehicle. (Han et al. Citation2014). In this study, all rats immunized with C500 or C500/pVAX-asd-IS survived, and no signs of disease were observed in the immunized mice throughout the experimental period. Active immunization against INH induced a good antibody response. Especially, after the booster immunization, there was a sharp increase in C500/pVAX-asd-IS-M and C500/pVAX-asd-IS-H groups of the P/N values in serum.
In the present study, INH gene vaccine immunization led to increased testicular weight and volume in C500/pVAX-asd-IS-M and C500/pVAX-asd-IS-H groups, and the increased testis coefficient in C500/pVAX-asd-IS-M group at decapitation. Furthermore, the epididymal weight was also raised in C500/pVAX-asd-IS-M and C500/pVAX-asd-IS-H groups and the sperm count of epididymis was improved in the C500/pVAX-asd-IS-H group, while the sperm deformity rate of epididymis was reduced in C500/pVAX-asd-IS-M and C500/pVAX-asd-IS-H groups. In agreement with our findings, a previous study has reported that active immunization against INH affected spermatogenesis and testicular development in goose ganders (Akhtar et al. Citation2019), and improved reproductive efficiency and testes weight of rams (Voge and Wheaton Citation2007). Sperm production is directly related to testicular volume in cockerels (De Reviers and Williams Citation1984), and the increase in testicular weight may probably also be reflected in an enhanced sperm production rate although the measurement of sperm production rate was beyond the scope of the current study. Active immunization against inhibin caused a similar increase in testis diameter and increased daily sperm output in male lambs (Al-Obaidi et al. Citation1987), thus, we propose that INH gene vaccine immunization could promote testicular development and enhance sperm production rate in rats.
The immunization elevated the P/N value of antibodies against INH which was associated with an increase in T concentration (Bame et al. Citation1999). Testicular Leydig cells secrete a large amount of T, which promotes and maintains spermatogenesis and testicular development (Han et al. Citation2016). In this study, T concentrations of serum were increased in the C500/pVAX-asd-IS-M group at 1 and 3 weeks post primary immunization. But the T levels of serum were reduced in C500/PVAX-asd-IS-H after the first booster because the main increase of T concentration would occur after the first booster immunization onwards. Exogenous immunization against inhibin may inhibit endogenous inhibin secreted by Sertoli cells, which subsequently increases T concentrations (Akhtar et al. Citation2020). In general, the LH concentrations in serum were reduced, and the FSH concentrations were increased and our data corroborated with the earlier reports (Bame et al. Citation1999). LH and FSH are under positive control of hypothalamic GnRHgonadal steroids and INH (Themmen and Huhtaniemi Citation2000). It has been proposed that INH immunization increases FSH concentrations and stimulates follicular development by neutralizing endogenous INH (Wang et al. Citation2012). INH immunization produces specific antibodies to neutralize endogenous INH, which promotes FSH secretion and inhibits LH secretion in a feedback manner (Mitchell et al. Citation2011). In inhibin-immunized bulls, sperm production, total sperm production and sperm density were positively correlated with serum FSH (Bame et al. Citation1996). Thus, high concentrations of T are beneficial to the spermatogenesis and maturation of male animals, but inhibit LH levels in a negative feedback manner.
Earlier reports showed that some spermatogenic markers, such as ALP, ACP, LDH, α-neutral glucosidase and carnitine, play a critical role in the development of the male reproductive organs and maintenance of spermatogenesis (Mai et al. Citation2020). The GGT activities markedly were increased in the C500/pVAX-asd-IS groups. GGT is present at the plasma membrane of Sertoli cellsinvolved in cell secretion and transferrin synthesis and is considered to be a marker of Sertoli cell function (Batista-Silva et al. Citation2020). The increased LDH activity leads to the deterioration of germinal epithelium of seminiferous tubules in rat testis (Mai et al. Citation2020). The degeneration of the testis parenchyma and the increase of lytic activity were positively correlated with ALP activity (Aly and Azhar Citation2013). While our data display a significant reduction in LDH and α-neutral glucosidase in the C500/pVAX-asd-IS-M group and ALP levels show a significant decrease in theC500/pVAX-asd-IS-L group, which is beneficial to testicular development in rats. The activities of these enzymes are regulated by T (Jana et al. Citation2006) and alterations in these enzyme activities might be due to improved T levels in INH immunization rats.
Apoptosis is a normal phenomenon in proliferating and continuously rejuvenating tissues and seminiferous epithelium and seasonality in breeding enhances the apoptosis rate (Banerjee and Chaturvedi Citation2017). A previous study has shown that the numbers of testicular cells started decreasing after INH immunization in Yangzhou ganders, and the concomitant effect of INH and seasonality may be the reason for testicular apoptosis (Akhtar et al. Citation2021). In our results, although the apoptosis rate of testicular cells was extremely increased in the C500/pVAX-asd-IS-M group, no significant increase occurred in the other groups, the increased apoptosis rate of testicular cells in the C500/pVAX-asd-IS-M group cannot be explained by single reason of INH immunization in this study. For normal spermatogenesis, high fertility and semen quality, normal development of germ cells is necessary (Akhtar et al. Citation2020). Sperm production is also directly related to Sertoli cell number (De Reviers and Williams Citation1984). In this regard, we speculate that the other cells of the testis have caused apoptosis after INH immunization in rats. However, which types of cells caused apoptosis of the testis in INH immunization rats requires further investigation.
5. Conclusion
This study characterized the neutralization of endogenous INH through intramuscular injection vaccination with INH DNA delivered by C500 strain, and successfully elicited a humoral immune response. These findings illustrate a functional cross-link between FSH, T and spermatogenic markers via inhibin to maintain spermatogenesis. INH immunization may have a positive effect on spermatogenesis and testicular development in male rats.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available on request from the corresponding author (Shuilian Wang), upon reasonable request.
Additional information
Funding
References
- Akhtar MF, Ahmad E, Ali I, Shafiq M, Chen Z. 2021. The effect of inhibin immunization in seminiferous epithelium of yangzhou goose ganders: A histological study. Animals (Basel). 11(10):2801–2809. doi:10.3390/ani11102801.
- Akhtar MF, Ahmad E, Mustafa S, Chen Z, Shi Z, Shi F. 2020. Spermiogenesis, stages of seminiferous epithelium and variations in seminiferous tubules during active states of spermatogenesis in yangzhou goose ganders. Animals (Basel). 10(4):570–583. doi:10.3390/ani10040570.
- Akhtar MF, Wei Q, Zhu H, Chen Z, Ahmad E, Zhendan S, Shi F. 2019. The role of active immunization against inhibin α-subunit on testicular development, testosterone concentration and relevant genes expressions in testis, hypothalamus and pituitary glands in Yangzhou goose ganders. Theriogenology. 128:122–132. doi:10.1016/j.theriogenology.2019.01.039.
- Al-Obaidi S, Bindon B, Hillard M, O'shea T. 1987. Reproductive characteristics of lambs actively immunized early in life with inhibin-enriched preparations from follicular fluid of cows. Reproduction. 81:403–414. doi:10.1530/jrf.0.0810403.
- Aly HA, Azhar AS. 2013. Methoxychlor induced biochemical alterations and disruption of spermatogenesis in adult rats. Reprod Toxicol. 40:8–15. doi:10.1016/j.reprotox.2013.05.002.
- Ball BA, Davolli GM, Esteller-Vico A, Fleming BO, Wynn MAA, Conley AJ. 2019. Inhibin-A and inhibin-B in stallions: seasonal changes and changes after down-regulation of the hypothalamic-pituitary-gonadal axis. Theriogenology. 123:108–115. doi:10.1016/j.theriogenology.2018.09.036.
- Bame J, Dalton J, Degelos S, et al. 1996. Immunization against inhibin enhances sperm production in dairy bulls. In: Biology of reproduction. Soc study reproduction 1603 Monroe St, Madison, WI 53711-2021. pp. 328–328.
- Bame J, Dalton J, Degelos S, Good TEM, Ireland JLH, Jimenez-Krassel F, Sweeney T, Saacke RG, Ireland JJ. 1999. Effect of long-term immunization against inhibin on sperm output in Bulls1. Biol Reprod. 60:1360–1366. doi:10.1095/biolreprod60.6.1360.
- Banerjee S, Chaturvedi CM. 2017. Apoptotic mechanism behind the testicular atrophy in photorefractory and scotosensitive quail: involvement of GnIH induced p-53 dependent bax-caspase-3 mediated pathway. J Photochem Photobiol, B. 176:124–135. doi:10.1016/j.jphotobiol.2017.09.023.
- Batista-Silva H, Dambros BF, Rodrigues K, Cesconetto PA, Zamoner A, Sousa de Moura KR, Gomes Castro AJ, Van Der Kraak G, Mena Barreto Silva FR. 2020. Acute exposure to bis(2-ethylhexyl)phthalate disrupts calcium homeostasis, energy metabolism and induces oxidative stress in the testis of Danio rerio. Biochimie. 175:23–33. doi:10.1016/j.biochi.2020.05.002.
- Bohring C, Krause W. 1999. Serum levels of inhibin B in men with different causes of spermatogenic failure. Andrologia. 31:137–141. doi:10.1111/j.1439-0272.1999.tb01400.x.
- Dan X, Liu X, Han Y, Liu Q, Yang L. 2016. Effect of the novel DNA vaccine fusing inhibin α (1-32) and the RF-amide related peptide-3 genes on immune response, hormone levels and fertility in Tan sheep. Anim Reprod Sci. 164:105–110. doi:10.1016/j.anireprosci.2015.11.018.
- De Reviers M, Williams, JB. 1984. Testis development and production of spermatozoa in the cockerel (Gallus domesticus). In: Cunningham FJ, Lake PE, Hewitt D, editors. Reproductive biology of poultry. Harlow, UK: British Poultry Science Ltd.; p. 183–202.
- Han L, Mao DG, Zhang DK, Liang AX, Fang M, Moaeen-ud-Din M, Yang LG. 2008. Development and evaluation of a novel DNA vaccine expressing inhibin α (1–32) fragment for improving the fertility in rats and sheep. Anim Reprod Sci. 109:251–265. doi:10.1016/j.anireprosci.2007.12.012.
- Han L, Zhen YH, Liang AX, Zhang J, Riaz H, Xiong J, Guo A, Yang L. 2014. Oral vaccination with inhibin DNA delivered using attenuated Salmonella choleraesuis for improving reproductive traits in mice. J Basic Microbiol. 54:962–968. doi:10.1002/jobm.201300052.
- Han XF, Li JL, Zhou YQ, Ren X-H, Liu G-C, Cao X-H, Du X-G, Zeng X-Y. 2016. Active immunization with GnRH-tandem-dimer peptide in young male rats reduces serum reproductive hormone concentrations, testicular development and spermatogenesis. Asian J Androl. 18:485–491. doi:10.4103/1008-682X.156856.
- Jana K, Jana S, Samanta PK. 2006. Effects of chronic exposure to sodium arsenite on hypothalamo-pituitary-testicular activities in adult rats: possible an estrogenic mode of action. Reprod Biol Endocrinol. 4:1–13. doi:10.1186/1477-7827-4-9.
- Knight P. 1996. Roles of inhibins, activins, and follistatin in the female reproductive system. Front Neuroendocrinol. 17:476–509. doi:10.1006/frne.1996.0013.
- Li Y, Fortin J, Ongaro L, Zhou X, Boehm U, Schneyer A, Bernard DJ, Lin HY. 2018. Betaglycan (TGFBR3) functions as an inhibin A, but not inhibin B, coreceptor in pituitary gonadotrope cells in mice. Endocrinology. 159:4077–4091. doi:10.1210/en.2018-00770.
- Lovell TM, Knight PG, Groome NP, Gladwell RT. 2000. Measurement of dimeric inhibins and effects of active immunization against inhibin α-subunit on plasma hormones and testis morphology in the developing Cockerel. Biol Reprod. 63:213–221. doi:10.1095/biolreprod63.1.213.
- Mai X, Dong Y, Xiang L, Er Z. 2020. Maternal exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin suppresses male reproductive functions in their adulthood. Hum Exp Toxicol. 39:890–905. doi:10.1177/0960327120903489.
- Martin T, Williams G, Lunstra D, Ireland J. 1991. Immunoneutralization of inhibin modifies hormone secretion and sperm production in Bulls1. Biol Reprod. 45:73–77. doi:10.1095/biolreprod45.1.73.
- McKeown R, O'Callaghan D, Roche J, Boland M. 1997. Effect of immunization of rams against bovine inhibin 1-26 on semen characteristics, scrotal size, FSH, LH and testosterone concentrations. Reproduction. 109:237–245. doi:10.1530/jrf.0.1090237.
- Meachem S, Nieschlag E, Simoni M. 2001. Inhibin B in male reproduction: pathophysiology and clinical relevance. Eur J Endocrinol. 145:561–571. doi:10.1530/eje.0.1450561.
- Mitchell V, Robin G, Boitrelle F, Massart P, Marchetti C, Marcelli F, Rigot JM. 2011. Correlation between testicular sperm extraction outcomes and clinical, endocrine and testicular histology parameters in 120 azoospermic men with normal serum FSH levels. Int J Androl. 34:299–305. doi:10.1111/j.1365-2605.2010.01094.x.
- Rehman A, Ahmad E, Arshad U, Riaz A, Akhtar MS, Ahmad T, Khan JA, Mohsin I, Shi Z, Sattar A. 2021. Effects of immunization against inhibin α-subunit on ovarian structures, pregnancy rate, embryonic and fetal losses, and prolificacy rate in goats where estrus was induced during the non-breeding season. Anim Reprod Sci. 224:106654. doi:10.1016/j.anireprosci.2020.106654.
- Schanbacher B. 1991. Pituitary and testicular responses of beef bulls to active immunization against inhibin alpha. J Anim Sci. 69:252–257. doi:10.2527/1991.691252x.
- Themmen AP, Huhtaniemi IT. 2000. Mutations of gonadotropins and gonadotropin receptors: elucidating the physiology and pathophysiology of pituitary-gonadal function. Endocr Rev. 21:551–583. doi:10.1210/edrv.21.5.0409.
- Voge J, Wheaton J. 2007. Effects of immunization against two inhibin antigens on daily sperm production and hormone concentrations in ram lambs. J Anim Sci. 85:3249–3255. doi:10.2527/jas.2007-0506.
- Wang S-L, Han L, Ahmad S, Cao S-X, Xue L-Q, Xing Z-F, Feng J-Z, Liang A-X, Yang L-G. 2012. Effect of a DNA vaccine harboring two copies of inhibin α (1-32) fragments on immune response, hormone concentrations and reproductive performance in rats. Theriogenology. 78:393–401. doi:10.1016/j.theriogenology.2012.02.019.
- Yalti S, Gürbüz B, Fiçicioğlu C. 2002. Serum levels of inhibin B in men and their relationship with gonadal hormones, testicular volume, testicular biopsy results and sperm parameters. J Obstet Gynaecol. 22:649–654. doi:10.1080/0144361021000020466.
- Ying S-Y. 1988. Inhibins, activins, and follistatins: gonadal proteins modulating the secretion of follicle-stimulating hormone. Endocr Rev. 9:267–293. doi:10.1210/edrv-9-2-267.
- Zhao Z, Xu Y, Wu B, Cheng X, Li Y, Tang X, Zhang C, Chen H. 2009. Characterization of attenuated Salmonella C500 strain with a delta asd mutant and use as an asd+ balanced-lethal host-vector system. Sheng wu Gong Cheng xue bao = Chin J Biotechnol. 25:29–36.