Abstract
Reedbed in the UK has been classified as priority habitat for most regional Biodiversity Partnerships. However, critical information pertaining to the quality and spatial coverage of reedbed is currently lacking. This paper presents the results of a project conducted in collaboration with the Cumbria Wildlife Trust and Environment Agency aimed at monitoring and understanding variations in the spectral reflectance and biophysical properties of reedbed canopies across Leighton Moss Nature Reserve in Lancashire, northwest England. Throughout the seasonal phenological cycle of the reedbed habitats in the study area, optimal spectral indices required for quantifying its biophysical properties were determined using field spectroscopy and ground-based measurements. Results of the experiment showed that the narrow-band-derived Difference Vegetation Index (DVI) and Renormalised Difference Vegetation Index (RDVI), with the correlation coefficient R2 of 0.77 and 0.72, respectively, provided the most accurate estimates of the leaf area index for the reedbed canopies.
1. Introduction
Leighton Moss Nature Reserve is dominated by vast distribution of Phragmites austrilis (Phragmites) existing alongside the reedmace (Typha) plant species. The reserve is classified as a site of special and scientific interest and a protected area of high conservation interests in the UK. The Phragmites is a perennial plant that grows up to a maximum height of three meters with a thick mass of roots called the rhizomes. The height, density, and stem thickness (i.e. diameter) are some of the major biophysical measures used in estimating the quality of the reedbed canopy (Hawke and José Citation1996).
Remotely sensed data have been widely used in quantifying biophysical properties of different vegetation types (Liu et al. Citation2007; Wang, Huang, and Wang Citation2008). The use of high-resolution field spectral data has also been shown to assist in the effective differentiation of plant species and an in-depth understanding of vegetation physiology over time (Bork, West, and Price Citation1999; Bork et al. Citation1999; Schmidt and Skidmore Citation2003; Armitage, Kent, and Weaver Citation2004; Gao and Zhang Citation2006). Gao and Zhang (Citation2006) investigated the spectral characteristics of four plant communities (Phragmites australis community, Spartina alterniflora community, Scirpus mariqueter community, and Carex scabrifolia community) in the seasons of spring, summer, and autumn at the Chongming Dongtan Nature Reserve in Shanghai using a ground FieldSpec Pro JR spectroradiometer. The results of the study showed that the spectral characteristics of the four plant communities were unique during the three seasons studied and the near-ground spectral reflectance varied with the growing season, community type, and its phenology.
In addition to the use of only spectral information, both spectral characteristics and biophysical measures have been used as parameters for investigating the conditions of diverse vegetation canopies (Elvidge and Chen Citation1995; McDonald, Gemmell, and Lewis Citation1998; Blackburn and Pitman Citation1999; Blackburn and Steele Citation1999; Cochrane Citation2000). Blackburn and Steele (Citation1999) investigated the relationships between spectral reflectance characteristics, concentrations of photosynthetic pigments, and biophysical attributes for semiarid bush land canopies. They derived relationships between spectral features and biophysical properties of the matorral vegetation type and identified the factors influencing such relationships. The relationships between spectral reflectance and biophysical measures were determined using empirical methods of data analysis based on mathematical regression models such as linear, logarithmic, exponential, or power functions. The first derivative and red edge reflectance properties of vegetation canopies were shown to have strong correlations with increasing vegetation cover. Shaw, Malthus, and Kupiec (Citation1998) established a clear potential for monitoring changes in vegetation canopy cover using very high-resolution indices in the red edge spectral region. The relative proportion of two derivative red edge maxima (D719/D703) and derivative red edge shoulders (D730/D700) were shown to contain vital information about the proportion of species compared to the red edge position or single-wavelength indices. Apart from vegetation indices (VIs) calculated by multiple single bands within the red edge region, indices are derived by different simulated sensor images from ground-acquired spectral data. Previous studies have developed correlations between the leaf area index (LAI) and red edge parameters in the first derivative reflectance region (Horler, Dockray, and Barber Citation1983; Filella and Penuelas Citation1994; Shaw, Malthus, and Kupiec Citation1998). Armitage, Kent, and Weaver (Citation2004) used simulated Compact Airborne Spectrographic Imager (CASI) sensor to calculate series of VIs. The derivations of different VIs employ a variety of spectral information and relate these measures with different biophysical measures was investigated in this study.
The study aims at investigating the multi-seasonal spectral variation of Phragmites australis, a dominant species of the reedbed wetland habitats in the UK. The specific objectives of the study are as follows:
| (1) | To investigate the relationship between different biophysical properties of reedbed sample sites of varying canopy structure over summer, winter, spring, and autumn; | ||||
| (2) | To analyze the seasonal properties in spectral reflectance of varying reedbed canopy sample sites; and | ||||
| (3) | To compare the relationships between biophysical measures and spectral reflectance derivatives (such as VIs derived by broad band, narrow band, or single wavelengths; and red edge derivative reflectance) of different sample plots observed over the four seasons. | ||||
2. Materials and methods
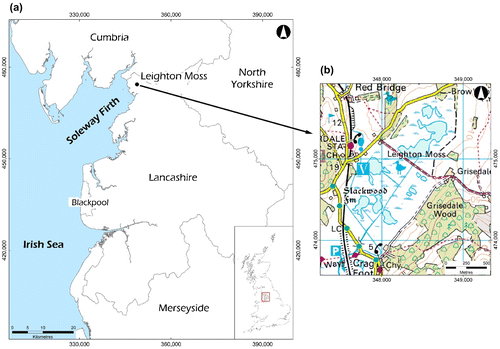
2.1. Study site
The site used for this study was Leighton Moss Nature Reserve (2°47′W, 54°10′N) situated in the northern part of Lancashire county, north-western England (Figure ). The study site covers an area of 175 ha in a wide shallow basin confined by limestone ridges and the high salt marshes of Morecambe Bay to the southwest (Middleton et al. Citation1995). At present, the basin is occupied by a large reedbed site, the largest in the north-western region (Ratcliffe Citation1977). Leighton Moss Nature Reserve is known to maintain suitable wetland habitat for endangered species such as the bitterns and other wildlife that continue to depend on its ecological diversity for continued existence.
2.2. Selection of sample plots
The sampling plots were selected based on the variation of reed canopy heights and habitat conditions. Most of the canopies had a homogenous floristic pattern of Phragmites across the reserve. Randomly selected sampling plots were located between the dry reedbed and willow carrs and in the wet reedbed habitats (Kent and Coker Citation1992). It was observed that the height of the Phragmites situated between the dry reedbed and willow carrs were smaller compared to those in the wet and dry reedbeds. During the study, field campaign to acquire spectral response readings and biophysical measurements were implemented in this order: summer (June–August 2008), winter (December 2008–February 2009), spring (April–May 2009) and autumn (September–October 2009). The coordinates of each sampling plot were taken by a Garmin eTrex handheld GPS.
2.3. Collection of biophysical measures
The biophysical measures of 63 randomly sampled plots were acquired over winter, autumn, summer, and spring. Field measurements included LAI, average diameter of reed stems per sample plot (D), canopy height (CH) of reed stems per sample plot, and the number of reed stems per sample plot (n). The calculated biophysical measures included the reed density (RD = 4 × n) and basal area (BA = 4πr2, where r = D/2) Here, the multiplier factor of four was used to derive measures for 1 m2 from sampling plots of 0.25 m2. The LAI measurements were performed employing the Li-COR LAI-2000 Plant Canopy Analyzer and processed by the FV2000 software. The LAI-2000 estimates LAI through radiation transmission values obtained by ratioing radiation measured at the top and the bottom of the canopy. A measurement with the LAI-2000 consists of a minimum of 10 numbers: five of the numbers are signals from the five detectors when the optical sensor is above the vegetation, and the remaining five are the readings made with the sensor below the vegetation (Li-COR, Inc Citation1992).
2.4. Spectral reflectance measurements and processing
For each sample plot, a GER 1500 spectroradiometer was used to measure the spectral reflectance of the reedbed canopies. The instrument has a spectral range of 350–1050 nm covering the ultraviolet (UV), visible and near infrared (NIR) wavelengths. It has a bandwidth of 1.5 nm and 512 channels. The spectroradiometer was used in standalone mode with a notebook interface. The sensor head of the instrument had a field of view of 15° and every measurement was made from a nadir position with the sensor head above the canopy. The measurements were made between 10:49 and 14:10 local time under clear sky and sunny weather conditions in order to obtain consistent spectral readings (Milton Citation1987; McCoy Citation2005). A Spectralon panel, SRT 99-050, was used as the reference panel when making measurements. All the spectral data were converted to reflectance values using the GER 1500 Version 1.30 Software and the results analyzed. For each sample plot, a minimum of 10 spectral readings were taken and the mean obtained to eliminate any potential source of variation (Blackburn and Pitman Citation1999; Gao and Zhang Citation2006).
After processing the high-resolution field spectroradiometric readings obtained by the GER 1500, the percentage reflectance values were simulated to represent the Landsat ETM+ (bands 1, 2, 3, and 4) and the CASI used by the UK Natural Environment Research Council (Armitage, Kent, and Weaver Citation2004). Details of the bandwidth settings of both simulated sensors are shown in Table . Landsat and CASI bands were generated by the average values of field-measured spectral reflectance and subsequently used to derive the VIs. The relationship between biophysical measures and VIs were derived by high-resolution field reflectance spectra data. The broad-band and narrow-band VIs were computed by simulated Landsat ETM+ and CASI bands, respectively. The single reflectance wavelength VIs was based on correlation peaks derived from a correlogram of all biophysical and single-wavelength percentage reflectance. The VIs evaluated in this study are presented in Table . There were 15 possible combinations of CASI Red and NIR bands used in computing the six VIs.
Table 1. Simulated Landsat ETM+ and CASI bands used for calculating the broad- and narrow-band VIs.
Table 2. Vegetation indices used in the study.
The continuum-removal analysis of Kokaly and Clark (Citation1999) enables the isolation of absorption features of interest, thus increasing the coefficients of determination and facilitating the identification of more sensible absorption features (Huang et al. Citation2004). It can also be considered as an improvement on the standard derivative analysis as demonstrated in a variety of applications (Huang et al. Citation2004; Mutanga and Skidmore Citation2004; Mutanga, Skidmore, and Prins Citation2004; Adam et al. Citation2011). The process of continuum-removal analysis entails choosing specific parts of the spectrum where it is believed that there is useful information for a particular study area. Different matrices (such as band depth and band depth normalized by area) are calculated and investigations are made to determine which biophysical measure best relates with the calculated matrix. The continuum-removal analysis pulls out more of the intricate differences in the spectra between the plots. For this study, the empirical methods of data analysis based on mathematical regression models (such as linear, logarithmic, exponential, or power functions) were used as demonstrated by Blackburn and Steele (Citation1999).
The spectral readings of the Phragmites obtained for each season were averaged. The first derivative of the spectral reflectance curve was computed in order to identify the red edge characteristics of the Phragmites vegetation by , where
is the wavelength,
is the first derivative amplitude at
,
is the reflectance at the wavelength
, and Δλ is the wavelength interval between
or
and
.
3. Results and discussion
3.1. Relationships between different biophysical measures
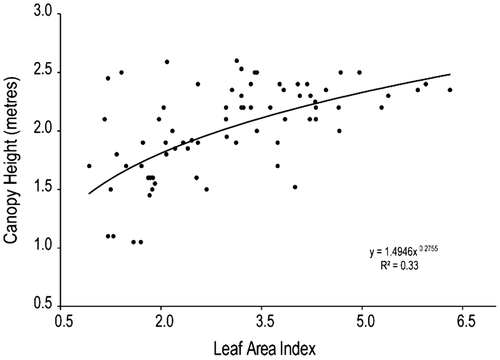
The results of a linear correlation matrix showed no significant relation between most measured biophysical measures with the exception of LAI and canopy height (CH) (correlation coefficient R = 0.56, p-value p < 0.01). Further analysis using logarithmic, exponential and power function mathematical models indicated that the power model (y = 1.4946x0.2755, R = 0.58) could best describe the relationship between CH and LAI (as shown in Figure ). The significant correlation between LAI and CH of reedbed canopies is similar to results of Yuan et al. (Citation2013), which demonstrates the existence of significant correlation between both aforementioned vegetation biophysical measures. It is also demonstrated that as LAI increases, so does CH and vice versa (Yuan et al. Citation2013). The poor correlation between LAI and other biophysical measures (namely BA and RD) could be attributed to seasonal variation of reedbed canopies over different phenological stages and the physical structure of reed stands. Sampson, Vose, and Allen (Citation1998) noted that the relationship between BA and LAI was strongly linear during early stages of tree canopy growth, but at latter stages of phenological development this relationship declines. The result of this study showed a negative correlation between LAI and BA (R = −0.15) and RD (R = −0.15). Considering LAI was the most significant factor of all biophysical measures, subsequent analyses of this study focused on its relationship with different spectral properties. Accurate estimates of the LAI are important in ecosystem analysis because of the importance of canopy structure on gas, water, carbon, and energy exchange (Gower and Norman Citation1991).
3.2. Characteristics of multi-seasonal reedbed spectral reflectance
Figure (a)–(c) show the multi-seasonal mean reflectance spectra of the Phragmites acquired over summer (June–August 2008), autumn (September–October 2009), winter (December 2008–February 2009), and spring (April–May 2009) for three sampling sites 2A, 2B, and 3A. The results show the mean reflectance spectra of all across monitored sites tend to vary depending on the canopy structure and timing of the spectra data acquisition. The shape of the mean reflectance for the sample sites is presented in Figure (d). Figure (e) and (f) present the varying LAI of derivative spectra curves over the four seasons (summer, autumn, winter, and spring). The derivative spectra revealed a clear distinction in plots of varying LAI. On average, LAI of the monitored sites over the four seasons varied from 1.2 in winter to 4 in summer.
Figure 3. Multi-seasonal mean reflectance spectra for sampling plots (a): Site 2A, (b): Site 2B, (c): Site 3A, and (d): All sampling plots. First derivative curves of Phragmites over two wavelength regions (e): 400–1000 nm and (f): 600–800 nm.
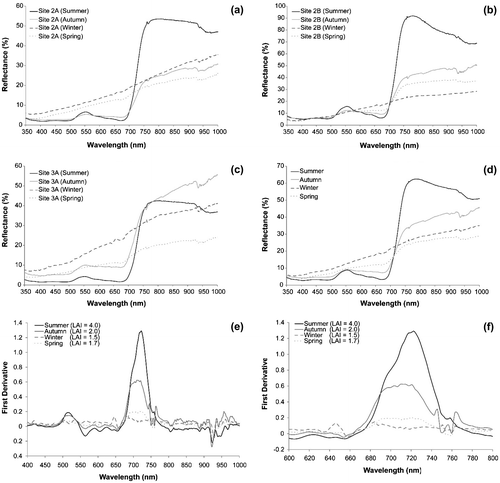
The spectra reflectance of the Phragmites at the NIR and red edge region (around 750 nm) was the highest in site 2B (summer) which had a reflectance percentage of 87% at wavelength of 750.58 nm (Figure (b)). The reflectance percentage of the other sites at the same wavelength were site 2A (summer) 48%, site 2B (autumn) 39%, site 3A (autumn) 38%, site 3A (summer) 37%, site 2B (spring) 33%, site 3A (winter) 31%, site 2B (winter) 23%, site 2A (winter) 22%, site 2A (autumn) 21%, site 2A (spring) 19%, and site 3A (spring) 18%, respectively (Figure (a)–(c)). The mean reflectance spectra for all the sampling sites over the four seasons (Figure (d)) revealed that the spectral reflectance curves of the Phragmites during winter and spring were quite similar. The spectral reflectance curves for summer and autumn had pronounced changes in the visible and NIR regions. The “green peak” around 550 nm was obviously pronounced in the summer spectra reflectance curve but diminished in the autumn, winter, and spring reflectance curves.
The red edge was determined using the first derivative curve in order to identify more subtle changes within the vegetation spectra (Figure (e) and (f)). Results of the field experiments indicated that the “red edge peak” was highest in summer (late June), followed by autumn (early October), spring (early May), and lowest in winter (early February). The spectral characteristics of reedbeds appeared to be related to the phenology of plants (Pu and Gong Citation2000), which in turn determine the canopy structure, growth stage, water content, and chlorophyll content of reedbed canopies. These results indicate that the architecture of the reedbed canopy and the seasonal variations affect the spectral reflectance of the Phragmites.
3.3. Relationship between broad-band Landsat-derived spectral indices and LAI
The correlations between Landsat-derived VIs and biophysical measures indicated that the best linear relationship was with LAI. Pearson’s correlations of Landsat-derived VIs by linear regression model were as follows: 0.716 (SR), 0.742 (NDVI), 0.738 (TNDVI), 0.532 (DVI), 0.741 (SAVI), and 0.658 (RDVI). As indicated in the aforementioned R values, NDVI was most correlated with LAI. Further analysis of curve fitting for NDVI with LAI revealed that the power-based model could best predict the LAI (regression model y = 3.9803x0.8032; R = 0.78; p < 0.001).
Past studies have demonstrated the NDVI values generated by broad-band multispectral satellite sensor could be used to estimate LAI for wetland and grassland vegetation (Cayrol et al. Citation2000; Fernandes et al. Citation2003).
3.4. Relationship between narrow-band CASI-derived spectral indices and LAI
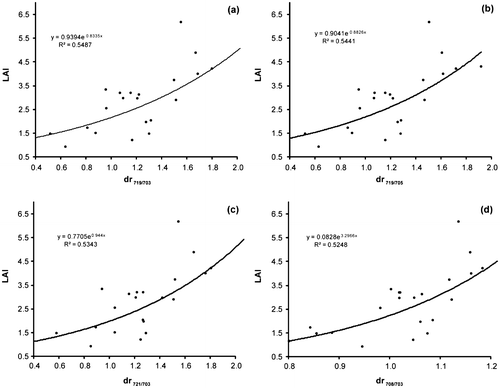
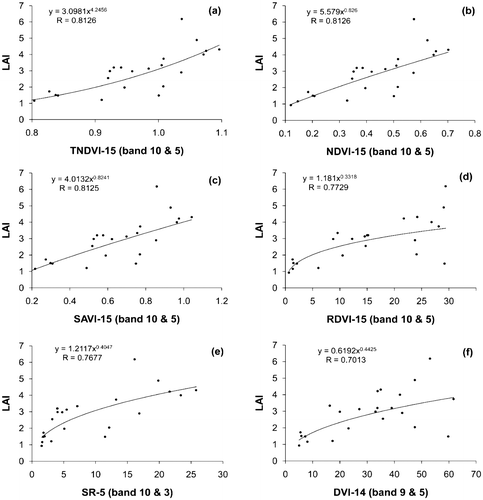
Figure presents scatter plots of LAI vs. six CASI-derived VIs: TNDVI (a), NDVI (b), SAVI (c), RDVI (d), SR (e) and DVI (f) respectively. All scatter plots were modeled by power regression equations. The simulated CASI bands used to calculate the vegetation indices were band 5 (705–711 nm) and band 10 (860–870 nm) (Table ). The CASI-derived VIs most correlated with LAI were TNDVI (0.8126), NDVI (0.8126), and SAVI (0.8125) (Figure (a)–(c)). The least correlated VIs with LAI were RDVI (R = 0.7729), SR (R = 0.7677), and DVI (R = 0.7013) (Figure (d)–(f)). The poor performance of the DVI in comparison to TNDVI or NDVI was attributed to effects of topography, shadow, or atmosphere (Akkartal, Türüdüa, and Erbekb Citation2004). In a study by Fu, Wang, and Jiang (Citation2010), both exponential and power curve models of TNDVI and NDVI were proved to be useful inputs in estimating LAI of Masson pine vegetation. In comparison to the broad-band Landsat-derived NDVI, the narrow-band CASI-derived TNDVI and NDVI were demonstrated to be more effective in this study.
Figure 4. Scatter plots of LAI vs. CASI-derived VIs by power regression models (a) TNDVI-15, (b) NDVI-15, (c) SAVI-15, (d) RDVI-15, (e) SR-5, and (f) DVI-14.
3.4.1. Relationship between single-wavelength indices and biophysical measures
In order to identify spectral regions most strongly related to the biophysical measures, correlograms were constructed by sequentially regressing the value of percentage reflectance at each GER1500 channel (Rλ) against the biophysical measures and plotting the coefficient of correlation (R2) against wavelength (Blackburn and Steele Citation1999). Figure (a) shows a correlogram that illustrates how the correlation between percentage reflectance and biophysical measures changes with wavelength. The correlation of coefficients for each biophysical measure was plotted against the wavelengths of a reference spectrum, showing the reflectance of a well-developed reedbed canopy in summer. The correlograms showed that the LAI had a similar form to the reedbed reflectance spectrum, with little correlation in the visible region and high correlations in the NIR regions. Blackburn and Steele (Citation1999) discovered that for matorral vegetation there was a positive relationship between the LAI and NIR reflectance at 783.2 nm (R783.2) (R2 = 0.58 by the model y = 0.22e0.11x).
Figure 5. (a) Correlograms constructed by correlation coefficient values (of percent reflectance against LAI, CH, BA and RD) against variations with wavelength and scatter plots showing power regression models that best predicts the LAI using (b) SAVI (R602 and R1037), (c) NDVI (R602 and R1037), (d) (R700 and R1037) and NDVI (R700 and R1037).
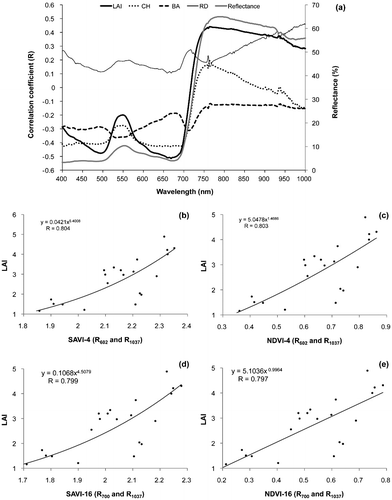
For the reedbed canopy in all the sites observed over the four seasons (winter, summer, spring and autumn), there was a positive relationship between the LAI and NIR reflectance at 763.03 nm (R763.03) based on an exponential regression model (R = 0.46 by the model y = 1.4955e0.011x). The canopy height correlogram followed a similar pattern with the reflectance spectra and had a positive relationship with the LAI. The peak correlations in the green, red, and NIR were at wavelengths of 501, 665, and 702 nm, respectively. The correlogram of the stem basal area shows a weak relationship within the visible region of the reflectance spectrum. The peak of the correlation occurs at the near infrared wavelength R766.13 (R2 = 0.0136 or R = –0.1165; model y = 0.00005x + 0.0035). There was a negative relationship between the stem basal area and peak near infrared reflectance at 766.13 nm. From the correlogram, the correlation coefficient of the reed density indicates a positive relationship with the percentage reflectance of all the sample plots. The correlation coefficient values in the blue spectral region (400–500 nm) gradual declines from wavelengths R400–R493.06 (R range was 0.27–0.11) similar to the LAI pattern in the same region of the correlogram. The correlation coefficients gently increase from the bottom peak R494.71 in the blue region to R529.41 in the chlorophyll absorption green region. The peak correlation in the green region was at wavelength R560.76 (R = 0.20). A prominent peak was observed at wavelength R700.23 (R = 0.23) in the red region. In the near infrared region, the maximum peak was at wavelength 1036 nm (R1036) with R value of 0.47. Generally, the percentage reflectance of the sample plots was poorly related to most of the biophysical measures with the exception of the LAI. From the correlograms (Figure (a)), the red and NIR peak wavelengths were used to generate the VIs and correlated with the biophysical measures (LAI: R501, R668, and R763; CH: R501, R665, and R702; RD: R564, R700, and R1037; and BA: R533, R602, and R713, respectively).
The results indicated that for CH, BA, and RD, the R value was quite low. However, the LAI showed some correlation with SAVI and NDVI generated by the red (R602 and R700) and NIR (R1037) reflectance channels, respectively. Results of further analysis based on other mathematical models indicated the power regression model relating LAI to both SAVI-4 (R = 0.804) and NDVI-4 (R = 0.803) were most significant (Figure (b) and (c)). Though NDVI and SAVI show strong correlation with foliage density, the effects of sensitivity to external factors such as solar and viewing geometry, soil background, and atmospheric effects are compensated in SAVI (Rondeaux, Steven, and Baret Citation1996).
3.4.2. Relationship between biophysical measures and red edge reflectance
The red edge spectral reflectance (R680–760) was compared with the biophysical measures using a linear regression mathematical model. The results showed that the only biophysical measure related to the red edge spectra was the LAI. Table shows selected red edge spectral reflectance wavelengths that most correlated with LAI (p < 0.05). The selected red (680–695 nm) and NIR (749–760 nm) wavelengths within the red edge region were subsequently used to calculate the six VIs and compared with LAI for correlation. The red edge spectral values with p values less than 0.05 were selected and used for calculating the VIs. The selected wavelengths in the red edge region were used to develop six vegetation indices (SR, NDVI, TNDVI, DVI, SAVI, and RDVI) and the most effective VI for predicting LAI was selected. Table presents regression models that best predict LAI by red edge-derived VIs. The exponential regression model relating DVI calculated by wavelengths R695 and R758 had the best relationship with LAI (R = 0.88).
Table 3. Selected red edge reflectance spectra and LAI derived using linear regression model.
Table 4. Regression models that best predicted LAI using red edge indices.
3.4.3. Relationship between biophysical measures and amplitude of first derivative in red edge region
Table summarizes the results of the correlation coefficients between LAI and amplitude of the first derivative in the red edge region. In this experiment, the maximum R value for first derivative in the red edge region was used to determine variations to reedbed biophysical measures. The relative ratios (R719/R703), (R719/R705), (R721/R703) and (R708/R703) contain useful information for estimating LAI of reedbed canopies by comparing them with other red edge derivatives. Figure presents the four most effective red edge parameters used for accurate prediction of LAI in the study area. However, the orthogonal and hybrid VIs, DVI, and RDVI computed using the red edge reflectance (Table ) had a stronger correlation with the LAI when compared to these ratios.
Table 5. List of effective regression models for LAI prediction by amplitude of first derivatives in red edge region.
4. Conclusions
The results of this study show that there is no strong relationship between the different biophysical measures evaluated with the exception of LAI and canopy height. The mean spectral reflectance of all the monitored plots varied depending on the canopy structure and season when the reflectance spectral data were acquired. The mean reflectance and spectral derivative graphs revealed a clear distinction in sample plots of varying LAI. Notable changes were in the NIR, green and red wavelength regions caused by chlorophyll absorption in the region. The spectral reflectance of the Phragmites during winter and spring were quite similar, unlike the summer and autumn reflectance curves, which had pronounced changes in the visible and NIR regions. The experiments also indicated that the amplitude of the first derivative in the red region was the highest in the summer (late June), followed by autumn (early October), spring (early May), and lowest in winter (early February). Table summarizes the reflectance features that best predicts the LAI derived in the study. The orthogonal and hybrid VIs, DVI and RDVI, computed using the red edge reflectance at specific wavelengths (i.e. 681, 695, and 758 nm) had the strongest correlation with the LAI. For biophysical measures with VIs, the performance of the narrow band red edge-derived DVI using wavelengths R695 and R758 outperformed all simulated broad-band Landsat, narrow-band CASI or first derivative features investigated in the study. The results show that DVI is a good predictor of deciduous plantations and considered more sensitive to vegetation density than other indices (Franklin Citation2001). Considering the valuable information contained in the red edge region of the electromagnetic spectrum, subtle information of chlorophyll content in vegetation cover can be effectively utilized in developing models that predict accurately biophysical measures such as LAI. The development of space-borne sensors with high spatial, temporal, and spectral (particularly in the red edge region) resolutions could be used to generate valuable information needed in management of reedbed or similar wetland habitats and agricultural applications such as quantifying nitrogen content through understanding chlorophyll content (Eitel et al. Citation2007) and forestry management (Eitel et al. Citation2011).
Table 6. Summary of reflectance and first derivative features having the most correlation with LAI within the study site.
Funding
This work was supported by the Cumbria Wildlife Trust, Environment Agency and Petroleum Technology Development Fund [grant number: GB09/05].
Notes on contributors
Alex O. Onojeghuo is a senior lecturer working at the Surveying and Geoinformatics department, Nnamdi Azikiwe University, Awka, Nigeria. Alex has a PhD in geography (specialization in remote sensing and GIS) from Lancaster University, UK, an MSc degree in surveying and geoinformatics from University of Lagos Nigeria and a BSc degree in surveying and photogrammetry from Enugu State University of Science and Technology, Nigeria. He conducts research in the use of environmental remote sensing and GIS in vegetation analysis, particularly tropical forests and wetlands.
George A. Blackburn is a senior lecturer with Lancaster Environment Centre, Lancaster University. George received his BSc degree in geography from the University of Bristol, UK and a PhD in remote sensing from the University of Southampton, UK. His research interests focus on developing remote sensing and GIS techniques for environmental applications, particularly in vegetation ecophysiology and ecology.
Acknowledgments
The authors would like to thank the following persons for their advice and assistance: Graham Jackson-Pitt (Local Biodiversity Manager, Cumbria Biodiversity Partnership), Neil Harnott (Cumbria Biodiversity Action Plan Manager, Cumbria Wildlife Trust), Judith Bennett (Biodiversity Technical Officer, Environment Agency), staff of RSPB in Leighton Moss for access to the site and the Petroleum Technology Development Fund (Nigeria) for funding the study.
References
- Adam, S., A. De Backer, A. De Wever, K. Sabbe, E. A. Toorman, M. Vincx, and J. Monbaliu. 2011. “Bio-physical Characterization of Sediment Stability in Mudflats Using Remote Sensing: A Laboratory Experiment.” Continental Shelf Research 31 (10): S26–S35.10.1016/j.csr.2009.12.008
- Akkartal, A., O. Türüdüa, and F. Erbekb. 2004. “Analysis of Changes in Vegetation Biomass Using Multitemporal and Multisensor Satellite Data.” In Proccedings of XXth ISPRS Congress. Istanbul, Turkey. http://www.isprs.org/proceedings/XXXV/congress/yf/papers/946.
- Armitage, R. P., M. Kent, and R. E. Weaver. 2004. “Identification of the Spectral Characteristics of British Semi-natural Upland Vegetation Using Direct Ordination: A Case Study from Dartmoor, UK.” International Journal of Remote Sensing 25 (17): 3369–3388.10.1080/01431160310001654464
- Blackburn, G. A., and J. I. Pitman. 1999. “Biophysical Controls on the Directional Spectral Reflectance Properties of Bracken (Pteridium Aquilinum) Canopies: Results of a Field Experiment.” International Journal of Remote Sensing 20 (11): 2265–2282.10.1080/014311699212245
- Blackburn, G. A., and C. M. Steele. 1999. “Towards the Remote Sensing of Matorral Vegetation Physiology: Relationships between Spectral Reflectance, Pigment and Biophysical Characteristics of Semi-arid Bushland Canopies.” Remote Sensing of Environment 70 (3): 278–292.10.1016/S0034-4257(99)00044-9
- Bork, E. W., N. E. West, and K. P. Price. 1999. “Calibration of Broad- and Narrow-band Spectral Variables for Rangeland Cover Component Quantification.” International Journal of Remote Sensing 20 (18): 3641–3662.10.1080/014311699211255
- Bork, E. W., N. E. West, K. P. Price, and J. W. Walker. 1999. “Rangeland Cover Component Quantification Using Broad (TM) and Narrow-band (1.4 NM) Spectrometry.” Journal of Range Management 52 (3): 249–257.10.2307/4003687
- Cayrol, P., A. Chehbouni, L. Kergoat, G. Dedieu, P. Mordelet, and Y. Nouvellon. 2000. “Grassland Modeling and Monitoring with SPOT-4 VEGETATION Instrument during the 1997–1999 SALSA Experiment.” Agricultural and Forest Meteorology 105 (1–3): 91–115.10.1016/S0168-1923(00)00191-X
- Cochrane, M. A. 2000. “Using Vegetation Reflectance Variability for Species Level Classification of Hyperspectral Data.” International Journal of Remote Sensing 21 (10): 2075–2087.10.1080/01431160050021303
- Deering, D. W., and J. W. Rouse. 1975. “Measuring ‘Forage Production’ of Grazing Units from Landsat MSS Data.” In Proccedings of the 10th International Symposium on Remote Sensing of Environment, 1169–1178. Ann Arbor, MI.
- Eitel, J. U. H., D. S. Long, P. E. Gessler, and A. M. S. Smith. 2007. “Using In‐situ Measurements to Evaluate the New RapidEye ™ Satellite Series for Prediction of Wheat Nitrogen Status.” International Journal of Remote Sensing 28 (18): 4183–4190.10.1080/01431160701422213
- Eitel, J. U. H., L. A. Vierling, M. E. Litvak, D. S. Long, U. Schulthess, A. A. Ager, D. J. Krofcheck, and L. Stoscheck. 2011. “Broadband, Red-edge Information from Satellites Improves Early Stress Detection in a New Mexico Conifer Woodland.” Remote Sensing of Environment 115 (12): 3640–3646.10.1016/j.rse.2011.09.002
- Elvidge, C. D., and Z. Chen. 1995. “Comparison of Broad-band and Narrow-band Red and Near-Infrared Vegetation Indices.” Remote Sensing of Environment 54 (1): 38–48.10.1016/0034-4257(95)00132-K
- Filella, I., and J. Penuelas. 1994. “The Red Edge Position and Shape as Indicators of Plant Chlorophyll Content, Biomass and Hydric Status.” International Journal of Remote Sensing 15 (7): 1459–1470.10.1080/01431169408954177
- Franklin, S. E. 2001. Remote Sensing for Sustainable Forest Management. New York: Lewis Publishers.10.1201/9781420032857
- Fernandes, R., C. Butson, S. Leblanc, and R. Latifovic. 2003. “Landsat-5 TM and Landsat-7 ETM+ Based Accuracy Assessment of Leaf Area Index Products for Canada Derived from SPOT-4 VEGETATION Data.” Canadian Journal of Remote Sensing 29 (2): 241–258.10.5589/m02-092
- Fu, Y. Z., X. Q. Wang, and H. Jiang. 2010. “The Correlation between LAI and Vegetation Index of Masson Pine.” [ In Chinese.] Remote Sensing for Land and Resources 22 (3): 41–46.
- Gao, Z. G., and L. Q. Zhang. 2006. “Multi-seasonal Spectral Characteristics Analysis of Coastal Salt Marsh Vegetation in Shanghai, China.” Estuarine, Coastal and Shelf Science 69 (1–2): 217–224.10.1016/j.ecss.2006.04.016
- Gower, S. T., and J. M. Norman. 1991. “Rapid Estimation of Leaf Area Index in Conifer and Broad-Leaf Plantations.” Ecology 72 (5): 1896–1900.10.2307/1940988
- Hawke, C., and P. José. 1996. Reedbed Management for Commercial and Wildlife Interests. Sandy, UT: Royal Society for the Protection of Birds.
- Horler, D. N. H., M. Dockray, and J. Barber. 1983. “The Red Edge of Plant Leaf Reflectance.” International Journal of Remote Sensing 4 (2): 273–288.10.1080/01431168308948546
- Huang, Z., B. J. Turner, S. J. Dury, I. R. Wallis, and W. J. Foley. 2004. “Estimating Foliage Nitrogen Concentration from HYMAP Data Using Continuum Removal Analysis.” Remote Sensing of Environment 93 (1–2): 18–29.10.1016/j.rse.2004.06.008
- Huete, A. R. 1988. “A Soil-adjusted Vegetation Index (SAVI).” Remote Sensing of Environment 25 (3): 295–309.10.1016/0034-4257(88)90106-X
- Jordan, C. F. 1969. “Derivation of Leaf-area Index from Quality of Light on the Forest Floor.” Ecology 663–666.10.2307/1936256
- Kent, M., and P. Coker. 1992. Vegetation Description and Analysis: A Practical Approach. Chichester: John Wiley and Sons.
- Kokaly, R. F., and R. N. Clark. 1999. “Spectroscopic Determination of Leaf Biochemistry Using Band-depth Analysis of Absorption Features and Stepwise Multiple Linear Regression.” Remote Sensing of Environment 67 (3): 267–287.10.1016/S0034-4257(98)00084-4
- Li-COR, Inc. 1992. LAI-2000 Plant Canopy Analyser Operating Manual. Lincoln, NE: Li-COR Inc.
- Liu, Z. Y., J. F. Huang, X. H. Wu, and Y. P. Dong. 2007. “Comparison of Vegetation Indices and Red-edge Parameters for Estimating Grassland Cover from Canopy Reflectance Data.” Journal of Integrative Plant Biology 49 (3): 299–306.10.1111/jipb.2007.49.issue-3
- McCoy, R. M. 2005. Field Methods in Remote Sensing. New York: Guilford Press.
- McDonald, A. J., F. M. Gemmell, and P. E. Lewis. 1998. “Investigation of the Utility of Spectral Vegetation Indices for Determining Information on Coniferous Forests.” Remote Sensing of Environment 66 (3): 250–272.10.1016/S0034-4257(98)00057-1
- Middleton, R., C. E. Wells, E. Huckerby, and C. Cox. 1995. The Wetlands of North Lancashire (North West wetlands survey). Lancaster University Archaeological Unit.
- Milton, E. J. 1987. “Review Article Principles of Field Spectroscopy.” International Journal of Remote Sensing 8 (12): 1807–1827.10.1080/01431168708954818
- Mutanga, O., and A. K. Skidmore. 2004. “Hyperspectral Band Depth Analysis for a Better Estimation of Grass Biomass (Cenchrus ciliaris) Measured under Controlled Laboratory Conditions.” International Journal of Applied Earth Observation and Geoinformation 5 (2): 87–96.10.1016/j.jag.2004.01.001
- Mutanga, O., A. K. Skidmore, and H. H. T. Prins. 2004. “Predicting in Situ Pasture Quality in the Kruger National Park, South Africa, Using Continuum-removed Absorption Features.” Remote Sensing of Environment 89 (3): 393–408.10.1016/j.rse.2003.11.001
- Pu, R. L., and P. Gong. 2000. Hyperspectral Remote Sensing and Its Applications. [ In Chinese.] Beijing: Higher Education Press.
- Ratcliffe, D. A. 1977. A Nature Conservation Review. London: Cambridge University Press.
- Richardson, A. J., and C. L. Wiegand. 1977. “Distinguishing Vegetation from Soil Background Information.” Photogrammetric Engineering and Remote Sensing 43: 1541–1552.
- Rondeaux, G., M. Steven, and F. Baret. 1996. “Optimization of Soil-adjusted Vegetation Indices.” Remote Sensing of Environment 55 (2): 95–107.10.1016/0034-4257(95)00186-7
- Roujean, J. L., and F. M. Breon. 1995. “Estimating PAR Absorbed by Vegetation from Bidirectional Reflectance Measurements.” Remote Sensing of Environment 51 (3): 375–384.10.1016/0034-4257(94)00114-3
- Rouse Jr., J., R. H. Haas, J. A. Schell, and D. W. Deering. 1974. “Monitoring Vegetation Systems in the Great Plains with ERTS.” NASA Special Publication 351: 309–317.
- Sampson, D., J. Vose, and H. Allen. 1998. “A Conceptual Approach to Stand Management Using Leaf Area Index as the Integral of Site Structure, Physiological Function, and Resource Supply.” Proceedings of the Ninth Biennial Southern Silvicultural Research Conference, Gen. Tech. Rep. SRS-20. 447–451. Asheville, NC: U.S. Department of Agriculture, Forest Service, Southern Research Station.
- Schmidt, K. S., and A. K. Skidmore. 2003. “Spectral Discrimination of Vegetation Types in a Coastal Wetland.” Remote Sensing of Environment 85 (1): 92–108.10.1016/S0034-4257(02)00196-7
- Shaw, D. T., T. J. Malthus, and J. A. Kupiec. 1998. “High-spectral Resolution Data for Monitoring Scots Pine (Pinus sylvestris L.) Regeneration.” International Journal of Remote Sensing 19 (13): 2601–2608.10.1080/014311698214668
- Tucker, C. J. 1979. “Red and Photographic Infrared Linear Combinations for Monitoring Vegetation.” Remote Sensing of Environment 8 (2): 127–150.10.1016/0034-4257(79)90013-0
- Wang, F. M., J. F. Huang, and X. Z. Wang. 2008. “Identification of Optimal Hyperspectral Bands for Estimation of Rice Biophysical Parameters.” Journal of Integrative Plant Biology 50 (3): 291–299.10.1111/jipb.2008.50.issue-3
- Yuan, Y., X. Wang, F. Yin, and J. Zhan. 2013. “Examination of the Quantitative Relationship between Vegetation Canopy Height and LAI.” Advances in Meteorology 2013: 964323. doi:10.1155/2013/964323.