Abstract
Objectives: Treatment regimens tested in major clinical trials, conducted by cooperative groups, are often adapted as standard of care by cancer centers with the hope to replicate the treatment outcomes reported in these landmark studies. It is therefore postulated that applying clinical trial regimens in a non-clinical trial setting yield similar outcomes. The aim of the present study was to explore this hypothesis in the context of childhood acute lymphoblastic leukemia (ALL) in our institution.
Methods: We retrospectively evaluated 224 consecutive pediatric ALL cases treated between January 2001 and December 2007. Standard-risk (SR) patients were treated on CCG-1991 (regimen OD) while high-risk (HR) patients were treated on CCG-1961 (regimen D). Results were compared with those of the equivalent regimen in the original clinical trials. Statistical analysis was carried using chi-square or Fisher's exact test, Kaplan–Meier and log-rank tests.
Results: Comparison of treatment outcomes revealed that SR patients had inferior 5-year overall survival (OS) of (89.0 ± 2.9 vs. 96.0% ± 0.9%); event-free survival of (82.3 ± 3.5 vs. 88.7% ± 1.4%); and relapse rate of (15.8 vs. 9.3% (P = 0.034)) compared to patients treated in the clinical trial. However, no statistically significant difference in treatment outcomes was observed between HR patients.
Conclusions: Despite using comparable regimens, suboptimal outcomes were noted in SR patients implying that similar treatments do not necessarily yield similar outcomes. This underscores the need to evaluate outcomes of adapted regimens to identify areas that need further improvement in centers not enrolling patients on prospective collaborative clinical trials.
Introduction
The treatment of childhood acute lymphoblastic leukemia (ALL) has advanced significantly over the years with survival rates now approaching 90%.Citation1,Citation2 This success is the result of collaborative trials that have improved the risk- and response based-treatment approach of childhood ALL. However, treatment regimens continue to be refined and protocols modified to improve outcomes and minimize acute and late effects of leukemia treatment.
New and more effective cancer treatments are usually discovered and proven through clinical trials. The results of these trials are disseminated within the medical community. If a new treatment regimen turns out to be effective, it may be adapted as a new standard of care. Eventually, the treatment regimen, which led to the success, will be available to both clinical trial participant and non-participant centers alike. As a result, a non-participant center will have the opportunity of adapting the treatment protocol as published by the major cooperative groups (as it is) or with modifications to control for center-specific limitations and optimize treatment outcomes. The idea of comparing disease outcomes between studies is a useful approach that requires further consideration. In line with this, the present study proposes a regimen-to-regimen framework that simultaneously takes into account the effect of patient's risk group and regimen used to identify areas that need further improvement.
From January 2001 to December 2007, our institution has been following the CCG-1991 (regimen OD) and the CCG-1961 (regimen D) to treat pediatric patients with standard-risk (SR) and high-risk (HR) ALL, respectively. Despite this, the outcome of these regimens has not been evaluated against the outcomes of equivalent regimens from the CCG-1961 and CCG-1991 clinical trials.Citation3,Citation4 In light of this, the purpose of the present study was to conduct regimen-to-regimen comparison between patients treated on the adapted regimen in a non-clinical trial setting and those treated on the comparable regimen from the clinical trial to determine if comparable regimens lead to similar survival and relapse rates in ALL patients.
Methods
Patients
This is a retrospective study of 224 consecutive children newly diagnosed with ALL and treated at the Princess Norah Oncology Center, Jeddah, Saudi Arabia from January 2001 to December 2007. Patients were retrospectively analyzed (as treated) based on the treatment regimen adapted by the center. The study was approved by the Hospital Research and Ethics Review Committee. To allow for regimen-to-regimen comparisons, the eligibility and exclusion criteria used in the clinical trial were adapted in this study. However, the pediatric age group in Saudi Arabian institutions is up to the age of 14 years. Therefore, the eligibility criteria included children aged 1–14 years and rapid early response (RER). Patients younger than 1 year of age, slow early response (SER), Philadelphia chromosome positive, severe hypodiploidy (less than 45 chromosomes), induction failure, central nervous system disease (CNS-3) at diagnosis, or induction death were excluded. Data collected from patients' medical records included: age at diagnosis, sex, white blood cell (WBC) count, date of: diagnosis, remission, induction, relapse, death, and last follow-up; cytogenetics, immunophenotype at diagnosis, CNS status, response to chemotherapy on day 7, day 14, and day 28. Outcome data were prospectively collected to estimate 5-year survival rates.
Risk stratification
Patients were stratified based on the National Cancer Institution-Rome criteria and immunophenotype into two groups: SR group was defined as patients with precursor B-cell immunophenotype, age ≥1 year and <10 years, and WBC <50 × 109/l. HR group was defined as patients with T-cell immunophenotype or precursor B-cell immunophenotype and age ≥10 years and/or WBC ≥50 × 109/l.
CNS stratification was based on the presence or absence of red blood cells (RBC), WBC, and leukemic blasts in the cerebral spinal fluid into: CNS1 (no blasts present on cytospin regardless of cell count), CNS2 (blasts present but WBC <5 or traumatic lumbar puncture with blasts), or CNS3 (blasts present but WBC ≥5, clinical signs of CNS leukemia, or traumatic lumbar puncture with blasts but WBC exceeds that expected due to traumatic contamination as per the Steinherz/Bleyer equation).
Treatment regimen
In the COG trial, 1036 SR patients were treated according to the oral methotrexate arm of the CCG-1991 (regimen OD/OS), which utilized dexamethasone for induction, oral methotrexate during interim maintenance, and one (regimen OS) or two (regimen OD) delayed intensification phases.Citation3 In the present study, SR patients were treated with CCG-1991 (regimen OD).Citation5 In contrast, 650 HR patients were treated on the increased intensity regimen (IPII) of the original CCG-1961 and received vincristine, prednisone, asparaginase, daunorubicin, and intrathecal methotrexate during induction and one (regimen C) or two (regimen D) delayed intensification phases.Citation4 In the present study, HR patients were treated on adapted CCG-1961 (regimen D) in which prednisone was replaced with dexamethasone.Citation5
Statistical analysis
Descriptive statistics was used to summarize demographic, clinical characteristics of patients and responses to therapy. Data from the clinical trial studies were extrapolated from the published trials as it was not possible to retrieve patient-level data.Citation3,Citation4 However, regimen-specific data (regimen IPII from the CCG-1961 study and regimen OD/OS from the CCG-1991 study) were used.Citation3,Citation4 Relapse and death as first event were computed from event tables reported in these studies.Citation3,Citation4 Categorical variables were compared using chi-square test or Fisher's exact test. The definition of SER was extrapolated from the clinical trial studies to allow for regimen-to-regimen comparison as follows: M3 bone marrow (BM) on day 7 for HR patients; and M3 BM on day 14 for SR patients.Citation3,Citation4 Induction failure was defined as M2 or M3 BM on day 28. Adverse events were relapse or death from any cause. Induction death was defined as death from any cause within 35 days from starting date of induction; remission death as death from any cause while in first complete remission; event-free survival (EFS) as the time elapsed from the date of diagnosis to the date of the first adverse event (relapse at any site, second malignant neoplasm, or death from any cause) or last contact date; and overall survival (OS) as the time interval between the date of diagnosis and the date of either the last follow-up or death from any cause. Time to event analysis was performed using Kaplan–Meier method. The log-rank test was used to evaluate differences in survival. P values less than 0.05 were considered statistically significant.
Results
Clinical characteristics
A total of 224 consecutive patients were diagnosed with ALL during the study period. One patient had missing data for appropriate risk stratification and thus 223 patients were included in the analysis. SR patients constituted 56.5% (n = 126) of the study population and 43.5% (n = 97) were HR. B-cell immunophenotype comprised 89.7% (n = 200) while 10.3% (n = 23) had T-cell immunophenotype. Patients with Philadelphia chromosome or severe hypodiploidy (less than 45 chromosomes) were excluded from the study. However, patients with 11q23 rearrangements and t(1;19) were treated based on the risk stratification indicated previously. In total, Philadelphia chromosome fusion was found in two HR patients; severe hypodiploidy was not observed in our patient cohort, however, four HR patients had mild hypodiploidy with 45 chromosomes; 11q23 rearrangement was reported in one SR patient with t(1;11)(q21;q23); and t(1;19) was found in nine (six SR and three HR) patients.
SR patients
Demographics and clinical characteristics
Of the 126 SR patients treated following CCG-1991 (regimen OD), 3 had induction death and 3 CNS3 were excluded. Thus, 120 patients were eligible for the analysis. The demographic characteristics and clinical features of patients treated with the adapted CCG-1991 (OD regimen) and those treated on the equivalent regimen from the CCG-1991 clinical trial are reported in Table . The two treatment groups were similar in terms of age, gender, and WBC. All our patients were of Arab/Middle Eastern origin. In contrast, patients treated on CCG-1991 included 705 Whites, 40 Blacks, 196 Hispanics, 28 Asian, and 67 children of other racial/ethnic origin. CNS status, BM day 7, and BM day 14 had missing data in both the present and the CCG-1991 studies and no comparison was made.
Table 1 Demographics and clinical features of patients treated on CCG-1991 (regimen OD) versus comparable regimen from the CCG-1991 clinical trial
Treatment outcome
The OS and EFS for SR patients in the present study are depicted in Fig. A. The 5-year OS and EFS rates resulting from the regimen-to-regimen comparison for SR patients treated with equivalent regimens are reported in Table . The 5-year OS and EFS rates were 89.0 ± 2.9 and 82.3% ± 3.5%, respectively. In contrast, the 5-year OS and EFS rates from the equivalent regimen in the CCG-1991 clinical trial were 96.9 ± 0.9 and 88.7% ± 1.4%, respectively.
Figure 1 OS and EFS of: (A) SR patients treated on CCG-1991 (regimen OD), and (B) HR patients treated on CCG-1961 (regimen D).
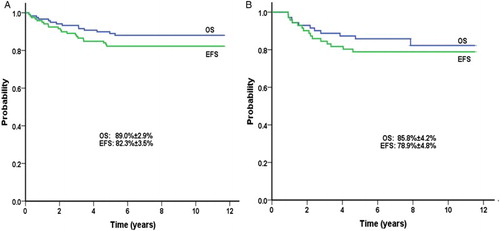
Table 2 Comparison of outcomes of patients treated on CCG-1991 (regimen OD) versus comparable regimen from the CCG-1991 clinical trial
Relapse and death events
Overall, 19 (15.8%) SR patients in our study compared to 96 (9.3%) patients in the equivalent regimen from the CCG-1991 clinical trial experienced relapse (P = 0.034), as depicted in Table . The incidence of relapse by site was similar: 9 (7.5%) vs. 43 (4.2%) isolated BM (P = 0.149); 6 (5.0%) vs. 26 (2.5%) isolated CNS (P = 0.134); 1 (0.8%) vs. 7 (0.7%) testicular relapse (P = 0.585), respectively. The incidence of any relapse occurring in the CNS (isolated or combined) was 9 (7.5%) vs. 36 (3.5%); respectively (P = 0.056). Death in remission occurred in 2 (1.7%) patients in the current study compared to 5 (0.5%) in the CCG-1991 clinical trial study (P = 0.159), as shown in Table .
HR patients
Demographics and clinical characteristics
HR patients were treated following CCG-1961 (regimen D). Out of the 97 HR patients, 25 (2 infants, 3 induction deaths, 2 Philadelphia positive, 4 induction failures, 3 SER, and 11 CNS3) were excluded to allow for regimen-to-regimen comparison. These patients were compared with the 650 patients treated on the comparable regimen from the CCG-1961 clinical trial (Table ).
Table 3 Demographics and clinical features of patients treated on CCG-1961 (regimen D) versus comparable regimen from the CCG-1961 clinical trial
Patients were grouped into three age categories: 1–9.9 years, 10–15.9 years, and 16 years and older. There was statistically significant difference between age groups with a P-value of 0.001 for age groups 1–9.99 years and 10–15.9 years. Comparison was restricted to these two groups because of the upper age limit of 14 years in our study. Patients in the present study were younger with 70.8% in the 1–9.9-year age group compared to 35.2% in the original regimen. No significant gender difference was noted between patients in the two regimens (P = 0.839). Furthermore, no WBC-specific difference was detected when patients were grouped into three major classes as shown in Table . Approximately 87.5% of patients in our study had WBC count of 50 × 109/l or greater and 12.5% of patients had WBC count less than 50 × 109/l. Patients in the two regimens were comparable in terms of CNS status. However, race/ethnicity of patients was different in the two regimens (Table ).
Treatment outcome
The OS and EFS for HR patients are shown in Fig. B. The survival rates were not significantly different between regimens (Table ). The 5-year OS for HR patients was 85.8 ± 4.2% compared to 88.7 ± 1.9% for those on equivalent regimen in the CCG-1961 clinical trial and the corresponding 5-year EFS was 78.9 ± 4.8 and 81.2 ± 2.4%, respectively.
Table 4 Comparison of outcomes of patients treated on CCG-1961 (regimen D) versus comparable regimen from the CCG-1961 clinical trial
In the present study, no significant difference in survival rates was found between patients younger than 10 years versus 10 years and older. The younger group had a 5-year OS rate of 83.6 ± 5.9% compared with 85.0 ± 8.0% for the older (P = 0.759). Similarly, the 5-year EFS was 82.0 ± 5.4% compared to 76.2 ± 9.3%, respectively (P = 0.826).
Relapse and death events
The relapse rate for HR patients was 9.7% compared to 14.8% for those on the comparable regimen from the clinical trial (P = 0.325). The distribution of relapse by site was: 1 (1.4%) vs. 49 (7.5%) isolated BM (P = 0.05); 1 (1.4%) vs. 29 (4.5%) isolated CNS (P = 0.349); and 5 (6.9%) vs. 15 (2.3%) combined (P = 0.04); respectively. In addition, remission death occurred in 4 (5.6%) patients compared to 12 (1.8%) in the clinical trial study (P = 0.066).
Discussion
Regimen-to-regimen comparisons were conducted to examine if equivalent treatments in clinical trial versus non-clinical trial settings lead to similar survival and relapse rates. Two regimen-specific comparisons were performed. First, SR patients treated on CCG-1991 (regimen OD) were compared with patients treated with the equivalent regimen in the CCG-1991 clinical trial. Despite receiving similar treatments, our data revealed that SR patients had inferior survival rates and higher relapse. However, remission death was similar in both patient groups. Second, HR patients treated on CCG-1961 (regimen D) were compared with patients treated with the comparable regimen in the CCG-1961 clinical trial. In contrast to SR patients, HR patients had similar survival rates, relapse rates, and death as first events.
The regimen-to-regimen comparison revealed differences in treatment outcomes that were confined only to SR patients. We therefore postulated that these variabilities might have resulted from differences in: socioeconomic factors, supportive care measures, race/ethnicity, and/or study design between the two patient groups. However, the fact that HR patients had similar survival and death in remission rates compared to patients in the clinical trial suggests that these factors had little or no influence in determining the discrepancy in treatment outcomes. Furthermore, despite the use of more treatment/double delayed intensification in all patients in the current study, death in remission was similar to that of the clinical trial group. Nevertheless, there was a statistically significant difference in relapse rate between the two SR patient groups, which may explain the observed variability. This indicates the need for further refinement of risk stratification in SR patients. In a clinical trial setting, a variety of clinical trial effects can potentially lead to improved outcomes including: stringent selection criteria for patients to be eligible for protocol therapy; improved routine care and treatment delivery as strictly set by the clinical trial protocol; improved patient and/or clinician compliance and adherence (the ‘Hawthorne’ effect) as the result of closer observation, quality control, and/or centralized review within clinical trials; and the possibility that patients consenting to participate in clinical trials may be inherently more compliant and adherent to therapy.Citation6 It is possible that these factors may have contributed to the higher relapse rate observed in the SR group of the present study compared to the clinical trial group.
Further refinement of risk stratification may be accomplished by objectively measuring response to therapy and performing detailed cytogenetic/molecular testing.Citation7–Citation11 Evaluation of response by morphology can be subjective. Therefore, objective measurement of response by flow-cytometry and response stratification based on minimal residual disease (MRD) is now considered the standard of care. Recently, two important findings regarding integrating MRD assessment into the clinical management of ALL emerged. First, MRD has been identified as the most important independent predictor of disease-free survival and OS for pediatric ALL.Citation8–Citation10 Second, augmenting therapy for SR patients with persistent MRD at end of induction has also been reported to improve EFS.Citation11,Citation12 In addition, carrying out detailed cytogenetic/molecular evaluations is needed to identify potential novel signatures associated with inferior outcomes, which may help refine risk-based therapy.Citation7,Citation13 In the current study, only conventional cytogenetics and targeted molecular testing for recurrent HR molecular abnormalities were performed. Therefore, it is possible that further detailed molecular testing may identify novel molecular abnormalities that require further intensification of therapy in the future.
Despite using comparable regimens, this study demonstrated that treatment outcomes could still vary between patients in the same risk group. Although the study is limited by the number of patients in each group and being a retrospective design compared to the multicenter randomized prospective collaborative clinical trial, the regimen-to-regimen approach was a useful framework in identifying which of the adapted regimens yield optimal/suboptimal outcomes compared to the equivalent regimens in the clinical trial. Adapted regimens that yield similar treatment outcomes, as those reported in the original clinical trials, might be considered optimal. In that respect, our HR patients achieved optimal outcomes compared to the clinical trial, which supports continued use of the equivalent HR regimen in our center. In contrast, adapted regimens that are associated with suboptimal outcomes indicate the need for improvement as demonstrated in the SR patients. However, detecting the causes that lead to poor outcomes is critical for effective intervention and optimizing treatment outcomes.
Presently, most of cancer research is conducted in developed countries led by cooperative groups. These groups have a proven track record of success in helping cancer patients live longer through conducting collaborative clinical trials. In contrast, many centers in developing countries do not enroll patients on prospective collaborative clinical trials. In the absence of center-specific clinical trials, these institutions adapt treatment regimens developed by cooperative groups with or without modifications with the hope of achieving similar outcomes for their patients.
Conclusions
The present study demonstrated that adapting a treatment regimen may not always lead to similar outcomes as those of the clinical trial. In light of this, we suggest that centers not enrolling patients on prospective clinical trials determine the effectiveness of the adapted regimen using a regimen-to-regimen approach and identify areas that need further improvement.
Disclaimer statements
Contributors W.J.: concept, design, data review, data validation, data cleaning, analysis, preparing manuscript draft, submission, and all correspondance. N.E.: helped in design and concept, data collection, study coordination, manuscript draft review. K.A.: data collection, manuscript draft and review. S.F.: data collection, manuscript draft review. M.B.A.: statistical analysis, data coding and cleaning, manuscript writing and review.
Funding None.
Conflicts of interest The authors declare no conflicts of interest or financial support to disclose.
Ethics approval The study was approved by the hospital research and ethics review committee (HRERC ref. #: S1108–106).
References
- Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354:166–78. doi: 10.1056/NEJMra052603
- Tucci F, Aricò M. Treatment of pediatric acute lymphoblastic leukemia. Haematologica. 2008;93:1124–8. doi: 10.3324/haematol.13517
- Matloub Y, Bostrom BC, Hunger SP, Stork LC, Angiolillo A, Sather H, et al. Escalating intravenous methotrexate improves event-free survival in children with standard-risk acute lymphoblastic leukemia: a report from the children's oncology group. Blood. 2011;118:243–51. doi: 10.1182/blood-2010-12-322909
- Siebel NL, Steinherz PG, Sather HN, Nachman JB, Delaat C, Ettinger LJ, et al. Early postinduction intensification therapy improves survival for children and adolescents with high-risk acute lymphoblastic leukemia: a report from the children's oncology group. Blood. 2008;111:2548–55. doi: 10.1182/blood-2007-02-070342
- Jastaniah W, Elimam N, Abdalla K, Iqbal BA, Khattab TM, Felimban S, et al. Identifying causes of variability in outcomes in children with acute lymphoblastic leukemia treated in a resource-rich developing country. Pediatr Blood Cancer. 2014;62:945–50. doi: 10.1002/pbc.25374
- Unger JM, Barlow WE, Martin DP, Ramsey SD, Leblanc M, Etzioni R, et al. Comparison of survival outcomes among cancer patients treated in and out of clinical trials. J Natl Cancer Inst. 2014;106:1–13. doi: 10.1093/jnci/dju002
- Schultz KR, Pullen DJ, Sather HN, Shuster JJ, Devidas M, Borowitz MJ, et al. Risk- and response-based classification of childhood B-precursor acute lymphoblastic leukemia: a combined analysis of prognostic markers from the Pediatric Oncology Group (POG) and Children's Cancer Group (CCG). Blood. 2007;109:926–35. doi: 10.1182/blood-2006-01-024729
- Stow P, Key L, Chen X, Pan Q, Neale GA, Coustan-Smith E, et al. Clinical significance of low levels of minimal residual disease at the end of remission induction therapy in childhood acute lymphoblastic leukemia. Blood. 2010;115:4657–4663. doi: 10.1182/blood-2009-11-253435
- Eckert C, von Stackelberg A, Seeger K, Groeneveld TW, Peters C, Klingebiel T, et al. Minimal residual disease after induction is the strongest predictor of prognosis in intermediate risk relapsed acute lymphoblastic leukaemia – long-term results of trial ALL-REZ BFM P95/96. Eur J Cancer. 2013;49:1346–55. doi: 10.1016/j.ejca.2012.11.010
- Eckert C, Hagedorn N, Sramkova L, Mann G, Panzer-Grümayer R, Peters C, et al. Monitoring minimal residual disease in children with high-risk relapses of acute lymphoblastic leukemia: prognostic relevance of early and late assessment. Leukemia. 2015;29:1648–1655. doi: 10.1038/leu.2015.59
- Vora A, Goulden N, Mitchell C, Hancock J, Hough R, Rowntree C, et al. Augmented post-remission therapy for a minimal residual disease-defined high-risk subgroup of children and young people with clinical standard-risk and intermediate-risk acute lymphoblastic leukaemia (UKALL 2003): a randomised controlled trial. Lancet Oncol. 2014;15:809–18. doi: 10.1016/S1470-2045(14)70243-8
- Vora A, Goulden N, Wade R, Mitchell C, Hancock J, Hough R, et al. Treatment reduction for children and young adults with low-risk acute lymphoblastic leukaemia defined by minimal residual disease (UKALL 2003): a randomised controlled trial. Lancet Oncol. 2013;14:199–209. doi: 10.1016/S1470-2045(12)70600-9
- Harrison CJ, Moorman AV, Schwab C, Carroll AJ, Raetz EA, Devidas M, et al. An international study of intrachromosomal amplification of chromosome 21 (iAMP21): cytogenetic characterization and outcome. Leukemia. 2014;28:1015–21. doi: 10.1038/leu.2013.317
