Abstract
Background and objectives: The microenvironment of acute myeloid leukemia (AML) is suppressive for immune cells. Regulatory T cells (Tregs) have been recognized to play a role in helping leukemic cells to evade immunesurveillance. The mesenchymal stem cells (MSCs) are essential contributors in immunomodulation of the microenvironment as they can promote differentiation of Tregs via the indoleamine 2,3-dioxygenase (IDO) pathway.
The aim of the present work was to evaluate the expression of IDO in bone marrow derived MSCs and to study its correlation to percentage of Tregs.
Methods: Thirty-seven adult bone marrow samples were cultured in appropriate culture medium to isolate MSCs. Successful harvest of MSCs was determined by plastic adherence, morphology, and positive expression of CD271 and CD105; negative expression of CD34 and CD45 using flowcytometry. MSCs were examined for IDO expression by immunocytochemistry using anti-IDO monoclonal antibody. CD4+ CD25+ cells (Tregs) were measured in bone marrow samples by flowcytometry.
Results: MSCs were successfully isolated from 20 of the 37 bone marrow samples cultured. MSCs showed higher expression of IDO and Tregs percentage was higher in AML patients compared to control subjects (P = 0.002 and P < 0.001, respectively). A positive correlation was found between IDO expression and Tregs percentage (P value = 0.012, r = 0.5).
Conclusion: In this study, we revealed an association between high IDO expression in MSCs and elevated levels of Tregs which could have an important role in the pathogenesis of AML, providing immunosuppressive microenvironment.
Introduction
Acute myeloid leukemia (AML) arises from a series of genetic alterations in a hematopoietic stem cell (HSC) leading to uncontrolled cell growth. It has been postulated that the hematopoietic microenvironment (HM) is implicated in the pathogenesis of AML with defects in HSCs themselves arising secondarily.Citation1,Citation2
Mesenchymal stem cells (MSCs) are the key component of the HM as they are essential element of both healthy and leukemic HM.Citation3 MSCs are capable of promoting growth, survival and drug resistance of leukemic cells by providing the necessary cytokines and cell contact-mediated signals to leukemic stem cells (LSCs).Citation4,Citation5 These LSC are resistant to chemotherapy and are the cause of disease relapse.Citation6,Citation7
MSCs have immunosuppressive effects on cells of the innate and adaptive immune responses. They can inhibit B cell function and differentiation,Citation8 inhibit dendritic cells generation from monocytes,Citation9 inhibit production of the proinflammatory cytokines such as IL-2, IFN-γ, and TNF-α and promote production of IL-10.Citation10–Citation12 Also, MSCs affect T cells, MSCs inhibit T cell proliferation in response to various stimulants,Citation13,Citation14 inhibit the production of IL-2, TNF-α by T cells,Citation15 induce the differentiation of CD4+CD25hiFOXP3+ T regulatory cells (Tregs) and maintain their inhibitory function.Citation16,Citation17 MSCs immunosuppression is mediated by different mechanisms including indoleamine 2,3-dioxygenase (IDO), nitric oxide, transforming growth factor β1, prostaglandin E2, and IL-10.Citation18
Tregs are immune suppressive T-cells; they inhibit T cell proliferation and cytokine production.Citation19 IDO plays important role in Tregs regulation by enhancing the suppressive phenotype and preventing Tregs reprogramming into non-suppressive helper-like cells thus promoting stabilization of Tregs.Citation20,Citation21 IDO may help tilting the tumor microenvironment from hostile to supportive for tumor cells and may be considered as one of the mechanisms of leukemia escape from immune control and so IDO inhibition can be regarded as a potential target in anti-leukemia therapy.Citation22–Citation24
We studied the expression of IDO in bone marrow derived MSCs and its correlation to percentage of regulatory T cells to evaluate its role in providing immunosuppressive microenvironment in AML.
Subjects and methods
The study was conducted in Kasr Alainy Hospitals, Cairo University on 37 subjects; 21 denovo AML patients with age range 18–60 years; 12 males and 9 females. Sixteen age- and sex-matched patients who came for diagnostic bone marrow aspirates to check the marrow cellularity prior to splenectomy operations were included in the study as a control group. The study was performed in accordance with the Helsinki Declaration, and the protocols were approved by the ethics committee of Cairo University. All participants provided informed consent before enrolment into the study.
Sample collection
From each participant bone marrow samples were collected; 0.5 ml on EDTA-anticoagulant for flowcytometric quantification of Tregs and 2 ml on heparin anticoagulant for isolation of MSCs and subsequent estimation of IDO expression by immunohistochemical staining.
Flowcytometric analysis of Tregs
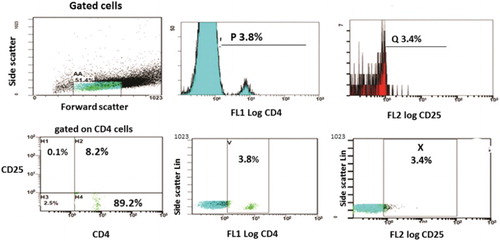
For each sample, two tubes were prepared; the first tube was used as control tube containing 50 µl of the sample with no monoclonal antibodies added in order to allow the flowcytometer adjustment and to obtain the basic histogram showing the main cell population and adjust the auto-fluorescence region. In the second tube, 50 µl of the sample was mixed with 5 µl of FITC conjugated anti-CD4 monoclonal antibody and 5 µl of PE conjugated anti-CD25 monoclonal antibody and was incubated in the dark for 20 minutes. Erythrocytes were eliminated by adding 500 µl of Opti Lyse C Lysing solution. A minimum of 10 000 leukocytes was analyzed in each tube by means of FACScan flow cytometer (Becman Coulter Cytomics FC500). Forward and side scatter gates were established to exclude cell debris and clumps before analysis. The control tube was then introduced; the laser scatter was received on both forward and side scatter detectors showing the cell population on a basic histogram and adjustment of the auto-fluorescence on the corresponding PMT. The tube for CD4 and CD25 determination was then introduced into the flowcytometer. Lymphocytes were gated first and CD4+ cells were detected, then CD4+ cells were gated and we detected CD25+ population within this gate. Results were expressed as a percentage of cells expressing positive CD25 within the CD4 positive population.Citation25
Isolation and identification of MSCs
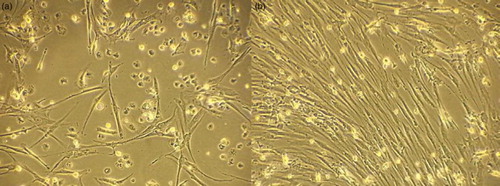
Bone marrow mononuclear cells were separated using ficoll-hypaque density gradient centrifugation, and the cell count was adjusted to 1.0 × 106 cells/ml. The cells were plated in 25 cm² tissue culture flask containing 5 ml complete culture medium (CCM: 1% l-glutamine, 2% antibiotic–antimycotic, 10% fetal calf serum in DMEM) with a density of 1.0 × 106 cells/cm2 and the flasks were incubated at 37°C with 5% humidified CO2. Two days later the media and non-adherent cells were removed, 5 ml of fresh CCM was added to the flask and incubated. The cells were examined every other day by inverted microscopy and medium change was performed every 3 days until the cells reached 70% confluence. Cell harvest was performed at 70% confluence using Trypsin-EDTA and counted. 125 000 cells were suspended in 5 ml CCM and cultured in 25 m2 tissue culture flasks with CCM changed every 3 days until cells reached 70% confluence and cells were harvested and a second passage was performed. Cells harvested after second passage were identified as MSCs by plastic adherence property, fibroblast-like morphology and flowcytometric analysis; harvested cells were stained with monoclonal anti-CD271 and anti-CD105 as MSCs markers; anti-CD45 (panleucocytic marker) and anti-CD34 (HSCs marker) were used as exclusion markers.
Estimation of MSCs expression of IDO
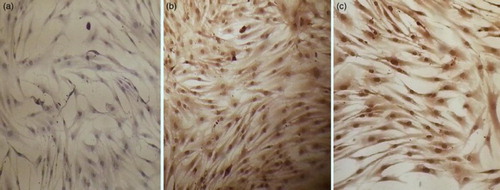
A total of 50 000 cells harvested after the second passage were suspended in 600 µl CCM and cultured in Labtek wells (two wells per each participant, one used for staining using monoclonal anti-IDO and the other used as a negative control), incubated at 37°C with 5% humidified CO2. Cells were examined 2–3 days later and after reaching 70% confluence, all the wells were fixed in ice cold methanol for 5 minutes and washed three times with PBS followed by cell permeabilization with 0.1% PBS-Tween for 20 minutes. Ultra V Block was applied and wells were incubated for 5–10 minutes at room temperature to block nonspecific background staining. Staining of MSCs was done using mouse monoclonal antibody to IDO 1 mg/ml (Abcam: catalog No. ab55305) according to the manufacturer's instructions. IDO expression was evaluated by two independent assessors using light microscopy. Reading of tissue slides was blind, and both assessors were unaware of clinical outcome. The total IDO immunostaining score was calculated by multiplying the proportion score by an intensity score. The proportion score reflects the estimated fraction of positively stained cells (score 0, none; score 1, <10%; score 2, 10–50%; score 3, 51–80%; score 4, >80%). The intensity score represents the estimated staining intensity (score 0, no staining; score 1, weak; score 2, moderate; score 3, strong) giving a total score ranging from 0 to 12. IDO overexpression was defined as a total score > 4.Citation26 Intensity score performed was strictly controlled by using negative controls for individual cases and control samples. In addition, each slide was compared, regarding intensity scoring to its negative control.
Statistical analysis
Data were statistically described in terms of mean, standard deviation, median, minimum, and maximum for quantitative data and frequencies (number of cases) and relative frequencies (percentages) for qualitative data. Comparison of quantitative variables was done using Mann–Whitney test when comparing two groups and Kruskal–Wallis when comparing more than two groups. For comparing categorical data, Chi square (χ2) test was performed. Exact test was used instead when the expected frequency is less than 5. Correlations were done between variables using spearman correlation coefficient. A probability value (P value) less than 0.05 was considered statistically significant. All statistical calculations were done using SPSS (Statistical Package for the Social Science; SPSS Inc., Chicago, IL, USA) version 21.
Results
The study included 37 participants; 21 denovo AML, 12 males (57.1%) and 9 females (42.9%) with age range from 18 to 60 years (mean ± SD, 45.5 ± 13.9) and 16 control patients, 10 males (62.5%) and 6 females (37.5%) with age range from 16 to 60 years (mean ± SD, 43.5 ± 12.8). Bone marrow samples of only 12 cases and 8 controls gave successful culture of MSCs. The other 17 samples were excluded from the study due to the following; 14 samples were discarded due to contamination, one sample failed to show any growth of spindle shaped cells and two samples showed few adherent spindle cells, however these cells failed to grow further and expand in culture.
Flowcytometric analysis of the Tregs was performed to all samples (n = 37). In AML patients (n = 21), percentage of CD25+/CD4+ cells ranged from 4.2 to 20% with a mean ± SD of 9 ± 4.2% and median 8%. While among the controls (n = 16), percentage of CD25+/CD4+ cells ranged from 0.2 to 4.7% with a mean ± SD of 1.99 ± 1.5% and median 1.4% with statistically significant difference between AML patients and control group (P < 0.001) (Table and Fig. ).
Table 1 Tregs (CD25+/CD4+ cells) percentage in patients and controls (n = 37)
Identification of MSCs was based on the presence of plastic adherent cells with spindle shaped fibroblast-like morphology (Fig. ) that showed positive expression of CD271, CD105 and negative expression of CD45, CD34.
MSCs showed higher expression of IDO among AML patients than controls with P-value of 0.002. MSCs of three patients (25%) had score 4, of four patients (33.3%) had score 8 and of five patients (41.7%) had score 12. All MSCs of the control patients had score 4. Mean IDO expression was shown in Table and Fig. .
Table 2 IDO expression of MSCs in patients and controls (n = 20)
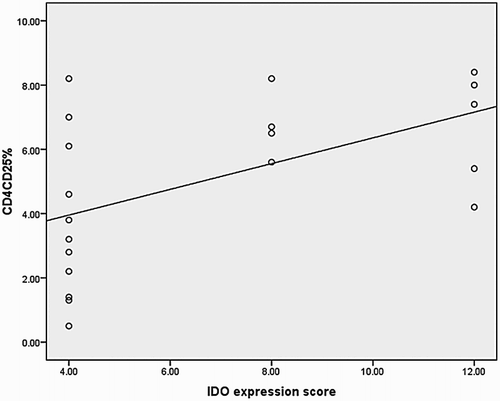
IDO expression in MSCs had positive correlation with Tregs percentage and bone marrow blasts percentage, with P = 0.012, r = 0.5 and P = 0.011, r = 0.703, respectively (Fig. ).
Discussion
Successful isolation of BM-MSCs was determined by flowcytometric characterization of cells morphologically identified as MSCs by positive expression of CD271, CD105 and negative for CD45, CD34 according to the proposed criteria of the International Society for Cellular Therapy.Citation27
In our study, the low affinity nerve growth factor receptor (LNGFR; CD271) was used in characterization of MSCs as several studies described CD271 as the most selective marker for the characterization and purification of human BM-MSCs.Citation28–Citation31 MSCs selected by CD271 expression have a 10- to 1000-fold higher proliferative capacity in comparison to MSCs isolated by plastic adherence.Citation31 These observations led to the recent commercialization of CD271 as a preferred marker for the purification of a homogeneous population of cells that contains all the BM-MSCs activity. In a study by Jarocha et al. comparison of different strategies of MSCs isolation revealed advantage to expand MSC directly from purified CD105 + and CD271 + cells.Citation32
Tregs were characterized in bone marrow samples by measuring positive CD25 expression on gated CD4+ cells. Although the expression of FoxP3, a member of the forkhead/winged-helix family of transcriptional factors, is considered an optimal marker for Tregs.Citation33,Citation34 Nevertheless, some studies used only CD4 and CD25 or other parameters such as absolute counts and percentages in total lymphocytes.Citation35,Citation36 Also, FOXP3 protein expression may not be stable, depending on FOXP3 gene methylation, and can disappear in 10–15% of FOXP3 + Tregs (so-called exFOXP3 cells) in mice.Citation37 Moon et al. showed that CD4 + CD25high/CD4 and CD4 + CD25highFoxP3+/CD4 cell populations were significantly correlated (P < 0.0001).Citation25
Our results showed a significant increase in the percentage of Tregs in AML patients in comparison with the control group (P < 0.001). These finding are in agreement with Moon et al. who found that the AML and high-grade MDS groups had significantly increased Tregs populations in both peripheral blood and bone marrow compared to the control groups.Citation25 This can be explained by the previous studies which have shown that AML cells secrete factors, which inhibit T-cell activation and proliferation and limit proinflammatory T helper-1 cytokine production.Citation38,Citation39 This suppressive effect is reversed, however, when Tregs and other T lymphocytes were removed from the microenvironment in vitro, leading to augmented immune responses to AML.Citation39 This can also be explained by the effect of MSCs in the marrow microenvironment on generating CD4 + CD25 + FOXP3+ cells. Ivanova-Todorova et al. found a stable tendency towards an increase in CD4 + CD25 + FOXP3 + cells number in the presence of MSCs compared with the respective control cultures without MSCs.Citation40 It was also stated that CD271 + MSCs secrete higher levels of cytokines and have greater immunosuppressive properties.Citation41
Our results showed a significant increase in MSCs IDO expression in AML patients when compared to the control group (P = 0.002). The increase in IDO expression in AML patients in comparison to the controls may be explained by the ability of leukemic cells to induce changes in MSCs. Civini et al. showed that there is a dynamic relationship between BM-MSCs and leukemia cells.Citation42 They confirmed that BM-MSCs affect leukemia cells and found that leukemia cells change the profile of cytokines produced by BM-MSCs to a proinflammatory signature through soluble factors and does not require direct contact between BM-MSCs and leukemia cells.
A positive correlation was found between IDO expression and Tregs (r = 0.5, P = 0.012), which may be explained by the role of IDO in inducing the conversion of CD25-FOXP3-T cells into CD25 + foxp3+.Citation43 The percentage of CD4 + CD25 + cells was significantly increased in IDO + AML patients compared to IDO-AML patients or controls,Citation44 which may also explain the correlation that was found in our study between blast cell percentage and the percentage of Tregs further supporting the proinflammatory effect of leukemia cells on BM-MSCs.Citation42
Data from several studies proved the poor prognostic effect of elevated Tregs on outcome of AML; Tregs at diagnosis were lower in patients who had achieved CR compared with those with persistent leukemia or death.Citation45 Studies in murine models have shown that the frequencies of Tregs are increased in AML in vivo, that these Tregs have suppressive functions on Teffs in vitro, and that removing/depleting Tregs improves the function of Teffs in vitro and improves treatment outcome.Citation46
In conclusion, our study revealed an association between high IDO expression in MSC and elevated levels of Tregs which has an important role in the pathogenesis of AML and supposed to affect treatment outcome. Current treatments for AML have not changed for several decades and have not resulted in satisfactory outcomes. Modulating the immune system may improve survival in patients with AML because the immune system is highly active against leukemic cells. It would be interesting to determine whether inhibiting IDO in conjugation with chemotherapy would result in better outcomes since it persistently breaks the tolerance to tumors, thus eliciting an effective immune response.
Disclaimer statements
Contributors No contributors other than the authors.
Funding None.
Conflict of interest Authors state that there is no conflict of interests.
Ethics approval The study was approved by the ethics committee of Cairo University.
References
- Ramakrishnan A, Deeg HJ. A novel role for the marrow microenvironment in initiating and sustaining hematopoietic disease. Expert Opin Biol Ther. 2009;9:21–8. doi: 10.1517/14712590802603093
- Wang H, Zhang P, Liu L, Zou L. Hierarchical organization and regulation of the hematopoietic stem cell osteoblastic niche. Crit Rev Oncol Hematol. 2013;85(1):1–8. doi: 10.1016/j.critrevonc.2012.05.004
- Sacchetti B, Funari A, Michienzi S, DI Cesare S, Piersanti S, Saggio I, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–36. doi: 10.1016/j.cell.2007.08.025
- Dazzi F, Ramasamy R, Glennie S, Jones SP, Roberts I. The role of mesenchymal stem cells in haemopoiesis. Blood Rev. 2006;20:161–71. doi: 10.1016/j.blre.2005.11.002
- Ramasamy R, Lam EW, Soeiro I, Tisato V, Bonnet D, Dazzi F. Mesenchymal stem cells inhibit proliferation and apoptosis of tumor cells: impact on in vivo tumor growth. Leukemia. 2007;21:304–10. doi: 10.1038/sj.leu.2404489
- Warner JK, Wang JC, Hope KJ, Jin L, Dick JE. Concepts of human leukemic development. Oncogene. 2004;23:7164–77. doi: 10.1038/sj.onc.1207933
- Lane SW, Scadden DT, Gilliland DG. The leukemic stem cell niche: current concepts and therapeutic opportunities. Blood. 2009;114:1150–7. doi: 10.1182/blood-2009-01-202606
- Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–72. doi: 10.1182/blood-2005-07-2657
- Djouad F, Charbonnier LM, Bouffi C, Louis-Plence P, Bony C, Apparailly F, et al. Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin-6-dependent mechanism. Stem Cells. 2007;25:2025–32. doi: 10.1634/stemcells.2006-0548
- Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–22. doi: 10.1182/blood-2004-04-1559
- Beyth S, Borovsky Z, Mevorach D, Liebergall M, Gazit Z, Aslan H, et al. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005;105:2214–9. doi: 10.1182/blood-2004-07-2921
- Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y, Yu XD, et al. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105:4120–6. doi: 10.1182/blood-2004-02-0586
- Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101:3722–9. doi: 10.1182/blood-2002-07-2104
- Polchert D, Sobinsky J, Douglas G, Kidd M, Moadsiri A, Reina E, et al. IFN-gamma activation of mesenchymal stem cells for treatment and prevention of graft versus host disease. Eur J Immunol. 2008;38:1745–55. doi: 10.1002/eji.200738129
- Eljaafari A, Tartelin ML, Aissaoui H, Chevrel G, Osta B, Lavocat F, et al. Bone marrow-derived and synovium-derived mesenchymal cells promote Th17 cell expansion and activation through caspase 1 activation: contribution to the chronicity of rheumatoid arthritis. Arthritis Rheum. 2012;64:2147–57. doi: 10.1002/art.34391
- Prevosto C, Zancolli M, Canevali P, Zocchi MR, Poggi A. Generation of CD4+ or CD8+ regulatory T cells upon mesenchymal stem cell-lymphocyte interaction. Haematologica. 2007;92:881–8. doi: 10.3324/haematol.11240
- DI Ianni M, Del Papa B, De Ioanni M, Moretti L, Bonifacio E, Cecchini D, et al. Mesenchymal cells recruit and regulate T regulatory cells. Exp Hematol. 2008;36:309–18. doi: 10.1016/j.exphem.2007.11.007
- Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–50. doi: 10.1016/j.stem.2007.11.014
- Kondelkova K, Vokurkova D, Krejsek J, Borska L, Fiala Z, Ctirad A. Regulatory T cells (TREG) and their roles in immune system with respect to immunopathological disorders. Acta Medica (Hradec Kralove). 2010;53:73–7.
- Munn DH. Indoleamine 2,3-dioxygenase, Tregs and cancer. Curr Med Chem. 2011;18:2240–6. doi: 10.2174/092986711795656045
- Bonanno G, Mariotti A, Procoli A, Folgiero V, Natale D, De Rosa L, et al. Indoleamine 2,3-dioxygenase 1 (IDO1) activity correlates with immune system abnormalities in multiple myeloma. J Transl Med. 2012;10:247. doi: 10.1186/1479-5876-10-247
- Sharma MD, Baban B, Chandler P, Hou DY, Singh N, Yagita H, et al. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J Clin Invest. 2007;117:2570–82. doi: 10.1172/JCI31911
- Baban B, Chandler PR, Sharma MD, Pihkala J, Koni PA, Munn DH, et al. IDO activates regulatory T cells and blocks their conversion into Th17-like T cells. J Immunol. 2009;183:2475–83. doi: 10.4049/jimmunol.0900986
- Curti A, Aluigi M, Pandolfi S, Ferri E, Isidori A, Salvestrini V, et al. Acute myeloid leukemia cells constitutively express the immunoregulatory enzyme indoleamine 2,3-dioxygenase. Leukemia. 2007;21:353–5. doi: 10.1038/sj.leu.2404485
- Moon HW, Kim BH, Park CM, Hur M, Yun YM, Kim SY, et al. CD4+ CD25highFoxP3+regulatory T-cells in hematologic diseases. Korean J Lab Med. 2011;31:231–7. doi: 10.3343/kjlm.2011.31.4.231
- Brandacher G, Winkler C, Schroecksnadel K, Margreiter R, Fuchs D. Antitumoral activity of interferon-gamma involved in impaired immune function in cancer patients. Curr Drug Metab. 2006;7:599–612. doi: 10.2174/138920006778017768
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells: the International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7. doi: 10.1080/14653240600855905
- Jones EA, English A, Henshaw K, Kinsey SE, Markham AF, Emery P, et al. Enumeration and phenotypic characterization of synovial fluid multipotential mesenchymal progenitor cells in inflammatory and degenerative arthritis. Arthritis Rheum. 2004;50:817–27. doi: 10.1002/art.20203
- Jones EA, Kinsey SE, English A, Jones RA, Straszynski L, Meredith DM, et al. Isolation and characterization of bone marrow multipotential mesenchymal progenitor cells. Arthritis Rheum. 2002;46:3349–60. doi: 10.1002/art.10696
- Gronthos S, Simmons PJ. The growth factor requirements of STRO-1-positive human bone marrow stromal precursors under serum-deprived conditions in vitro. Blood. 1995;85:929–40.
- Quirici N, Soligo D, Bossolasco P, Servida F, Lumini C, Deliliers GL. Isolation of bone marrow mesenchymal stem cells by anti-nerve growth factor receptor antibodies. Exp Hematol. 2002;30:783–91. doi: 10.1016/S0301-472X(02)00812-3
- Jarocha D, Lesko E, Ratajczak MZ, Majka M. Comparison of different strategies of MSC isolation revels advantage to expand MSC directly from purified CD105(+) and CD271(+) cells. Blood. 2006;108:725A.
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–87. doi: 10.1016/j.cell.2008.05.009
- Alatrakchi N, Koziel M. Regulatory T cells and viral liver disease. J Viral Hepat. 2009;16:223–9. doi: 10.1111/j.1365-2893.2009.01081.x
- Kordasti SY, Ingram W, Hayden J, Darling D, Barber L, Afzali B, et al. CD4 + CD25high Foxp3 + regulatory T cells in myelodysplastic syndrome (MDS). Blood. 2007;110:847–50. doi: 10.1182/blood-2007-01-067546
- Solomou EE, Rezvani K, Mielke S, Malide D, Keyvanfar K, Visconte V, et al. Deficient CD4 + CD25 + FOXP3 + T regulatory cells in acquired aplastic anemia. Blood. 2007;110:1603–6. doi: 10.1182/blood-2007-01-066258
- Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martínez-Llordella M, Ashby M, et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009;10(9):1000–7. doi: 10.1038/ni.1774
- Buggins AG, Milojkovic D, Arno MJ, Lea NC, Mufti GJ, Thomas NS, et al. Microenvironment produced by acute myeloid leukemia cells prevents T cell activation and proliferation by inhibition of NF-kappaB, c-Myc, and pRb pathways. J Immunol. 2001;167:6021–30. doi: 10.4049/jimmunol.167.10.6021
- Orleans-Lindsay JK, Barber LD, Prentice HG, Lowdell MW. Acute myeloid leukaemia cells secrete a soluble factor that inhibits T and NK cell proliferation but not cytolytic function–implications for the adoptive immunotherapy of leukaemia. Clin Exp Immunol. 2001;126:403–11. doi: 10.1046/j.1365-2249.2001.01692.x
- Ivanova-Todorova E, Bochev I, Dimitrov R, Belemezova K, Mourdjeva M, Kyurkchiev S, et al. Conditioned medium from adipose tissue-derived mesenchymal stem cells induces CD4 + FOXP3 + cells and increases IL-10 secretion. J Biomed Biotechnol. 2012;2012:1295167–72. doi: 10.1155/2012/295167
- Kuci S, Kuci Z, Kreyenberg H, Deak E, Putsch K, Huenecke S, et al. CD271 antigen defines a subset of multipotent stromal cells with immunosuppressive and lymphohematopoietic engraftment-promoting properties. Haematologica. 2010;95:651–9. doi: 10.3324/haematol.2009.015065
- Civini S, Jin P, Ren J, Sabatino M, Castiello L, Jin J, et al. Leukemia cells induce changes in human bone marrow stromal cells. J Transl Med. 2013;11:298. doi: 10.1186/1479-5876-11-298
- Fallarino F, Grohmann U, You S, Mcgrath BC, Cavener DR, Vacca C, et al. Tryptophan catabolism generates autoimmune-preventive regulatory T cells. Transpl Immunol. 2006;17:58–60. doi: 10.1016/j.trim.2006.09.017
- Curti A, Isidori A, Ferri E, Terragna C, Neyroz P, Cellini C, et al. Generation of dendritic cells from positively selected CD14 + monocytes for anti-tumor immunotherapy. Leuk Lymphoma. 2004;45:1419–28. doi: 10.1080/10428190310001653682
- Shenghui Z, Yixiang H, Jianbo W, Kang Y, Laixi B, Yan Z, et al. Elevated frequencies of CD4(+) CD25(+) CD127lo regulatory T cells is associated to poor prognosis in patients with acute myeloid leukemia. Int J Cancer. 2011;129:1373–81. doi: 10.1002/ijc.25791
- Zhou Q, Bucher C, Munger ME, Highfill SL, Tolar J, Munn DH, et al. Depletion of endogenous tumor-associated regulatory T cells improves the efficacy of adoptive cytotoxic T-cell immunotherapy in murine acute myeloid leukemia. Blood. 2009;114(18):3793–802. doi: 10.1182/blood-2009-03-208181