Abstract
Objectives: Sickle cell disease (SCD) is associated with a pro-inflammatory state, characterized by an elevated baseline leukocyte count and inflammatory cytokines. Inflammation, white blood cell (WBC) adhesion to vascular endothelium with subsequent endothelial injury, and repeated ischemia–reperfusion injury contribute to disease pathogenesis. Identification of genetic polymorphisms that may modulate disease severity in SCD is becoming a field of interest. The Duffy blood group antigen has been identified as a receptor for various chemokines involved in neutrophil activation and trafficking. This study aimed at investigating the effect of RBCs’ Duffy antigen expression and its genetic polymorphisms on modulating disease severity and its complications among Egyptian sickle cell patients.
Methods
We analyzed the association of Duffy genotypes and phenotypes with clinical expression of SCD in 100 Egyptian patients. The Duffy phenotype expression was detected by indirect anti-globulin test while Duffy genotyping was conducted with polymerase chain reaction-restriction fragment length polymorphism-based assay.
Results
Total WBC count was strongly associated with Duffy genotype. WBCs were significantly higher in Duffy-positive patients (P = 0.002). No statistical significance was evident between individual measures of disease severity (pulmonary dysfunction, avascular necrosis, central nervous system dysfunction, kidney dysfunction, and leg ulcers) and Duffy genotype.
Conclusion
Our study suggests that RBC Duffy expression increases levels of WBCs in SCD patients and that Duffy genotype may not be a potential biomarker for end-organ damage in SCD.
Introduction
Despite identical genotypes, the clinical course of sickle cell disease (SCD) is extremely variable, prompting the search for genetic and biological predictors of disease severity. SCD is now recognized as a chronic inflammatory state in which inflammation, white blood cell (WBC) adhesion to vascular endothelium, subsequent endothelial injury, and repeated ischemia–reperfusion injury contribute to disease pathogenesis.Citation1 This chronic inflammatory state which differs from one patient to another may partly account to the variability of disease expression.Citation2 In addition, environmental, psychosocial, and genetic factors have been implicated. Therefore, active research has been directed to genetic polymorphisms that can modulate disease severity.Citation3
The Duffy antigens are carried by Duffy antigen receptor for chemokines (DARC, CD234). This glycoprotein is expressed on RBC membrane, endothelial cells lining post capillary venules as well as the epithelial cells of some organs.Citation4 The DARC binds a variety of chemokines including C–X–R (acute inflammation chemokine) and C–C (chronic inflammation chemokine), IL-8 (interleukin 8), and RANTES.Citation5
The function of DARC has not been clearly defined. It has been suggested that RBC Duffy antigens may play a dual role as a chemokine sink to prevent WBC activation in the circulation and hinder chemokine spread from the blood into organs.Citation6 As such, RBC Duffy expression potentially provides enhanced recruitment and protection from inadvertent chemokine release by neutralizing chemokines in the blood.Citation7 Therefore, it has been hypothesized that a Duffy-positive phenotype Fy (a+b+), Fy (a+b−), or Fy (a−b+) may be protective against inflammatory processes due to more effective clearance of chemokines from circulation.Citation8
A single nucleotide polymorphism (SNP) rs2814778 T/C position-33 in the FY gene promoter results in absence of Duffy expression on red blood cells,Citation9 and the substitution 265C>T, together with 298G>A, weakens the Fyb antigen (Fyx).Citation10
Although the associations between Duffy phenotype and markers of disease severity in SCD have been studied, no clear consensus was reached.Citation8 This study aimed at investigating the effect of RBCs’ Duffy antigen expression and its genetic polymorphisms on modulating SCD severity and its complications.
Subjects and methods
Study population
This case–control study was conducted at New Children's Hospital, Cairo University, Egypt, from 2011 to 2014. It included 100 patients with SCD (54 males and 46 females) with mean age of 12.04 ± 6.21 years. Diagnosis of SCD was established by hemoglobin electrophoresis and/or high-performance liquid chromatography. Of the total number of patients, 59 patients (59%) had homozygous HbSS, 40 (40%) HbSβ thalassemia, and 1 (1%) with HbSC. All recruited patients were in a steady-state attending routine follow-up visits at the time of enrolment. The study protocol was approved by the Research Ethics Committee of Cairo University. Informed consents were obtained willingly from all patients and/or their guardians.
Clinical events
Detailed history-taking, review of medical records, and thorough clinical examinations were carried out. Subjects were stratified into complicated and non-complicated SCD patients. Complications were determined according to a modified version of a previously validated chronic disease severity score.Citation11 It was based on the presence or absence of each of the following: (i) Pulmonary dysfunction (as defined by the presence of pulmonary hypertension previously diagnosed by Doppler echocardiography when tricuspid regurgitant jet velocity was equal to or above 2.5 m/s), (ii) avascular necrosis of the hip or shoulder (AVN), (iii) central nervous system (CNS) abnormality (as defined by a history of stroke, seizure, or transient ischemic attack), (iv) kidney dysfunction (as defined by the presence of micro-albuminuria when urinary albumin/creatinine (A/C) ratio is 30–300 g/g or macro-albuminuria when A/C ratio is > 300 g/g), and (v) history of leg ulcers. For each item, one point was assigned if present. The points were added to obtain the final score, ranging from 0 points, representing the mildest disease, to a maximum of five points, representing the most severe disease.Citation11 Complicated group included patients having one or more of the clinical complications and had score 1, 2, or 3, whereas non-complicated group included those who had none of the above clinical complications and had score 0. The number of vaso-occlusive crisis (VOC) requiring treatment in the past 12 months was recorded. VOC was defined as seeking medical treatment for not otherwise explained pain in extremities, back, abdomen, chest, or head.Citation12
Laboratory methods
Steady-state laboratory testing for all patients included complete blood picture, reticulocytic count, and hemoglobin electrophoresis. The expression of Duffy phenotype was detected by indirect anti-globulin test using both: tube and gel methods.
Genotyping of Duffy antigen (-33T>C GATA box motif, 125G>A, 265C>T, and 298G>A)
DNA extraction
Genomic DNA extraction from peripheral blood leucocytes was done using the Gene JET Whole Blood Genomic DNA purification kit (Fermentas Life Sciences, Canada) following the manufacturer's instructions. Genotyping of Duffy antigen was performed by polymerase chain reaction–restriction fragment length polymorphism (PCR–RFLP). All PCRs were performed in a total volume of 25 µl containing 150 ng genomic DNA, 2X Dream Taq Green PCR Master Mix (Fermentas, Lithuania), and 25 pM each of forward and reverse primers (Fermentas, Lithuania). Amplified products were visualized by gel electrophoresis using UV light.
PCR–RFLP genotyping
RFLP genotyping was performed by a PCR-based approach as described previously.Citation13,Citation14 Briefly, two DNA fragments encompassing the FY locus were obtained, one fragment A of 186-pb length (primers FYN1 5′-CAAGGCTGACCCCCATA-3′ and FYN2 5′-CATGGCACCGTTTGGTTCAG-3′), and one fragment B of 392-pb length long (FYAB1 5′-TCCCCCTCAACTGAGAACTC-3′ and FYAB2 5′-AAGGCTGAGCCATACCAGAC-3′) using the following thermocycling conditions: 20 seconds at 94°C, 15 seconds at 60°C, and 20 seconds at 72°C for 35 cycles for the former and 30 seconds at 95°C, 30 seconds at 58°C, and 40 seconds at 72°C for 35 cycles for the latter. Restriction enzyme digestion was performed following supplier-recommended protocols (New England Biolabs, Beverly, MA) as follows: fragment A/StyI, fragment B separate assay by using BanI, MsPA1I, and MwoI. Restriction fragments were visualized after gel electrophoresis.
Interpretation of results
Genotypically, patients were considered to be Duffy negative when genotyping showed two mutated GATA site (FY*B-33) or had one FY*B-33 associated with one of the other mutations observed on the Duffy gene (FY*B-33/FY*B-33, FY*B1/FY*B-33).Citation3 Patients were considered Duffy positive when one of the following genotypes was obtained FY*A/FY*A, FY*A/FY*B, FY*B/FY*B, and FY*B1/FY*B.
N.B.: FY*B1 Duffy allele has the SNP at nucleotide 298 from G to A (298 G>A).Citation13
Duffy phenotyping
Duffy phenotypes were determined by an indirect anti-globulin test according to the manufacturer's procedures (CLB, Netherlands), in which the tube method was used for 40% of subjects and the Gel method for 60% of subjects.
Four phenotypes were determined; patients with Fy (a−b−) phenotype were considered Duffy negative, whereas those with Fy (a+b−), Fy (a−b+), or Fy (a+b+) were considered Duffy positive.
Statistical analysis
All statistical calculations were carried out using computer programs SPSS (Statistical Package for the Social Science; SPSS Inc., Chicago, IL, USA) version 15 for Microsoft Windows. Quantitative variables were expressed as mean, standard deviation, median, and range and compared using the unpaired t-student test for independent samples when comparing two groups and the Kruskal–Wallis test when comparing more than two groups. Qualitative variables were expressed as frequency and percentage and compared using the chi-square test. Fisher's exact test was used when expected frequency was less than 5. The P value was considered to be significant if less than 0.05.
Results
Distribution of Duffy genotypes and phenotypes in sickle cell patients
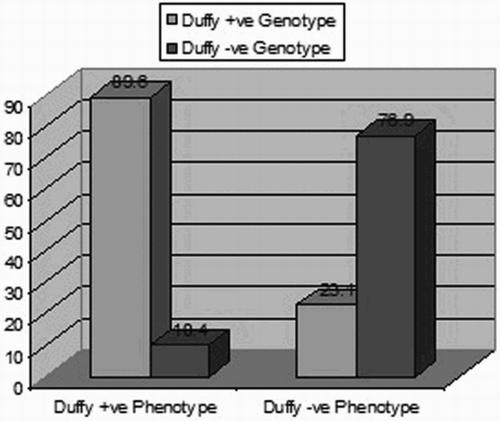
Fifty-five of 100 sickle cell patients (55%) had a positive Duffy (FY+) genotype; 24 were FY*A/FY*B, 23 were FY*B/FY*B, 5 were FY*A/FY*A, 2 were FY*A/FY*B-33, and 1 was FY*B/FY*B1. Forty-five patients (45%) were Duffy-negative (FY−) genotype, 43 patients were FY*B-33/FY*B-33, and 2 were FY*B1/FY*B-33. Phenotypically, 48 patients (48%) were Duffy positive: 23 (a+b−), 16 (a−b+), and 9 (a+b+), whereas 52 patients (52%) were Duffy negative (a−b−).
Comparing Duffy genotypes with phenotypes showed a statistically significant difference, indicating discordance between Duffy phenotype and genotype in our cohort (Fig. ).
Characteristics of sickle cell patients according to disease-related complications
Sixty-two (62%) of our patients fulfilled criteria of complicated group. The highest disease severity score for any of our patients was three out of possible five. Studying the frequency of disease-related complications among our cohort (n = 100) showed that 40% had kidney dysfunction, 26% had pulmonary dysfunction, 11% had CNS dysfunction, 8% had AVN of hip or shoulder joints, and none had history of leg ulcer. Demographic, clinical, laboratory, and Duffy genotypes/phenotypes characteristics in complicated and non-complicated patients are outlined in Table . Complicated group showed significantly a higher median transfusion frequency/year, lower hemoglobin level, and higher urinary A/C ratio (P = 0.048, 0.01, and 0.001, respectively).
Table 1 Demographic, clinical, laboratory, and Duffy genotypes/phenotypes in complicated and non-complicated sickle cell patients
Relationship of Duffy genotype with sickle cell patients’ clinical characteristics, VOC, and disease severity score
Duffy-positive genotype was significantly more prevalent among male patients (P = 0.04). There were no statistically significant differences in disease genotypes, VOC, frequency of disease-related complications (pulmonary dysfunction, AVN of hip or shoulder joints, CNS abnormalities, kidney dysfunction, or history of leg ulcers), transfusion frequency/year, or hydroxyurea treatment dose between Duffy-positive and -negative patients (Table ). Despite the statistically significant difference observed in overall disease severity score between Duffy-positive and -negative patients (P = 0.048), no constant trend in distribution of score number was evident among both groups (Table ).
Table 2 Clinical variables and disease severity score among Duffy-positive (FY+) and Duffy-negative (FY−) sickle cell patients
Relationship of Duffy genotype with hematological parameters of sickle cell patients
Comparing standard hematological parameters between Duffy-positive and -negative patients showed that the WBC count was significantly higher in Duffy-positive patients (P = 0.002) (Table ).
Table 3 Hematological parameters among Duffy-positive (FY+) and Duffy-negative (FY−) genotype sickle cell patients
Discussion
The pathophysiology underlying complications in SCD remains incompletely understood; genetic polymorphisms that may modulate disease severity are of great interest. In this context, the role of individual blood group antigens as functional molecules modifying SCD expression is one important avenue to explore.Citation1
The Duffy blood group antigen has been identified as a receptor for various chemokines involved in neutrophil activation and trafficking. It has been postulated that Duffy glycoprotein may mitigate the inflammatory state and thus modulate the occurrence of clinical complications in SCD patients.Citation15
In the present study, with regard to Duffy genotype in SCD patients, the percentage of Duffy negative genotype was relatively lower in our studied patients compared with previous studies (8, 11 and 16). This lower frequency of Duffy-negative genotype in our studied population might be contributed to racial differences and geographical distribution of the Duffy allele.
As for the correlation between genotype and phenotype results, in the present study, we demonstrated a discrepancy between the patients’ Duffy genotyping as determined by PCR–RFLP and their Duffy phenotype detected by hemagglutination testing where 55 patients were considered Duffy positive by genotyping while only 48 were considered Duffy positive by serological testing. Similarly, Castilho et al.,Citation13 Afenyi-Annan et al.,Citation11 and Parasol et al.Citation6 found discrepancy between Duffy phenotype and genotype in their studied groups. They attributed this discordance between Duffy genotype and Duffy phenotype to the SNPs, which cause weakening of Fyb antigen expression to the extent that only some anti-fyb reagents detect the antigen by hemagglutination. It should be noted that the serological testing used in this study here does not distinguish between Fy(a−b−) and Fy(a−bweak) erythrocyte phenotypes. Fy(a−bweak) erythrocytes often type as Fy(a−b−) if only the usual anti-Fyb were used by routine methods. Also, our studied group was multi-transfused patients, which may alter the result of the serologic testing of the expression of the Duffy antigen on the RBCs.
In the current study, we demonstrated a significant association of total leucocytic count with the Duffy genotype. Duffy-positive patients had significantly higher WBC count than that detected in Duffy-negative patients. This comes in approval with Nebor et al.,Citation8 Afenyi-Annan et al.,Citation11 and Drasar et al.Citation16 who also found that Duffy-positive patients had significantly higher WBC counts compared with those detected in Duffy-negative patients, unlike Schnog et al.Citation15 who could not report a statistically significant difference between Duffy phenotype and the total leucocytic count. Several lines of evidence suggest that leucocyte adhesion to red blood cells and vascular endothelium is a key event in the pathogenesis of several Sickle cell anemia (SCA) complications.Citation8 High total leucocytic count is a strong risk factor for several sickle cell clinical manifestations such as hemorrhagic stroke in children and adults, acute chest syndrome, and early death.Citation11 We speculate that our finding of higher WBC count could potentiate the inflammatory process in the Duffy-positive sickle cell anemia patients and that red cell expression of Duffy antigen has a role in leukocyte trafficking. Whether and how expression of RBCs Duffy protein may promote high WBC count could be a new avenue of investigation that could improve our understanding of genes that regulate inflammation.
Conflicting results have been reported regarding the clinical relevance of RBC Duffy expression in sickle cell anemia.Citation3 Comparison of each measure of disease severity (pulmonary dysfunction, AVN, CNS dysfunction, and leg ulcers) between Duffy-positive and -negative patients in our study revealed no statistically significant difference. Similar to our study, Afenyi-Annan et al.Citation11 found no difference between FY+ and FY− patients for measures of lung dysfunction, AVN of hip or shoulder, and leg ulcers. This was also the case with Nebor et al.Citation8 who found no difference between the two groups for the occurrence of osteonecrosis of femoral and humeral head or leg ulcers. However, Drasar et al.Citation16 found the incidence of leg ulcers to be the only sickle-related complication that is significantly higher in Duffy-positive patients compared with Duffy-negative patients. However, several factors including genetic and environmental contribute to the development and persistence of leg ulcers in SCA patients; this might be explanation for the difference between our results.
By comparing the disease severity score between the two groups of patients, there was a statistically significant value (P = 0.048), yet it is not clinically important because there is no constant trend in the distribution of the score number among Duffy-positive and -negative patients. On the contrary, Afenyi-Annan et al.Citation11 concluded that Duffy-negative status was strongly associated with the presence of end-organ damage. This difference is likely due to the difference in sample size between the two studies, which may have allowed more power to detect differences between the studied groups. Also, the racial difference between the two studied populations may contribute to the association between FY-genotype and end-organ dysfunction that may be due to the increased likelihood of other polymorphisms of African origin or in genes situated close to FY locus.
In conclusion, we did not detect any association between DARC RBC expression and the five degenerative complications studied. However, further studies, based on larger samples and including other clinical events, are warranted to validate the hypothesis that the raised WBC associated with Duffy-positive phenotype could potentiate increased rates of vaso-occlusion and potentially be a risk factor for the development of sickle-related complications and clarify the role of Duffy genotype in the modulation of the clinical phenotypes associated with sickle cell anemia.
Disclaimer statements
Contributors Clinical data by M.E.G. Laboratory work and supervision by R.S., H.M.F., M.S.F., H.A.A.R., and S.Y. Paper writing and review by S.Y., H.M.F., M.S.F., H.A.A.R., and M.E.G.
Funding This study was funded by the authors only.
Conflict of interest None.
Ethics approval The study protocol was approved by the Research Ethics Committee of Cairo University.
Acknowledgments
The authors would like to thank all the patients and controls enrolled in this study.
References
- Belcher JD, Bryant CJ, Nguyen J, Bowlin PR, Kielbik MC, Bischof JC, et al. Transgenic sickle mice have vascular inflammation. Blood. 2003;101:3953–9. doi: 10.1182/blood-2002-10-3313
- Durpès MC, ELNemer W, Picot JN, Elion J, Decastel M. Activation state of α4β1 integrin on sickle red blood cells is linked to the Duffy antigen receptor for chemokines (DARC) expression. J Biol Chem. 2011;286(4):3057–64. doi: 10.1074/jbc.M110.173229
- Mecabo G, Hayashida DY, Azevedo-Shimmoto MM, Vicari P, Arruda MMAS, Bordin JO, et al. Duffy-negative is associated with hemolytic phenotype of sickle cell anemia. Clin Immunol. 2010;136:458–9. doi: 10.1016/j.clim.2010.06.006
- Helias V, Saison C, Ballif BA, Peyrard T, Takahashi J, Takahashi H, et al. ISBT Committee on terminology for red cell surface antigens. Nat Genetics. 2009;44(2):643–5.
- Pogo AO, Chaudhuri A, Oscar Pogo A. The Duffy protein: a malarial and chemokine receptor. Semin Hematol. 2000;37:122–9. doi: 10.1016/S0037-1963(00)90037-4
- Parasol N, Reid M, Rios M, Castilho L, Harari I, Kosower NS. A novel mutation in the coding sequence of the FY*B allele of the Duffy chemokine receptor gene is associated with an altered erythrocyte phenotype. Blood. 1998;92:2237–43.
- Pruenster M, Rot A. Throwing light on DARC. Biochem Soc Trans. 2006;34:1005–8. doi: 10.1042/BST0341005
- Nebor D, Durpes MC, Mougenel D, Elion J, Hardy-Dessources M-D, Romana M. Association between Duffy antigen receptor for chemokines expression and levels of inflammation markers in sickle cell anemia patients. Clin Immunol. 2010;136:116–22. doi: 10.1016/j.clim.2010.02.023
- Mange KC, Prak EL, Kamoun M, Goodman N, Danoff T, Hoy T, et al. Duffy antigen receptor and genetic susceptibility of African Americans to acute rejection and delayed function. Kidney Int. 2004;66:1187–92. doi: 10.1111/j.1523-1755.2004.00871.x
- Wang J, Ou ZL, Hou YF, Shen Z-Z, Ding J, Shao Z-M. Enhanced expression of Duffy antigen receptor for chemokines by breast cancer cells attenuates growth and metastasis potential. Oncogene. 2006;25(54):7201–11. doi: 10.1038/sj.onc.1209703
- Afenyi-Annan A, Kail M, Combs MR, Ashley-Koch A, Telen MJ. Lack of Duffy antigen expression is associated with organ damage in patients with sickle cell disease. Transfusion. 2008;48:917–24. doi: 10.1111/j.1537-2995.2007.01622.x
- Thein SL. Genetic modifiers of sickle cell disease. Hemoglobin. 2011;35:589–606. doi: 10.3109/03630269.2011.615876
- Castilho L, Rios M, Pellegrino Jr. J, Saad ST, Costa FF, Reid ME. A novel FY allele in Brazilians. Vox Sang. 2004;87:190–5. doi: 10.1111/j.1423-0410.2004.00554.x
- Yazdanbakhsh K, Rios M, Storry JR, Parasol N, Chaudhuri A, Reid ME. Molecular mechanisms that lead to reduced expression of Duffy antigens. Transfusion. 2000;40:310–20. doi: 10.1046/j.1537-2995.2000.40030310.x
- Schnog JB, Keli SO, Pieters RA, Duits AJ. Duffy phenotype does not influence the clinical severity of sickle cell disease. Clin Immunol. 2000;96:264–8. doi: 10.1006/clim.2000.4884
- Drasar E, Menzel S, Fulford T, Thein SL. The effect of Duffy antigen receptor for chemokines on severity in sickle cell disease. Haematologica. 2013;98(8):e87–9. doi: 10.3324/haematol.2013.089243