Abstract
Donor cell leukemia (DCL) is a rare complication of hematopoietic stem cell transplantation (HSCT). Its incidence has been reported between 0.12 and 5%, although the majority of cases are anecdotal. The mechanisms of leukemogenesis in DCL may be distinct from other types of leukemia. Here we describe a case of a 27-year-old woman with a diagnosis of biphenotypic acute leukemia who received a HSCT and developed a DCL. We briefly discuss the possible pathogenesis, diagnosis, and treatment of DCL.
Introduction
Hematopoietic stem cell transplantation (HSCT) is an efficient treatment for multiple hematologic malignancies, but disease relapse remains a major cause of posttransplant mortality.Citation1 Occasionally, relapsed disease may display different phenotypic or cytogenetic features from the original disease. This may be because of a lineage switch, clonal evolution, or emergence of latent surviving subclones,Citation2 but usually the relapse clone is host derived. Not often, acute leukemia can develop de novo in donor-derived cells. Donor cell leukemia (DCL) has been the focus of considerable interest for the unique insights into the mechanisms of leukemogenesis it might provide.
Case report
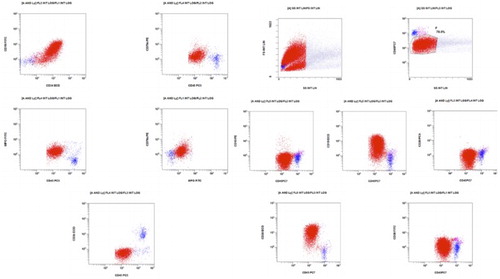
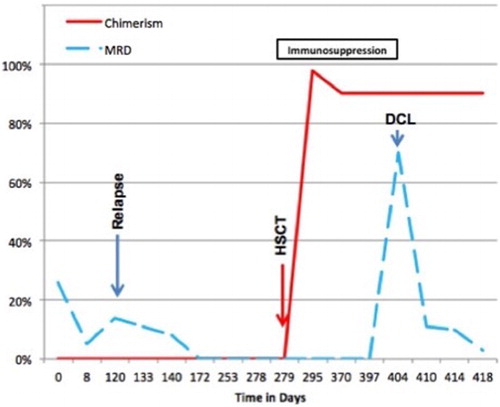
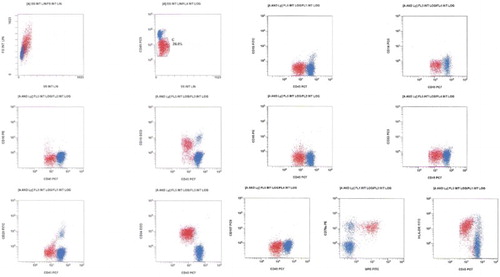
This 27-year-old female complained of generalized bone pain in the previous 3 months. She visited an orthopedic surgeon who ordered laboratory work up and found anemia, thrombocytopenia, and blasts in the blood count. She was referred to our center on July 2013. At physical examination she was pale, referred asthenia and bone pain, hepatosplenomegaly, rest of the examination with no other abnormalities. The initial laboratory work up revealed 29% blast cells in the peripheral blood, the flow cytometric analysis disclosing a biphenotypic acute leukemia (BAL). See Fig. BCR ABL(−), 46 XX, hyperdiploid, CD20 (−), CD 10 (−), CD13 (−), CD14 (−), CD15 (−), CD19 (+), CD33 (−), CD34 (+), CD45 (+/−), HLA (+), MPO (+), MLL (−), FLT3 (−), TEL AML (−). She was treated with a modification of the pediatric-inspired TOTAL XI scheduleCitation3 and a complete remission (CR) was achieved in October 2013. During the continuation therapy she abandoned treatment and subsequently had a relapse on November 2013. She was then treated with clofarabine and achieved a second CR on March 2014. Since she had no HLA-identical siblings, a female matched unrelated donor was identified and a reduced intensity conditioning allograft was conducted in April 2014, employing the Mexican method.Citation4 A total of 251 × 106/kg viable CD34 cells were grafted. On day +16, 98% chimerism was recorded, as assessed by means of short-tandem repeats. Later on, on day 41, grade I acute graft versus host disease (GVHD) was apparent with skin and mouth involvement. She was treated with prednisone, rituximab, and ruxolitinib obtaining an adequate response. Receiving oral cyclosporin A, on day +125, 3% blast cells appeared in the peripheral blood. In the bone marrow (BM) 70% blast cells were identified, the chimerism being 90%. The immunophenotype was again consonant with BAL at diagnosis CD20 (−), CD 10 (−), CD13 (−), CD15 (−), CD19 (+), CD34 (+), CD45 (+/−), MPO (+), CD79a (+/−), CD58 (−) (see Fig. ), but the number of blast cells and chimerism magnitude (90%) indicated a donor-cell origin. At this point cyclosporin A was stopped, the patient developing chronic GVHD. Maintaining a 90% chimerism, the assessment of minimal residual disease (MRD) became negative without any further chemotherapy (see Fig. ). Later on immunosuppression was re-started with sirolimus and several infectious complications ensued and were treated. Finally, on day +450 the patient died with 0% MRD, 90% chimerism on peripheral blood by means of short-tandem repeats, and a full blown hepatic sinusoidal obstructive syndrome.
Figure 1 Malignant cells (red) show large size as compared to normal lymphocytes and dim expression of CD45. Their phenotype is CD19+, CD20−+, CD10−, CD34++, CD79a++, MPO++, HLA-DR+, and CD117−. Myeloid markers CD13, CD14, CD15, and CD33 are all negative.
Discussion
DCL was first described by Fialkow et al.Citation5 in 1971. A 16-year-old female patient with acute lymphoblastic leukemia (ALL) relapsed 62 days after BM transplant (BMT) from an HLA-identical brother. Cytogenetics at relapse revealed only male (XY) metaphases, leading the authors to conclude that leukemic transformation had occurred in progeny of engrafted donor BM cells. They implicated activation of a leukemogenic agent in susceptible donor cells in this process. Further cases have been reported sporadically since, mostly as isolated reports and small series. Efforts to estimate the incidence of DCL are hampered by the sporadic nature of reports and habitual difficulties in confirming the diagnosis. In 1982, Boyd et al.Citation6 suggested that DCL might account for ≥5% of posttransplant leukemia relapses. Recently, frequency of reporting has accelerated with more cases reported since 2004 than during the previous 34 years. In 2005, a retrospective survey by the European Group for Blood and Marrow Transplantation asked all registered centers to report any cases of suspected or proven DCL encountered at their institutions, identifying 14 cases.Citation7 Because the 91 responding centers had performed a total 10 489 procedures, DCL incidence was estimated at 124 per 100 000 transplants. Most occurred within 4 years of HSCT, suggesting an annual incidence greatly exceeding the background incidence of acute leukemia. Sala-Torra et al.Citation8 reported six cases identified at their institutions since 1974, and our groupCitation9,Citation10 prospectively demonstrated donor origin in 2 of 40 consecutive relapses for a putative incidence of 5%: a figure intriguingly similar to that proposed by Boyd 24 years earlier.
The diagnosis of DCL depends on accurate and unequivocal demonstration of donor derivation of the leukemic clone. Several strategies have been employed, sharing the fundamental goal of identifying differences in genetic material between donor and recipient amenable to reliable investigation in the relapse clone. In practice, most approaches provide only indirect evidence of donor derivation through demonstration of complete donor chimerism. Conventional cytogenetics and fluorescent in situ hybridization, and molecular DNA markers are commonly used as diagnostic methods.Citation9,Citation11
Several putative mechanisms have been proposed to contribute critical ‘hits’ toward leukemogenesis in cells of donor origin following transplantation into a new host. Genetic factors might prime stem cells with a preleukemic phenotype within the donor, with a range of recipient- and therapy-specific factors probably interacting to contribute toward realization of malignant potential following engraftment into the more conducive BM environment of the recipient. A ‘multiple hit’ hypothesis has been proposed, with DCL probably the convergent endpoint of numerous distinct pathways (which may vary between individual cases).Citation11 In this case report, we can speculate that the immune manipulation with immunosuppressors withdrawal led to a graft versus leukemia effect that cleared the DCL, a phenomenon not previously reported, as far as we know.
DCL is considered to carry an extremely poor prognosis. Overall, this appears to hold, with at least 34 patients (53%) having died before publication by Wiseman,Citation11 at a median of 5.5 months after DCL diagnosis (range: 1 week to 64 months). However, at least 24 of the remaining 30 were evidently alive, after median follow-up of 14 months. Prognosis is hampered by the small number of reported cases, variable follow-up, and range of treatment strategies employed. Nevertheless, durable responses to salvage treatment were clearly achieved. Of the 24 surviving patients, at least 17 (71%) were reportedly in CR, with 4 others on active treatment. Mean overall survival for treated patients was 32.8 months.Citation11 We have shown that, since DCL is strictly a ‘new’ leukemia in the patient, a ‘de novo’ leukemia schedule should be tried; accordingly, we have been able to obtain sustained CR in patients with DCL treated with conventional chemotherapy and no further transplant.Citation12 In the paper by Hertenstein et al.,Citation7 reinduction chemotherapy with curative intent was attempted in 47 of 52 DCL patients, employing a range of standard AML- or ALL-specific regimens. No/minimal response occurred in 16 cases, and in another, reinduction resulted in aplasia precluding further treatment. Nevertheless, CR was achieved in at least 27 patients, remaining durable in 16 of these. CR was achieved in 13 of 17 (76%) donor-ALL patients, with 7 still in remission at median 12 months' follow-up. At least 16 of 34 (53%) donor-AML patients entered CR, with another still on treatment; remissions persisted in at least 10 of these (33%) at median 17 months' follow-up. A second allogeneic HSCT was performed in 17 patients of whom at least 7 (41%) remained alive (in CR) at a median of 29 months from diagnosis of DCL. At least seven patients subjected to second HSCT subsequently relapsed and died. One patient achieved a 44-month remission with reinduction before relapse of DCL prompted second BMT, but despite again responding, she relapsed and died 12 months later. Sala-Torra et al.'sCitation8 cohort included three DCL cases whose treatment included second HSCT, and although patient fate was not clearly stated, durable responses were probably achieved (because ‘last/latest follow-up’ ranged from 24 to 66 months). Thus, durable remissions can be obtained with standard reinduction strategies, particularly when consolidated with second HSCT.Citation11
DCL is an intriguing phenomenon, which was probably underdiagnosed for many years and could represent up to 5% or more of all leukemia relapses post-HSCT. Etiologic mechanisms are multifactorial. New molecular techniques for chimerism monitoring have elevated the ease, applicability, and reliability of DCL diagnosis to unprecedented levels, and it is perhaps not surprising that examples of DCL in the literature have exploded in parallel. DCL should be considered in cases of acute leukemia developing in the posttransplant period; the evolution of this case supports the notion that DCL is sensitive to the graft versus leukemia effect, which might be exploited in similar scenarios.
Disclaimer statements
Contributors Guillermo J. Ruiz-Delgado, Andrés A. León Peña, Andrés Gómez-de-León, Guillermo J. Ruiz-Argüelles.
Funding None.
Conflicts of interest The authors declare that they have no conflicts of interest.
Ethics approval Ethical approval was done by our local Research Ethics Committee.
ORCID
Andrés A. León Peña http://orcid.org/0000-0002-4010-9794
References
- Kumar L. Leukemia: management of relapse after allogeneic bone marrow transplantation. J Clin Oncol. 1994;12:1710–7.
- Giralt SA, Champlin RE. Leukemia relapse after allogeneic bone marrow transplantation: a review. Blood. 1994;84:3603–12.
- Ruiz-Delgado GJ, Macías-Gallardo J, Lutz-Presno J, Montes-Montiel M, Ruiz-Argüelles GJ. Outcome of adults with acute lymphoblastic leukemia treated with a pediatric-inspired therapy: a single institution experience. Leuk Lymphoma. 2011;52:314–6. doi: 10.3109/10428194.2010.529202
- Ruiz-Argüelles GJ, Gómez-Almaguer D, Ruiz-Argüelles A, González-Llano O, Cantú OG, Jaime-Pérez JC. Results of an outpatient-based stem cell allotransplant program using nonmyeloablative conditioning regimens. Am J Hematol. 2001 Apr;66(4):241–4. doi: 10.1002/ajh.1051
- Fialkow PJ, Thomas ED, Bryant JI, Neiman PE. Leukaemic transformation of engrafted human marrow cells in vivo. Lancet. 1971;1:251–5. doi: 10.1016/S0140-6736(71)90998-6
- Boyd CN, Ramberg RC, Thomas ED. The incidence of recurrence of leukemia in donor cells after allogeneic bone marrow transplantation. Leuk Res. 1982;6:833–7. doi: 10.1016/0145-2126(82)90067-4
- Hertenstein B, Hambach L, Bacigalupo A, Schmitz N, McCann S, Slavin S, et al. Development of leukemia in donor cells after allogeneic stem cell transplantation – a survey of the European Group for Blood and Marrow Transplantation (EBMT). Haematologica. 2005;90:969–75.
- Sala-Torra O, Hanna C, Loken MR, Flowers ME, Maris M, Ladne PA, et al. Evidence of donor-derived hematologic malignancies after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2006;12:511–7. doi: 10.1016/j.bbmt.2006.01.006
- Ruiz-Argüelles GJ, Ruiz-Delgado GJ, Garces-Eisele J, Ruiz-Arguelles A, Perez-Romano B, Reyes-Nuñez V. Donor cell leukemia after non-myeloablative allogeneic stem cell transplantation: a single institution experience. Leuk Lymphoma. 2006;47:1952–5. doi: 10.1080/10428190600693099
- Ruiz-Argüelles GJ, Ruiz-Argüelles A, Garcés-Eisele J. Donor cell leukemia: a critical review. Leuk Lymphoma. 2007;48:25–38. doi: 10.1080/10428190601003462
- Wiseman DH. Donor cell leukemia: a review. Biol Blood Marrow Transplant. 2011 Jun;17(6):771–89. doi: 10.1016/j.bbmt.2010.10.010
- Ruiz-Delgado GJ, Hernández-Reyes J, González-Ramírez MP, Martagón-Herrera NA, Garcés-Eisele J, Ruiz-Argüelles A, et al. Donor cell leukemia: a prospective study of its identification and treatment. Gac Méd Mex. 2015;151:582–7.