Abstract
Objective: To understand the responses of A, B, and O blood groups to oxidative stress (OS) induced through storage.
Methods: A, B, and O blood units were obtained from the blood bank at KIMS Hospital, Bangalore, and stored for 35 days at 4°C in citrate phosphate dextrose adenine-1 solution. Every fifth day, hemoglobin (Hb) was assessed in whole blood and erythrocytes were isolated from each group. OS markers such as (i) antioxidant enzymes [superoxide dismutase and catalase] and superoxides were assessed in hemolysate; (ii) lipid peroxidation product – malondialdehyde (MDA) and protein oxidation products [protein carbonyls, advanced oxidation protein products (AOPP), and protein sulfhydryls] were assessed in membrane ghosts.
Results: Antioxidant enzymes and Hb were similar in all groups. Superoxides increased in blood group O. MDA and AOPP differed between the groups, where levels in blood group O were lower than blood groups A and B. Sulfhydryls were maintained throughout storage.
Discussion: The antioxidant defense in A, B, and O groups were similar as evident from our results of Hb, antioxidant enzymes and sulfhydryls. However, the response of blood group O diverged from that of A and B, substantiated by the results of MDA, AOPP, and superoxides. Thus blood group O endured oxidative insult more efficiently than A and B. This study forms the basis for future studies on erythrocyte membrane and exploring blood group O as a potential candidate for prolonging storage.
Introduction
Blood storage is required to meet the demand for blood and its components, as blood transfusion is a life-saving treatment. It is used in cases of severe anemia (disease or chemotherapy) or by blood loss (due to trauma or major surgeries).Citation1 Red blood cells (RBCs) undergo a series of structural and functional changes resulting in reduced viability during storage. The oxidative modifications to lipids and proteins in the membrane are time-dependent and these changes destabilize them.Citation2,Citation3 RBCs are one of the first cells to be affected by reactive oxygen species due to high cell concentration of oxygen and hemoglobin (Hb) and the absence of cellular components to synthesize new proteins.Citation4 During storage, ATP and 2,3-diphospoglycerate concentrations drop due to metabolism, which cause dysfunction of the cation pumps, leading to oxidative damage of proteins and lipids. This results in morphological changes in the membrane leading to membrane vesiculation, changes in affinity and delivery of oxygen, adhesion to the endothelium and reduced life span of erythrocytes.Citation5Citation6–Citation7 Storage of blood attempts to bring the potential life-saving benefits of transfusion to maximum number of patients who require blood or its components. Thus, an in depth knowledge of the changes that occur during storage in different blood groups will help in the development of better storage techniques.
The ABO blood group system is characterized by the presence or absence of the carbohydrate determinants, antigens A and B. These antigens are synthesized by their corresponding glycosyltransferases. Transferase A encodes for α 1,3-N-acetylgalactosaminyltransferase and transferase B encodes for α 1,3-galactosyltransferase. These transferases catalyze the addition of specific monosaccharides to the precursor, i.e. the H antigen. Blood group O expresses only the H antigen on its surface.Citation8 The studies of He et al.,Citation9 Gill et al.,Citation10 and Dintenfass,Citation11 showed that there were variations in susceptibility between patients of different blood groups to cardiovascular disease and von Willebrand disease. Raval et al.,Citation12 reported that the changes in mechanical fragility were insignificant.
However, few studies have focused on the variations between blood groups during storage. Whole blood storage is still the most common practice that is employed in India. Hence, this study aims to understand the responses of erythrocytes isolated from A, B, and O blood groups to oxidative stress (OS) during storage. This study would give a better insight into the interactions of various blood groups under blood bank conditions.
Methods
Blood sampling
Blood was collected from healthy adults (males) of age 20–45 years from the blood bank at Kempegowda Institute of Medical Sciences (KIMS) Hospital, Bangalore and stored in citrate phosphate dextrose adenine-1 solution (CPDA-1) at 4°C. Ethical approval was obtained from the Ethics Committee of KIMS.
Experimental design
Blood units were grouped into A, B, and O blood groups (each group = 20 samples) and stored for 35 days at 4°C in CPDA-1. Every fifth day, Hb was assessed in whole blood and erythrocytes were isolated. The packed erythrocytes were converted into hemolysate and membrane ghosts. The superoxide levels and antioxidant enzymes were assessed in hemolysate while lipid peroxidation and protein oxidation products were assessed in membrane ghosts.
Erythrocyte separation
Erythrocytes were isolated from whole blood by centrifugation for 20 minutes at 1000 × g. The plasma was removed and the erythrocytes were washed and resuspended in isotonic phosphate buffer (pH 7.4).Citation13
Membrane separation
Hemolysis was performed on the erythrocyte suspension with hypotonic phosphate buffer yielding hemolysate and stored at −20°C. The ghost (membrane) was obtained by centrifuging the hemolysate at 20 000 × g, decanting the supernatant and washing in hypotonic phosphate buffer. The ghost was then resuspended in isotonic phosphate buffer and stored at −20°C.Citation13
Hemoglobin
Whole blood was incubated with Hemocor-D reagent, at room temperature and the absorbance was measured at 540 nm. Hb concentration was represented as g/dl.Citation14
Superoxides
Superoxide levels were estimated by the method of Olas and Wachowicz.Citation15 Hemolysate was treated with cytochrome C (160 µM), incubated at 37°C, centrifuged at 1000 × g and the absorbance was measured at 550 nm. Superoxides were represented as µM/mg protein.
Antioxidant enzymes
Superoxide dismutase [EC 1.15.1.1]
Hemolysate was treated with carbonate buffer (0.05 M) and epinephrine (30 mM). The change in absorbance was monitored at 480 nm. The amount of enzyme that inhibited oxidation of epinephrine by 50% was expressed as superoxide dismutase (SOD) activity.Citation16
Catalase [EC 1.11.1.6]
Hemolysate was treated with absolute alcohol and incubated at 0°C. H2O2 (6.6 mM) and phosphate buffer were added to the above mixture and the reduction in absorbance was detected at 240 nm. The enzyme activity was estimated using the extinction coefficient of 43.6 M cm−1.Citation17
Lipid peroxidation
Malondialdehyde (MDA)
SDS (8.1%) was added to the membrane suspension, mixed well and incubated at room temperature. Acetic acid (20%) and thiobarbituric acid (0.6%) were added and the samples were incubated at 100°C in a water bath. The cooled samples were treated with butanol-pyridine (15:1) mixture. After centrifugation, the absorbance of the colored layer was measured at 532 nm using the standard, 1,1,3,3-tetramethoxy propane. MDA levels were represented as µmol/mg protein.Citation18
Protein oxidation
Protein carbonyls (PrC)
2,4-Dinitrophenyl hydrazine (DNPH) (10 mM) was added to the membrane suspension. The reaction mixture was incubated in dark for 1 hour. Trichloroacetic acid (20%) was then added and cooled in ice for 10 minutes. The pellet obtained by centrifugation at 3000 × g was washed with ethanol-ethylacetate (1:1) mixture to eliminate free DNPH and contaminants. The pellet was resuspended in guanidine HCl (6 M) in 133 mM Tris. The absorbance was determined spectrophotometrically (370 nm) and PrC was computed using the extinction coefficient of 22 000 M−1 ml−1.Citation19
Advanced oxidation protein products (AOPP)
The membrane suspension was treated with isotonic phosphate buffer, 1.16 M l−1 potassium iodide, and glacial acetic acid. The absorbance of the mixture was determined at 340 nm, followed by the calculation of AOPP using the extinction coefficient of 26 mM−1 cm−1.Citation20
Protein sulfhydryls (P-SH)
Sodium phosphate buffer (0.08 M l−1) with Na2-EDTA (0.5 mg/ml) and SDS (2%) was treated with membrane suspension. 5,5′-Dithiobis-2-nitrobenzoic acid (DTNB) (20 mg in 10 ml) was added, mixed, incubated at room temperature and the absorbance was determined at 412 nm. The concentration of P-SH was calculated using the molar absorptivity, 13 600 M−1 l−1 cm−1.Citation21
Protein determination
The protein concentration in the membrane samples were determined with the standard, bovine serum albumin.Citation22
Statistical analyses
The results obtained were expressed as Mean ± SE. Two-way ANOVA and Bonferroni Post test was performed between the ABO blood groups (Groups) and storage days (sub groups) using GraphPad Prism 6 software and P < 0.05 was considered significant.
Results
Hemoglobin
Hb decreased in all groups over storage. Changes between the blood groups were insignificant.
Superoxides
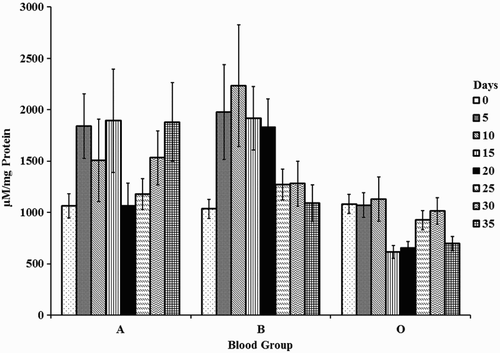
Changes in superoxides were significant in all groups with storage. Superoxides were high initially and reduced over storage in blood group A. Superoxides increased by one-fold on days 10, 20, and 25 in blood group A compared with day 5. Superoxides incremented by two-fold on day 10 with day 5 and one-fold on day 35 against day 20, whereas it decremented by 75% on day 20 against day 10 in blood group B. However, levels of superoxides were low initially and increased by four-fold (days 20, 25, 30, and 35) in blood group O against day 0.
Changes in superoxides between the blood groups were significant. Superoxides decreased by 80% (day 0) and 50% (day 10) in blood group O with respect to A and B. Increments of 212% (day 20) and 65% (day 25) were observed in blood group O with respect to B. Elevations of 50% and 86% were observed on day 35 in blood groups B and O, respectively against A (Fig. ).
Figure 1 Superoxides in erythrocytes of stored blood. A = Blood group A, B = Blood group B, and O = Blood group O. Values are represented as Mean ± SE. Changes between the blood groups were significantly different at P < 0.05. Changes within the sub groups (storage) are represented in special characters. * – significant with Day 0, # – significant with Day 5, ** – significant with Day 10, ## – significant with Day 15, ▪ – significant with Day 20, ○ – significant with Day 25.
Antioxidant enzymes
The antioxidant enzymes (SOD and catalase (CAT)) were similar in all groups with respect to storage. SOD was maintained throughout the storage period. CAT, however, was high on the initial days of storage and declined as the storage period increased. CAT decreased by 70% on days 25, 30, and 35 in all groups when compared with day 0 in all blood groups. Changes in SOD and CAT were insignificant between the blood groups.
Malondialdehyde
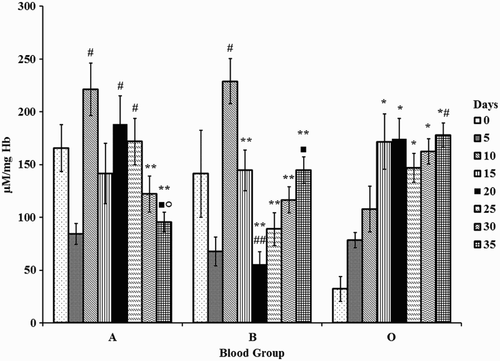
MDA increased in blood groups A, B, and O with storage. An increase of four-fold was observed on day 20 against day 5 in blood group B.
Changes between the blood groups were significant. Blood group O had lower levels of MDA when compared with A and B. MDA levels decreased by 80% in blood group O with respect to A (day 15) and B (days 15 and 20), respectively. Decrements of 66% were observed in blood groups B (day 25) and O (day 35) when compared with A (Fig. ).
Figure 2 Malondialdehyde in erythrocyte membranes of stored blood. A = Blood group A, B = Blood group B, and O = Blood group O. Values are represented as Mean ± SE. Changes between the blood groups were significantly different at P < 0.05. Changes within the sub groups (storage) are represented in special characters. # – significant with Day 5.
Protein carbonyls
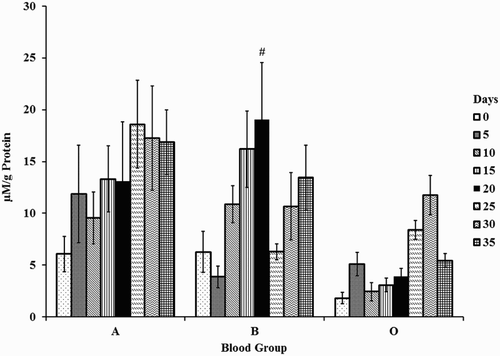
Changes in PrC were significant in blood groups B and O with storage. A decrement of 65% was observed on days 25 and 30 in blood group B with day 5. PrC showed a reduction of 60% (day 20) in blood group O when compared with day 5. Changes between the blood groups were insignificant.
Advanced oxidation protein products
Changes in AOPP were insignificant with storage. However, changes between the blood groups were significant. AOPP in blood group O were lower than in A and B. A decrement of 65% was observed in blood group O with A (days 15 and 35) and B (days 15 and 20), respectively (Fig. ).
Protein sulfhydryls
P-SH were maintained in all blood groups during storage. Sulfhydryls were significant in blood group B, where 55% decrement was observed on days 20, 30, and 35 against day 15. Changes in sulfhydryls were insignificant between blood groups.
Discussion
Every blood group system is genetically distinct. The A gene encodes N-acetylgalactosamine transferase and the B gene encodes for galactose transferase. These genes add the carbohydrate to galactose of the H antigen giving rise to the respective antigens. The presence or absence of A, B, and H antigens, which are localized on the same macromolecule, govern the ABO blood group system.Citation23
Blood group O had lower levels of MDA and AOPP, while it had higher levels of superoxides when compared with A and B. Blood group O, having lower levels of MDA suggests that the antioxidant system of blood group O could protect the cells from lipid peroxidation more efficiently than A and B. The reduction in AOPP in blood group O may be attributed to AOPP behaving like advanced glycation end products which at high concentrations can cause apoptosis and trigger NADPH oxidase that elevates superoxide anion production.Citation24 This was evident in the increased superoxides in blood group O. Although superoxides were high in blood group O, levels of Hb, antioxidant enzyme activities, protein sulfhydryls, and PrC were similar to A and B, suggesting that it could endure oxidative insult more efficiently than A and B. Thus blood group O can be a potential candidate for further storage studies.
This study shows that the ABO blood groups differ in terms of OS markers during storage. Hence there is a need to explore the responses of different blood groups to storage, which may contribute towards effective management of banked blood.
Disclaimer statement
Contributors Dr Leela Iyengar, Dr. Manohar SH, Dr. Suma, Ms. Manasa K and Jain University for support, Dr Venkatesh Prasad, Blood bank officer, Kempegowda Institute of Medical Sciences (KIMS) Hospital, Bangalore for his co-operation towards sample collection.
Conflicts of interest The authors have no conflict of interest to disclose.
Ethics approval Ethical approval was obtained from the Ethics Committee of KIMS.
Additional information
Funding
References
- Sparrow RL. Time to revisit red blood cell additive solutions and storage conditions: a role for “omics” analyses. Blood Transfus. 2012;10:7–11.
- Kucukakin B, Kocak V, Lykkesfeldt J, Nielsen HJ, Magnussen K, Rosenberg J, et al. Storage-induced increase in biomarkers of oxidative stress and inflammation in red blood cell components. Scand J Clin Lab Invest. 2011;71:299–303. doi: 10.3109/00365513.2011.563789
- Sachan DS, Hongu N, Johnsen M. Decreasing oxidative stress with choline and carnitine in women. J Am Coll Nutr. 2005;24:172–6. doi: 10.1080/07315724.2005.10719462
- Pandey KB, Rizvi SI. Markers of oxidative stress in erythrocytes and plasma during aging in humans. Oxid Med Cell Long. 2010;3:2–12. doi: 10.4161/oxim.3.1.10476
- Kato GJ. Understanding the erythrocyte storage lesion. Anesthesiology 2012;117:1159–61. doi: 10.1097/ALN.0b013e318272d8ac
- Van de Watering LM, Brand A. Effects of storage of red cells. Transfus Med Hemother. 2008;35:359–67. doi: 10.1159/000155221
- Jozwik M, Jozwik M, Jozwik M, Szczypka M, Gajewska J, Laskowska-Klita T. Antioxidant defence of red blood cells and plasma in stored human blood. Clin Chim Acta 1997;267:129–42. doi: 10.1016/S0009-8981(97)00148-4
- Zhang H, Ciarán JM, Muredach PR. ABO blood groups and cardiovascular diseases. Int J Vasc Med. 2012;2012: 641917-1-11.
- He M, Wolpin B, Rexrode K, Manson JE, Rimm E, Hu FB, et al. ABO blood group and risk of coronary heart disease in two prospective cohort studies. Arterioscler Thromb Vasc Biol. 2012;32:2314–20. doi: 10.1161/ATVBAHA.112.248757
- Gill JC, Endres-Brooks J, Bauer PJ, Marks Jr WJ, Montgomery RR. The effect of ABO blood group on the diagnosis of von Willebrand disease. Blood 1987;69:1691–5.
- Dintenfass L. The role of ABO blood groups in blood rheology of cardiovascular disorders. Angiology 1973;24:442–53. doi: 10.1177/000331977302400710
- Raval JS, Waters JH, Seltsam A, Scharberg EA, Richter E, Daly AR, et al. The use of the mechanical fragility test in evaluating sublethal RBC injury during storage. Vox Sang. 2010;99:325–31. doi: 10.1111/j.1423-0410.2010.01365.x
- Dodge JT, Mitchell C, Hanahan DJ. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963;100:119–30. doi: 10.1016/0003-9861(63)90042-0
- Hawk PB. Physiological chemistry. Vol. 392. Blakiston Division: McGraw-Hill; 1965.
- Olas B, Wachowicz B. Resveratrol and vitamin C as antioxidants in blood platelets. Thromb Res. 2002;106:143–8. doi: 10.1016/S0049-3848(02)00101-9
- Mishra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–5.
- Aebi H. Catalase in vitro. In: Packer L, (ed.) Methods enzymology. Cambridge: Acad Press; 1984. 105. p. 121–6.
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxidation in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3
- Reznick AZ, Packer L. Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Meth Enzymol. 1994;233:357–63. doi: 10.1016/S0076-6879(94)33041-7
- Witko V, Nguyen AT, Descamps-Latscha B. Microtiter plate assay for phagocyte-derived Taurine-chloramines. J Clin Lab Anal. 1992;6:47–53. doi: 10.1002/jcla.1860060110
- Habeeb AFSA. Reaction of protein sulfhydryl groups with Ellman's reagent. Meth Enzymol. 1972;25:457–64. doi: 10.1016/S0076-6879(72)25041-8
- Lowry OH, Rosenberg NJ, Farr AL, Randall RJ. Protein measurements with Folin-phenol reagent. J Biol Chem. 1951;193:265–75.
- Daniels G. Human blood groups. Oxford: John Wiley & Sons; 2008.
- Witko-Sarsat V, Friedlander M, Khoa TN, Capeillere-Blandin C, Nguyen AT, Canteloup S, et al. Advanced oxidation protein products as novel mediators of inflammation and monocyte activation in chronic renal failure. J Immunol. 1998;161:2524–32.