ABSTRACT
Objective and importance: Transplantation-mediated alloimmune thrombocytopenia (TMAT) occurs when leukocytes transferred in a donor organ from a patient with immune thrombocytopenia (ITP), mount a response against recipient platelets. We present the first fatal case of TMAT following liver transplantation and review its aetiology and treatment.
Clinical presentation: The liver donor had ITP and died from an intracranial haemorrhage. The recipient platelet count fell to 2 × 109/l on post-operative day 2. Treatment refractory thrombocytopenia resulted in pulmonary haemorrhage and death. TMAT did not occur in a kidney recipient from the same ITP donor.
Intervention: Extramedullary haematopoiesis was identified in the donor liver biopsy. Antibodies against platelet GPIb/IX were demonstrated in both donor and recipient. The thrombocytopenia was refractory to platelet transfusions, intravenous immunoglobulin, methylprednisolone, rituximab, romiplostim, plasmapheresis, vincristine and splenic artery embolization. On review of the literature, severe thrombocytopenia (<10 × 109/l) has started within 3 days of transplantation in all reported TMAT cases. Serious non-fatal bleeding was observed in 3/5 previously reported cases. The optimal treatment is unclear. TMAT should resolve as donor lymphocytes are eliminated but re-transplantation may be required in severe refractory cases. TMAT has been reported in recipients of a liver but not kidney or heart transplant from ITP donors, probably because of the greater burden of co-transplanted lymphoid tissue.
Conclusion: Before using the liver of an ITP donor, the recipient’s fully informed consent is required. However, the risk of TMAT from an ITP donor is currently unknown and systematic review of donor registries is needed.
Introduction
In patients with immune thrombocytopenia (ITP), auto-reactive plasma cells and lymphocytes accelerate platelet destruction. Production can also be suppressed by lymphocytes acting against bone marrow megakaryocytes [Citation1,Citation2]. ITP usually presents with bruising or bleeding and although intracranial haemorrhage is rare, fatal bleeding can lead to a patient becoming a deceased organ donor.
Orthotopic liver transplantation (OLT) results in the transfer of 109–1010 donor-derived leukocytes including pluripotent haematopoietic cells [Citation3]. Donor lymphocytes persist for weeks after transplantation [Citation4] but are eventually eliminated by the recipient’s immune system. However in 0.1–2% of OLT recipients, systemic graft-versus-host disease (GVHD) arises from donor lymphocytes engrafting in the recipients marrow and attacking the recipient tissues with a mortality rate of 80–100% [Citation3].
Transient, tissue-specific GVHD can also be caused by donor lymphocytes or plasma cells prior to their elimination. A common form of this ‘passenger lymphocyte syndrome’ (PLS) is the transient haemolysis caused by donor antibodies reactive against recipient red-cell antigens that occurs in 29% of ABO-mismatched OLT recipients [Citation5]. Transplantation-mediated alloimmune thrombocytopenia (TMAT) is a rare form of PLS in which leukocytes transferred in an ITP donor liver, act against the recipients platelets. TMAT is distinct from PLS-derived recipient thrombocytopenia arising from donors without ITP, who have allo-antibodies against human platelet antigens (HPA) [Citation6].
Of five previously documented TMAT cases, bleeding complicated three, but the platelet count always recovered. We present a TMAT case in which refractory thrombocytopenia and bleeding resulted in the recipient’s death.
Case report
The donor was a blood group A positive 73-year-old male, who died from intracranial haemorrhage (ICH) secondary to primary ITP with a platelet count of 6 × 109/l. ITP was diagnosed 2 years previously and bone marrow investigation was consistent with ITP. The donor had received steroids, intravenous immunoglobulins (IVIg), rituximab, romiplostim and splenectomy. He provided a liver and one kidney for transplant. The kidney recipient was unaffected by TMAT. The liver was procured by standard methods using University of Wisconsin preservation fluid and appeared healthy at retrieval.
The liver recipient was a blood group A positive 61 year-old male, with decompensated alcohol related liver disease. At transplant assessment he had a modified end-stage liver disease score of 20. His platelet count pre-surgery was 72 × 109/l.
The surgical procedure was uncomplicated apart from the need to perform portal vein thrombectomy. The cold ischaemia time was 515 minutes and the warm ischaemia time was 50 minutes. All anastamoses were standard including a lateral cavo-cavostomy. The patient needed 5 units of packed red blood cells, 6 units of fresh frozen plasma, 2 adult doses of platelets and 2 units of cryoprecipitate intra-operatively.
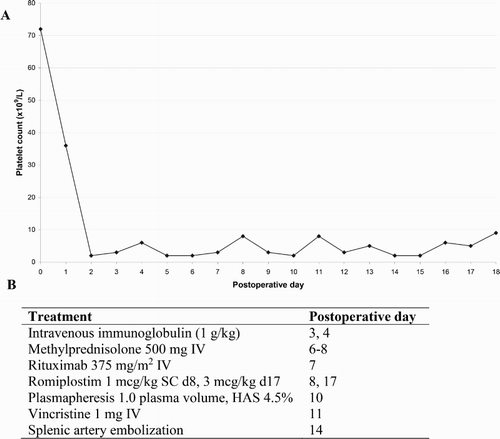
Post-operative graft function was good and he received immunosuppression with basiliximab on post-operative day (POD) 1 and 4, mycophenolate mofetil, prednisolone and tacrolimus. However, the platelet count fell from 36 × 109/l on POD1 to 2 × 109/l on POD2; at which time he developed spontaneous epistaxis. Thereafter, he remained haemorrhagic with bleeding from the gastrointestinal tract and lungs. Between POD2 and time of death POD18, he received 31 units of packed red blood cells and 50 adult doses of platelets. Despite this, he never achieved a sustained platelet count greater than 10 × 109/l (). Human leukocyte antigen allo-antibodies were not detected.
Figure 1. (a) Post-operative platelet count in a liver transplant recipient affected by transplantation mediated alloimmune thrombocytopenia (TMAT). (b) Timing of TMAT directed therapies. IV: intravenous; SC: subcutaneous; d: post-operative day; HAS: human albumin solution.
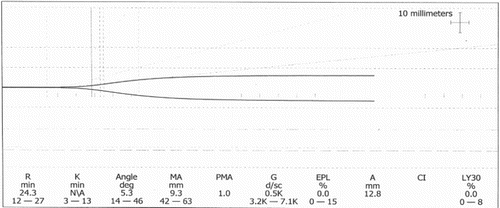
Thrombocytopenia was not precipitated by disseminated intravascular coagulation or systemic sepsis. A heparin-induced thrombocytopenia screen was negative. CMV and EBV serology were negative. Doppler ultrasound on POD 2&7 showed a patent portal vein. Drug-induced thrombocytopenia was considered and mycophenolate was stopped on POD2. A Von Willebrand disease screen was negative and a thromboelastogram on POD10 () showed a ‘champagne flute’ appearance typical of thrombocytopenia. Antibodies to human platelet antigens (HPA)-1, -2, -3, -4, -5 and -15 were not detected in donor or recipient, excluding post-transfusion purpura.
Figure 2. Thromboelastogram on day 10 post-transplantation. Thrombocytopenic picture. The reaction time (R) was normal, but clot formation time (K) was prolonged and maximum amplitude (MA) was decreased.
Platelet antibodies were detected in the recipient on POD7 and 17 by platelet immunofluorescence test. These antibodies were shown to be GPIb/IX specific by the monoclonal-antibody-immobilisation of platelet–antigens assay and the recipient was diagnosed with TMAT. Anti-GPIb/IX antibodies were later demonstrated in the donor’s plasma but were absent from the recipient’s serum sample taken 14 days prior to transplant. In addition to platelet transfusions, the patient received regular intravenous tranexamic acid 1 g × 4/day. TMAT directed treatments included IVIg, methylprednisolone, rituximab, romiplostim, plasmapheresis, vincristine and splenic artery embolization ().
As well as epistaxis, the patient developed extensive eccymosis. Melena started on POD3 and haemoptysis on POD5, both persisting until death. Computer tomography angiogram on POD6 did not identify a treatable point of gastrointestinal bleeding. Chest X-ray POD6 showed extensive bilateral consolidation consistent with pulmonary haemorrhage and the patient required non-invasive ventilation for type-2 respiratory failure from POD9. On POD10 he became agitated with worsening respiratory failure requiring intubation and mechanical ventilation. An extubation attempt on POD13 was unsuccessful with a marked decline in respiratory and haemodynamic stability. Re-listing for super-urgent OLT was considered on POD13 but the patient was considered not fit for retransplantation. On POD17, in light of the refractory nature of his haemorrhage, and the possibility that anti-platelet antibodies were functionally inhibiting GP-Ib/IX, he received eight 90 mcg/kg doses of recombinant factor VII (activated) concentrate (NovoSeven®). He did not respond and died on POD18 with multiorgan failure.
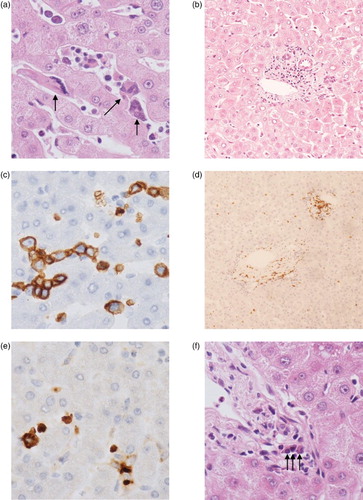
The donor liver time zero biopsy showed evidence of extramedullary haemopoiesis (EMH). Scattered T-cells and plasma cells were also noted in the portal tracts ().
Figure 3. Tri-lineage extramedullary haematopoiesis in the donor liver. (a) Extramedullary haematopoiesis (original magnification ×50). Three megakaryocytes highlighted with arrows. (b) Lymphocytes around the portal tract (×10). (C) Erythroid precursors stained with glycophorin C (×50). (D) Lymphocytes present in the parenchyma and around the portal tract with CD3 (×10). (E) Myeloid precursors stained with myeloperoxidase (×50). (F) Three plasma cells in the portal tract highlighted with arrows (×50).
Discussion
Similarities between this and five previously reported cases () will aid future recognition of TMAT. In all reports, the ITP donor died of ICH and had platelets <20 × 109/l at time of death. In all reports, the recipients platelet count fell to <10 × 109/l by POD3 and had a nadir platelet count <5 × 109/l. In three of six cases, defined anti-platelet antibody specificities found in donor and recipient supported the diagnosis. However these antibodies are not routinely tested for in ITP patients as the clinical value is low and an absence of detectable antibody does not exclude the diagnosis.
Table 1. Cases of transplantation mediated alloimmune thrombocytopenia (TMAT).
Thrombocytopenia is a frequent early complication following OLT and in a series of 541 OLT recipients the platelet count fell in 91%, from the pre-transplant baseline to a mean nadir platelet count of 43 (±33) × 109/l [Citation7]. The platelet count typically reaches a nadir on the fourth post-operative day before gradually rising to exceed pre-transplant levels after the second post-operative week. Contributory causes may include reduced hepatic thrombopoietin production, platelet consumption (disseminated intravascular coagulation, sepsis), medication, viral infection, hypersplenism, heparin-induced thrombocytopenia, allograft sequestration and gastrointestinal haemorrhage [Citation8]. These may also contribute to the severity of thrombocytopenia in TMAT, and potentially reversible mechanisms should also be considered.
This is the first case in which uncontrolled bleeding led directly to the patients death, although significant bleeding complicated three of five previously reported cases including ICH [Citation9], melena [Citation8] and intra-abdominal bleeding [Citation8,Citation10].
Bleeding risk may be greater in TMAT than ITP for a number of reasons. First, the use of ITP donors with ICH might select those with a more severe bleeding phenotype. Second, other causes of post-OLT thrombocytopenia discussed above compound the problem. Thirdly, not all ITP patients bleed even with severe thrombocytopenia, however inflammation occurs following OLT from reperfusion injury resulting in increased pro-inflammatory cytokines such as tumour necrosis factor-α [Citation11]. This inflammation represents a challenge to vascular integrity that is normally supported by platelets, which block gaps in the vascular wall and release soluble factors that enhance the endothelial barrier function [Citation12]. Finally, inflammation activated macrophages in the donor liver may bind and remove opsonized platelets more avidly.
Interestingly, this is the first TMAT case involving platelet GPIb/IX antibodies, which have been associated with a poorer response to steroids and IVIg compared to ITP patients with GPIIb/IIIa autoantibodies [Citation13].
The frequency and severity of PLS-induced haemolysis in ABO-mismatched solid organ transplantation increase with the size of the organ, and its burden of co-transplanted lymphoid tissue [Citation14]. This pattern is followed in cases of liver-derived TMAT, since transplantation of six kidneys and a heart from the same ITP donors did not result in thrombocytopenia [Citation10,Citation15,Citation16] suggesting that TMAT is unlikely following transplantation of these organs.
PLS-induced haemolysis usually resolves within a few months because donor lymphocytes only have a brief period of proliferation and antibody production [Citation5]. In two TMAT cases, the platelet count recovered within 3 weeks with the original donor liver intact [Citation10,Citation15]. Interestingly, our donor had prominent EMH, which is also found in the spleen of 60% of ITP patients undergoing splenectomy [Citation17]. Plasma cells normally require a survival niche such as bone marrow for long-term survival, needing contact with soluble factors released by stromal cells, and with cells such as megakaryocytes, myeloid or dendritic cells [Citation18]. As well as increasing the burden of co-transplanted haematopoietic tissue, EMH in the donor liver could therefore potentially act as a protected site for prolonged survival of platelet antibody producing plasma cells.
The optimal treatment for TMAT is unknown because there are few reported cases which were treated with multiple agents, making the impact of an individual treatment difficult to assess. TMAT should resolve as donor leukocytes are eliminated but during this time, recipients are exposed to a significant bleeding risk. There has been no clear success with immunosuppression, which aims to act against donor leukocytes, but may also reduce the ability of the recipient’s immune system to remove them. Similarly, in GVHD post-OLT, both reduction and intensification of immunosuppression has been attempted, but without consensus on an optimal strategy [Citation3]. Plasma exchange does not appear effective for TMAT (), suggesting that passive transmission of anti-platelet allo-antibodies is not an important pathogenic mechanism. In ITP, IVIg inhibits phagocytosis of opsonized platelets by the reticuloendothelial system and can improve responsiveness to platelet transfusions and although dramatic responses have not been seen in TMAT, IVIg may have a role in the bleeding patient. Thrombomimetics such as romiplostim increase platelet production by stimulating the thrombopoietin receptor. They are a rational treatment since thrombopoietin levels can fall post-OLT, although the usual response time of several weeks is a limitation. Splenectomy leads to a rapid platelet count improvement in 80% of ITP patients. However TMAT patients did not respond to splenic embolization (current patient) or splenectomy [Citation8,Citation9]. In these cases, the ITP donor had also failed to respond to splenectomy suggesting that the transplanted liver was the major site of platelet destruction. This is further supported by resolution of thrombocytopenia following re-transplantation [Citation8,Citation9], which should be considered in patients with persistent symptomatic TMAT.
The best treatment is prevention and the safety of OLT from an ITP donor, especially when refractory to splenectomy, has been questioned [Citation8,Citation9]. However publication bias towards cases with serious adverse outcomes is likely. Between January 2000 and November 2015, this donor was one out of thirteen United Kingdom deceased organ donors with a diagnosis of ITP. All had died from ICH. In total, they donated two hearts, ten livers, 22 kidney transplants and one pancreas. Of these, the current TMAT case was the only one reported to the UK transplant registry (personal communication Dr James Neuberger, NHS Blood and Transplant). Demand for liver organs currently outstrips supply and the UK adult elective liver transplant waiting list 1-year mortality is 11% [Citation19]. The decision to proceed to OLT from an ITP donor should involve an informed discussion on the risk of not proceeding versus the risks of complications including TMAT. Systematic review of donor registries is needed to understand the risk of TMAT following OLT from ITP donors.
Acknowledgements
Thanks to Dr Sinisa Savic for useful discussion during the writing of this manuscript. Thanks also to Dr John Hudson for providing details and samples from the liver donor, and the staff of H&I in Bristol for performing the platelet immunology investigations.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCiD
Q. A. Hill http://orcid.org/0000-0002-0627-4358
A. D. Padmakumar http://orcid.org/0000-0003-0683-0243
References
- Cooper N, Bussel J. The pathogenesis of immune thrombocytopaenic purpura. Br J Haematol. 2006 May;133(4):364–374. doi: 10.1111/j.1365-2141.2006.06024.x
- Mahevas M, Patin P, Huetz F, et al. B cell depletion in immune thrombocytopenia reveals splenic long-lived plasma cells. J Clin Invest. 2013 Jan;123(1):432–442. doi: 10.1172/JCI65689
- Rogulj IM, Deeg J, Lee SJ. Acute graft versus host disease after orthotopic liver transplantation. J Hematol Oncol. 2012;5:50. doi: 10.1186/1756-8722-5-50
- Schlitt HJ, Kanehiro H, Raddatz G, et al. Persistence of donor lymphocytes in liver allograft recipients. Transplantation. 1993 Oct;56(4):1001–1007. doi: 10.1097/00007890-199310000-00042
- Ainsworth CD, Crowther MA, Treleaven D, et al. Severe hemolytic anemia post-renal transplantation produced by donor anti-D passenger lymphocytes: case report and literature review. Transfus Med Rev. 2009 Apr;23(2):155–159. doi: 10.1016/j.tmrv.2008.12.005
- West KA, Anderson DR, McAlister VC, Alloimmune thrombocytopenia after organ transplantation. N Engl J Med. 1999 Nov 11;341(20):1504–1057. doi: 10.1056/NEJM199911113412004
- Chatzipetrou MA, Tsaroucha AK, Weppler D, et al. Thrombocytopenia after liver transplantation. Transplantation. 1999 Mar 15;67(5):702–706. doi: 10.1097/00007890-199903150-00010
- Diaz GC, Prowda J, Lo IJ, et al. Transplantation-mediated alloimmune thrombocytopenia: guidelines for utilization of thrombocytopenic donors. Liver Transpl. 2008 Dec;14(12):1803–1809. doi: 10.1002/lt.21539
- Friend PJ, McCarthy LJ, Filo RS, Transmission of idiopathic (autoimmune) thrombocytopenic purpura by liver transplantation. N Engl J Med. 1990 Sep 20;323(12):807–811. doi: 10.1056/NEJM199009203231207
- Kaestner F, Kutsogiannis DJ. Successful kidney and liver transplantation from a donor with immune thrombocytopenia. Transplant Proc. 2013 Sep;45(7):2838–2840. doi: 10.1016/j.transproceed.2013.02.136
- Bellamy MC, Galley HF, Webster NR. Changes in inflammatory mediators during orthotopic liver transplantation. Br J Anaesth. 1997 Sep;79(3):338–341. doi: 10.1093/bja/79.3.338
- Thachil J. Why do thrombocytopenic patients bleed? Transfusion. 2013 Dec;53(12):3280–3281. doi: 10.1111/trf.12467
- Zeng Q, Zhu L, Tao L, et al. Relative efficacy of steroid therapy in immune thrombocytopenia mediated by anti-platelet GPIIbIIIa versus GPIbalpha antibodies. Am J Hematol. 2012 Feb;87(2):206–208. doi: 10.1002/ajh.22211
- Ramsey G. Red cell antibodies arising from solid organ transplants. Transfusion. 1991 Jan;31(1):76–86. doi: 10.1046/j.1537-2995.1991.31191096190.x
- de la Torre AN, Fisher A, Wilson DJ, et al. A case report of donor to recipient transmission of severe thrombocytopenia purpura. Transplantation. 2004 May 15;77(9):1473–1474. doi: 10.1097/01.TP.0000122417.61345.2D
- Pereboom IT, de Boer MT, Haagsma EB, et al. Transmission of idiopathic thrombocytopenic purpura during orthotopic liver transplantation. Transpl Int. 2010 Feb;23(2):236–238. doi: 10.1111/j.1432-2277.2009.00936.x
- Chang CS, Li CY, Cha SS. Chronic idiopathic thrombocytopenic purpura. Splenic pathologic features and their clinical correlation. Arch Pathol Lab Med. 1993 Oct;117(10):981–985.
- Tangye SG. Staying alive: regulation of plasma cell survival. Trends Immunol. 2011 Dec;32(12):595–602. doi: 10.1016/j.it.2011.09.001
- NHS Blood and Transplant. Annual Report on Liver Transplantation: Report for 2014/15. http://www.odt.nhs.uk 2015 September [cited 2016 May 23]. Available from: http://www.odt.nhs.uk/uk-transplant-registry/organ-specific-reports/
