ABSTRACT
Background: Mutations in Janus kinase 2 (JAK2), myeloproliferative leukemia (MPL), and CALR are highly relevant to Philadelphia chromosome (Ph)-negative myeloproliferative neoplasms.
Methods: Assessing the prevalence of molecular mutations in Chinese Han patients with essential thrombocythemia (ET), and correlating their mutational profile with disease characteristics/phenotype.
Results: Of the 110 subjects studied, 62 carried the JAK2 V617F mutation, 21 had CALR mutations, one carried an MPL (W515) mutation, and 28 had non-mutated JAK2, CALR, and MPL (so-called triple-negative ET). Mutations in JAK2 exon 12 were not detected in any patient. Two ET patients had both CALR and JAK2 V617F mutations. Comparing the hematological parameters of the patients with JAK2 mutations with those of the patients with CALR mutations showed that the ET patients with CALR mutations were younger (p = 0.045) and had higher platelet counts (p = 0.043).
Conclusion: Genotyping for CALR could be a useful diagnostic tool for JAK2/MPL-negative ET, since the data suggest that CALR is much more prevalent than MPL, therefore testing for CALR should be considered in patients who are JAK2 negative as its frequency is almost 20 times that of MPL mutation.
Introduction
Essential thrombocythemia (ET) is a chronic Philadelphia chromosome-negative myeloproliferative neoplasm (MPN) characterized by the overproduction of circulating platelets in the periphery due to the excessive proliferation of megakaryocytes in the bone marrow [Citation1]. The recurrent Janus kinase 2 (JAK2) V617F mutation has been an important molecular marker for MPNs since its discovery in 2005 [Citation2]. However, only 50–60% of ET cases are associated with the JAK2 V617F mutation. Among the 40% of patients with ET who lack the JAK2 V617F mutation, 3–5% carry mutations at codon 515 of the gene encoding the thrombopoietin receptor, a myeloproliferative leukemia (MPL) virus oncogene [Citation3]. In 2013, somatic mutations in calreticulin (CALR) were found in 20–25% of patients with ET or primary myelofibrosis (PMF) [Citation4,Citation5]. Like JAK2 and MPL mutations, somatic mutations of CALR have been identified as a potentially powerful diagnostic tool for patients with ET [Citation6].
In the current work, we studied a population of patients with ET and analyzed the frequency of JAK2, CALR, or MPL mutations as well as patients’ hematological characteristics.
Methods
Patients
The patients and the data were selected retrospectively from the myeloproliferative neoplasm database established for scientific research at the Department of Hematology of Drum Tower Hospital. A total of 110 patients with ET were enrolled (60 females and 50 males with a mean age of 55.7 years, range 13–88 years); they had been diagnosed at the Department of Hematology between 2012 and 2015 according to the WHO Classification of Tumours of Hematopoietic and Lymphoid Tissues (2008) [Citation7]. The clinical and laboratory data were reviewed from medical records. Written informed consent was obtained from all subjects, and the study design followed the principles of the Helsinki Declaration and was approved by the Ethics Committee of Drum Tower Hospital.
JAK2, CALR, and MPL mutation analysis
The patients with JAK2, CALR, and MPL mutations were identified by Sanger sequencing, as previously described [Citation8].
Statistical analysis
The differences in the distribution of continuous variables between the categories were analyzed by either a Mann–Whitney test (for nonparametric analyses) or an independent t-test (for parametric analyses). p < 0.05 was considered statistically significant. The statistical program SPSS 13.0 was used for all calculations.
Results
Among the 110 patients tested, JAK2 V617F was the most common mutation, observed in 62 patients (56.3%), while CALR mutations were detected in 21 patients (19.1%) (). One patient (0.9%) carried the MPL W515L mutation. A mutation in JAK2 exon 12 was not detected in any patient. Two ET patients had both CALR and JAK2 V617F mutations. The incidence of triple-negative (negative for JAK2/MPL/CALR) patients was 25.5% (28/110).
Figure 1. The two types of CALR mutations identified in this study. L367fs*46 (52 nucleotide deletion) and K385fs*47 (TTGTC insertion).
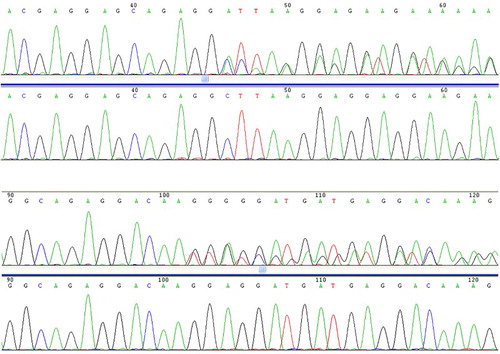
Next, we analyzed the patients’ biological features as a function of their mutational status, with a special focus on comparing the CALR-mutated versus JAK2-mutated subgroups, as these two mutations were predominant (). The ET patients with CALR mutations were younger (p = 0.045) and had higher platelet counts (p = 0.043). The leukocyte counts and hemoglobin levels were not significantly different between the two groups. More information about patients with both CALR and JAK2 V617F mutations has been provided in .
Table 1. Clinical and hematologic characteristics according to gene mutation status.
Table 2. Characteristics of two patients with both CALR and JAK2 V617F mutations.
Discussion
CALR is a highly conserved, multifunctional endoplasmic reticulum (ER) protein [Citation9]. The gene encoding the CALR protein is located on chromosome 19p13.2, contains 9 exons and spans a 4.2-kb region [Citation1]. Recently, CALR mutations were reported in ET patients with non-mutated JAK2/MPL [Citation10–14]. All CALR mutations reported in MPNs were located in exon 9 and were of the insertion/deletion type. Although the mutations varied, they all caused a frameshift to a unique alternative reading frame, which resulted in the loss of most of the acidic domain, including the KDEL signal, and created a novel C-terminus protein comprising a minimum of 36 amino acids [Citation4]. As reported in previous studies, several mutational types of CALR have been discovered [Citation13,Citation14]. However, in our study, L367fs*46 (52 nucleotide deletion) and K385fs*47 (TTGTC insertion), which were reported to have a incidence of over 60–80% in JAK2 and MPL mutation-negative ET and PMF patients [Citation4,Citation5], accounted for the mutation types. This study showed that each assay was able to detect CALR mutations, but targeted next generation sequencing (NGS) had the best limit of detection, detecting all mutations to a level of 1%. Among MPNs, CALR mutations have been found with ET and PMF but not with PV. CALR mutation has also been detected in other hematological malignancies such as MDS [Citation4,Citation5].
Although JAK2, MPL, and CALR mutations have been proposed to be mutually exclusive, we identified two ET patients with JAK2 V617F and CALR mutations, which was consistent with recent reports that described a rare concomitant mutation with these gene mutations [Citation14,Citation15]. In our study, the direct sequencing method identified a CALR mutation in 40.4% of patients with JAK2/MPL-negative ET, which was substantially less than the 53–71% reported by others [Citation4,Citation5,Citation8,Citation16,Citation17], probably owing to the small sample size. Many techniques have been used to detect CALR mutations, but not all are suitable for a clinical laboratory. Recently, Jones et al compared the limit of detection of Sanger sequencing (10–25%), fragment analysis PCR (5–10%), high-resolution melting (HRM) (5%), and targeted next generation sequencing (1.25%) [Citation18]. The effect of CALR mutation (which frequencies in ET are estimated between 15 and 32%) on the hematological phenotype has been reported in several studies [Citation10–14]. Compared with ET patients with a JAK2 mutation, those with a CALR mutation had lower leukocyte counts and hemoglobin values and higher platelet counts [Citation10–14]. We compared the hematologic and clinical features of patients grouped by mutation status, with the exception of two ET patients who carried concurrent JAK2 and CALR mutations. Compared with the JAK2 V617F mutation, the presence of a CALR mutation was associated with younger age and higher platelet counts. The leukocyte counts seemed to be lower in CALR-mutated ET patients than in those with the JAK2 mutation, although the difference did not reach statistical significance. Limited clinical and laboratory information of patients are provided in our study because of high rate of loss to follow-up. More detail on clinical characteristics (Hct, spleen size, symptoms, LDH, cytogenetics) histology (reticulin grade etc.), cytoreductive therapy used and response by IWG criteria, use of antithrombotic therapy, incidence of disease complications (thrombosis, bleeding and transformation) should be provided in our future research.
In summary, we have described the prevalence of molecular mutations in Chinese Han patients with ET, and their mutational profile with disease characteristics/phenotype. CALR mutations are a useful diagnostic marker for JAK2/MPL-negative ET patients because they are typically mutually exclusive with a JAK2 mutation and are present in a relatively high frequency.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Jing Wang is an attending doctor pursuing research in lymphoma.
Biao Zhang is an attending doctor, pursuing research in molecular pathology.
Bing Chen is a senior doctor, pursuing research in hematological malignancy.
Rong-Fu Zhou is a senior doctor, pursuing research in bleeding and coagulation.
Qi-Guo Zhang is a senior doctor, pursuing research in chronic myeloproliferative disease.
Juan Li is an associate senior doctor, pursuing research in lymphoma.
Yong-Gong Yang is an associate senior doctor, pursuing research in lymphoma.
Min Zhou is an associate senior doctor, pursuing research in lymphoma.
Xiao-Yan Shao is an associate senior doctor, pursuing research in myeloma.
Yong Xu is an associate senior doctor, pursuing research in myeloma.
Xi-Hui Xu is an associate senior doctor, pursuing research in lymphoma.
Jian Ouyang is a senior doctor, pursuing research in hematological malignancy.
Jingyan Xu is a senior doctor, pursuing research in hematological malignancy.
Qing Ye is a senior doctor, pursuing research in molecular pathology.
Additional information
Funding
References
- Tefferi A, Vardiman JW. Classification and diagnosis of myeloproliferative neoplasms: the 2008 World Health Organization criteria and point-of-care diagnostic algorithms. Leukemia. 2008;22(1):14–22. doi: 10.1038/sj.leu.2404955
- Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005 Apr 28;352(17):1779–1790. doi: 10.1056/NEJMoa051113
- Pikman Y, Lee BH, Mercher T, et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med. 2006;3(7):e270. doi: 10.1371/journal.pmed.0030270
- Klampfl T, Gisslinger H, Harutyunyan AS, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013;369(25):2379–2390. doi: 10.1056/NEJMoa1311347
- Nangalia J, Massie CE, Baxter EJ, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med. 2013;369(25):2391–2405. doi: 10.1056/NEJMoa1312542
- Cazzola M, Kralovics R. From Janus kinase 2 to calreticulin: the clinically relevant genomic landscape of myeloproliferative neoplasms. Blood. 2014;123(24):3714–3719. doi: 10.1182/blood-2014-03-530865
- Swerdllow S, Campo E, Harris NL. Who classification of tumours of haematopoietic and lymphoid tissues. France: IARC Press; 2008. 2008.
- Rumi E, Pietra D, Ferretti V, et al. JAK2 or CALR mutation status defines subtypes of essential thrombocythemia with substantially different clinical course and outcomes. Blood. 2014;123(10):1544–1551. doi: 10.1182/blood-2013-11-539098
- Coppolino MG, Woodside MJ, Demaurex N, et al. Calreticulin is essential for integrin-mediated calcium signalling and cell adhesion. Nature. 1997;386(6627):843–847. doi: 10.1038/386843a0
- Wu Z, Zhang X, Xu X, et al. The mutation profile of JAK2 and CALR in Chinese Han patients with Philadelphia chromosome-negative myeloproliferative neoplasms. J Hematol Oncol. 2014;7:48. doi: 10.1186/s13045-014-0048-6
- Trifa AP, Popp RA, Cucuianu A, et al. CALR versus JAK2 mutated essential thrombocythaemia – a report on 141 patients. Br J Haematol. 2015;168(1):151–153. doi: 10.1111/bjh.13076
- Shirane S, Araki M, Morishita S, et al. JAK2, CALR, and MPL mutation spectrum in Japanese patients with myeloproliferative neoplasms. Haematologica. 2015;100(2):e46–e48. doi: 10.3324/haematol.2014.115113
- Li N, Yao QM, Gale RP, et al. Frequency and allele burden of CALR mutations in Chinese with essential thrombocythemia and primary myelofibrosis without JAK2(V617F) or MPL mutations. Leuk Res. 2015;39(5):510–514. doi: 10.1016/j.leukres.2015.02.006
- Ha JS, Kim YK. Calreticulin exon 9 mutations in myeloproliferative neoplasms. Ann Lab Med. 2015;35(1):22–27. doi: 10.3343/alm.2015.35.1.22
- Lundberg P, Karow A, Nienhold R, et al. Clonal evolution and clinical correlates of somatic mutations in myeloproliferative neoplasms. Blood 2014;123(14):2220–2228. doi: 10.1182/blood-2013-11-537167
- Rotunno G, Mannarelli C, Guglielmelli P, et al. Impact of calreticulin mutations on clinical and hematological phenotype and outcome in essential thrombocythemia. Blood. 2014;123(10):1552–1555. doi: 10.1182/blood-2013-11-538983
- Chi J, Nicolaou KA, Nicolaidou V, et al. Calreticulin gene exon 9 frameshift mutations in patients with thrombocytosis. Leukemia. 2014;28(5):1152–1154. doi: 10.1038/leu.2013.382
- Jones AV, Ward D, Lyon M, et al. Evaluation of methods to detect CALR mutations in myeloproliferative neoplasms. Leuk Res. 2015;39(1):82–87. doi: 10.1016/j.leukres.2014.11.019
