ABSTRACT
Objectives: Our aim was to explore the relationship between JAK2V617F mutation allele burden and hematological parameters especially in coagulation function in Chinese population.
Methods: This study included 133 Ph-negative myeloproliferative neoplasms (MPNs) patients between 2013 and 2016. All the clinical and experimental data of patients were collected at the time of the diagnosis without any prior treatment, including blood parameters, coagulation function, splenomegaly, vascular events and chromosome karyotype. PCR and qPCR were used to detect JAK2V617F mutation and JAK2V617F mutation allele burden.
Results: In polycythemia vera patients, a positive correlation between the allele burden of JAK2V617F mutation and PLT counts was found; in essential thrombocythemia (ET) patients, WBC counts, RBC counts, HB, and HCT were higher in mutated patients than in wild-type patients. Furthermore, PT-INR was higher in ET and PMF mutated patients. In addition, a positive correlation between the allele burden of JAK2V617F mutation and activated partial thromboplastin time (APTT) was observed in JAK2V617F mutated ET patients.
Conclusions: Higher hematologic parameters including counts of WBC, RBC, and PLT are closely associated with JAK2V617F mutation and its burden in Ph-negative MPNs; importantly, PT-INR, APTT are also related to JAK2V617F mutation and allele burden. Thus, our data indicate that JAK2V617F mutation allele burden might not only represent the burden of MPN but also alter the coagulation function.
Introduction
Myeloproliferative neoplasms (MPNs) comprise a group of clonal malignancies derived from the myeloid lineage of hematopoietic stem cells. According to the existence of the Philadelphia chromosome, MPNs are classified into chronic myeloid leukemia and Ph-negative MPNs, and Ph-negative MPNs are further subclassified as polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF). Patients with these neoplasms present increased hematopoiesis and overproduction of mature and differentiated blood cells, and may have splenomegaly, myelofibrosis, and a higher risk of transformation to acute leukemia with the development of the disease. Besides, some patients usually present an increased risk of thrombotic and hemorrhagic complications [Citation1–4].
Recently, the discovery of the JAK2V617F [Citation4], CALR [Citation5], and MPL [Citation6] mutation provided new genetic markers and changed the diagnostic criteria of Ph-negative MPNs. The JAK2V617F mutation, resulting from a single G to T transversion occurs at base position 1849 in exon 14 of the JAK2 gene and leads to a valine to phenylalanine substitution at codon 617, which consequently increases the tyrosine kinase activity, thus resulting in clonal proliferation of one or several myeloid lineages [Citation7]
In patients with Ph-negative MPN, cases with a higher JAK2V617F allele burden were thought to have higher hematologic parameters, such as white blood cell counts, hemoglobin values, and platelet counts [Citation8–12]. In addition, accumulating studies about the association between JAK2V617F mutation and vascular events in Ph-negative MPN patients have been reported [Citation13,Citation14] and a higher risk of suffering with vascular events was investigated in patients with JAK2V617F mutation [Citation15,Citation16]. More recently, JAK2V617F mutation has been identified as an independent risk factor for thrombosis in ET [Citation17]. However, most studies were based on white populations, with only limited studies available from Chinese populations [Citation18,Citation19] and the role of coagulation function in MPN patients remains unknown.
In the current study, the aim was to investigate the relationship between JAK2V617F mutation, allele burden and clinical and laboratory parameters, especially in coagulation function, in Chinese Ph-negative MPNs patients.
Materials and methods
Patients and data collection
The study was conducted from May 2013 to April 2016, in the hematology department, the Second Hospital of Anhui Medical University, and Huaibei Miners General Hospital, and carried out according to the approval of the institutional review board and informed consent before patient enrollment. Hundred and thirty-three patients (70 males and 63 females) retrospectively diagnosed as Ph-negative MPNs were enrolled, the clinical diagnosis was made according to 2016 WHO criteria [Citation20]. All the patients’ clinical and experimental data were collected at the time of the diagnosis without any prior treatment, which included blood parameters, coagulation function, splenomegaly, and chromosome karyotype. The vascular events were recorded from the date of the diagnosis to the date of the last follow-up observation and the last follow-up assessment was conducted in October 2016.
JAK2V617F mutation detection
Total genomic DNA was extracted from EDTA-anticoagulated peripheral blood or bone marrow samples by using UNlQ-10 Column Clinical Sample DNA Isolation Kit (Sangon Biotech, Shanghai, China) following the manufacturer’s instructions. JAK2V617F mutation was detected by allele specific PCR (AS-PCR) as previously described [Citation4]. Allele specific real-time quantitative fluorescence PCR (AS-qPCR) was performed to detect JAK2V617F mutation by using a quantitative fluorescent DNA detection kit (Shenyou, Shanghai, China). JAK2 wild-type and mutation type were both amplified with respective primers, and JAK2V617F mutant allele burden was relatively quantified (JAK2V617F %) by determining the percentage of JAK2 mutation type quantity in the total JAK2 quantity.
Statistical analysis
Statistical significance was determined by the nonparametric unpaired Mann–Whitney U test and the parametric Student’s t test for samples when appropriate. Frequency differences of sex, splenomegaly, or vascular events according to the JAK2V617F mutation status were assessed by using a Chisquare or Fisher’s exact test. One-way ANOVA and LSD t test was used to compare the differences of the relative quantity of JAK2V617F mutation burden among the groups. Correlations were evaluated by using the Pearson’s coefficient test. All of the statistical analyses were carried out by using SPSS software version 19.0 for Windows (SPSS Inc., IL, USA) and PRISM 6.0 (GraphPad Software Inc., San Diego, CA, USA). P values less than 0.05 were considered to be statistically significant.
Results
Characteristics of the MPN patients enrolled andrates of the JAK2V617F mutation
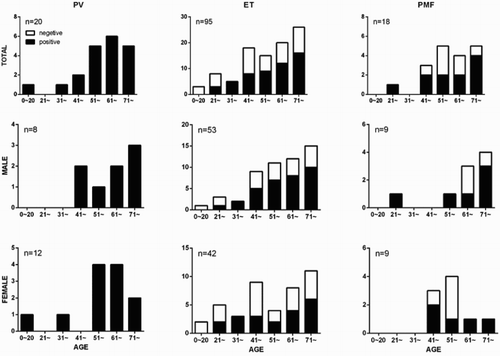
The enrolled patients with MPN comprised 95 ET, 20 PV, and 18 PMF patients, according to 2016 WHO criteria. The average ages were 59.3, 57.2, 60.7 years in PV, ET, and PMF patients, and M/F was 8/12, 55/40, 9/9, respectively. Chromosome karyotype was all normal. Splenomegaly was observed in 74 patients, and the rates were 75%, 47.3%, and 77.8% in PV, ET, and PMF patients, respectively. JAK2V617F mutation was detected in 84 of 133 (63.1%) patients with Ph-negative MPN, and the rates were 100%, 55.8%, and 61.1% in PV, ET, and PMF patients, respectively. Vascular events were presented in 6 PV patients, 21 ET patients (15 were JAK2 mutated and 6 were JAK2 wild-type, p = 0.182) and 2 PMF patients (2 were JAK2 mutated) during patients follow-up ().
Table 1. The clinical characteristics of Ph-negative MPN patients.
The rates of JAK2V617F mutation in different age groups of patients with Ph-negative MPN
In patients with Ph-negative MPN, the status of JAK2V617F mutation was assayed by AS-PCR and it was found that the patients with JAK2V617F mutation were older than wild-type ones (60.36 ± 15.62 vs. 54.32 ± 18.56, p = 0.045). In order to depict the JAK2V617F mutation rates in different sex and age groups, the patients were divided into two groups (male and female) and then categorized by age (). No significant difference in JAK2V617F mutation rates was observed between male and female.
The allele burden of the JAK2V617F mutation in MPN patients of this cohort
The above data showed the high rates of JAK2V617F mutation in Ph-negative MPNs including PV, ET, and PMF, but the allele burden of JAK2V617F mutation remained unknown. In order to investigate the level of burden in these patients, the burden of the JAK2V617F mutation was analyzed by AS-qPCR and it was found that the mean burden in 84 mutated Ph-negative MPN patients was (34.74 ± 18.93)%. To investigate whether the level of burden was different among PV, ET, and PMF, one-way ANOVA test was performed and showed that JAK2V617F mutation allele burden was higher in PV patients than in ET patients (45.13 ± 19.92 vs 31.12 ± 18.69% p = 0.007) and PMF patients (45.13 ± 19.92 vs 33.28 ± 10.79% p = 0.040) (a). Furthermore, it was found that the allele burden was higher in ET patients with vascular events than in ET patients without vascular events (40.18 ± 25.78% vs 27.54 ± 13.92% p = 0.026) (b).
Figure 2. The JAK2V617F mutation allele burden in ph-PMN patients was presented in (a). The JAK2V617F mutation allele burden between ET patients with vascular events and ET patients without vascular events were present in (b). The JAK2V617F mutation allele burden in the group age ≧65 were presented in (c). The JAK2V617F mutation allele burden in the group age <65 was presented in (d).
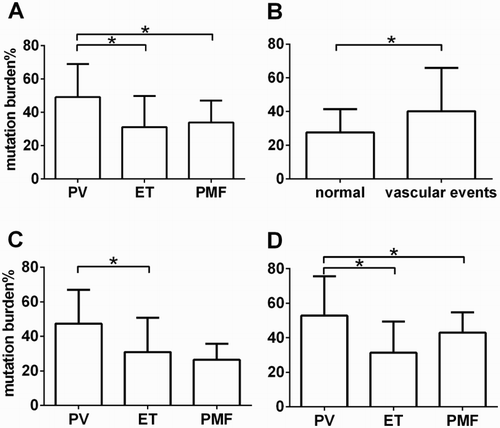
To further explore the relationship between burden and age, these patients with JAK2V617F mutation were classified into old people and young people (≧65 and <65) [Citation21]. The old people group (age ≧65) comprised PV (n = 8), ET (n = 22) and PMF(n = 5); the young people group (age <65) comprised PV (n = 12), ET (n = 31) and PMF (n = 6). Interestingly, significant differences in JAK2V617F mutation allele burden between PV and PMF patients disappeared in group age <65, while the significant difference between PV and ET patients still existed (c and d).
Distribution of MPNs in quantities according to the JAK2V617F mutation allele burden in this cohort
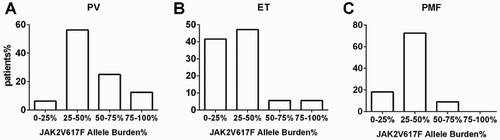
To further investigate the relationship between Ph-negative MPN patients and JAK2V617F allele burden, patients were grouped by quantities of JAK2V617F mutation allele burden and it was found that the cases were imbalanced distributed. ET and PMF patients were more often populated in the 0–25% or 25–50% groups, whereas PV patients more often classified into 25–50% and 50–75% groups ().
Relationship between JAK2V617F mutation and hematological parameters
Previous studies have illustrated the characteristics of Ph-negative MPNs with JAK2V1617F mutation. The association between JAK2V617F mutation and clinical and laboratory data in Ph-negative MPNs was also investigated in this study. Patients were divided into two groups (JAK2V6717F positive and JAK2V617F negative), and clinical and laboratory parameters were compared and analyzed between the two groups.
The results in the present study showed that in ET patients, WBC counts, RBC counts, HB, and HCT were higher in mutated patients than those in wild-type cases, while no difference of MCV, MCH, MCHC, PLT, and PDW was found. In addition, no significant difference was observed in PMF patients ().
Table 2. Hematological characteristics of MPN patients in this cohort.
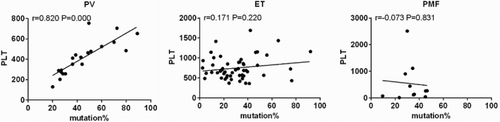
The above data indicated that the differences of hematological parameters in MPN were related to the JAK2V617F mutation or not. However, whether the allele burden of JAK2V617F mutation would affect the hematological parameters remains unknown. To investigate this point, Pearson test was used to analyze the relationship between JAK2V617F mutation allele burden and hematological parameters. In PV patients, the present results showed that PLT counts correlated with JAK2V617F mutation allele burden (R = 0.820, p < 0.001), while no significance was observed in other parameters. Additionally, no significant association was observed in ET and PMF patients ().
Relationship between JAK2V617F mutation and coagulation function
The clinical course of ET and PV was characterized by an increased frequency of thrombotic and hemorrhage complications, and coagulation function might be abnormal in these cases. The parameters of coagulation function involved prothrombin time-International normalized ratio (PT-INR), activated partial thromboplastin time (APTT), thrombin time (TT), plasma fibrinogen (FIB), and fibrin degradation products (FDP), which were used to measure patients’ hemostasis and coagulation function and to predict the risk of suffering from thrombotic and hemorrhage complications. Here the relationship between JAK2V617F mutation and coagulation function was analyzed.
Our data revealed a significant difference in the level of PT-INR between JAK2V617F mutation positive and negative groups. At the time of the diagnosis with MPN, PT-INR was higher in JAK2V617F mutated patients than that in ET and PMF patients with wild-type (1.12 ± 0.13 vs 1.05 ± 0.13, p = 0.029;1.00 ± 0.85 vs 1.19 ± 0.11, p = 0.002). With regard to APTT, TT, FIB, D-D, and FDP, no significant difference was observed ().
Table 3. Coagulation function of the patients in this cohort.
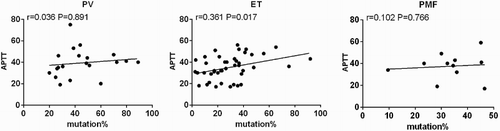
Then, we wonder whether the JAK2V617F mutation allele burden would influence coagulation function, and Pearson correlation test was performed. It was found that JAK2V617F mutation allele burden was correlated with APTT (R = 0.361 p = 0.017) in ET patients ().
Discussion
In this study, the relationship between JAK2V617F mutation and mutation allele burden with blood parameters and coagulation function in Chinese Ph-negative MPNs patients was investigated, and it was found that, JAK2V617F mutation ratio in Ph-negative MPNs was consistent with the previous studies [Citation4,Citation22,Citation23], JAK2V617F mutation allele burden was various in different subtypes of Ph-negative MPNs patients, and higher in ET patients with vascular events. Moreover, some correlations between JAK2V617F mutation and blood parameters were found. In addition, the results in the study demonstrated that the abnormal coagulation parameters, such as PT-INR and APTT, were related to JAK2V617F mutation and its allele burden.
Based on the evidences published to date, JAK2V617F mutation allele burden was various at different levels among PV, ET, and PMF [Citation24,Citation25], and its distribution among PV, ET, and PMF was characteristic [Citation25]. The present data based on Chinese population showed similar results to the previous studies, suggesting no difference existed in different ethnic populations. Vascular events showed no difference between JAK2V617F mutated patients and JAK2V617F wild-type patients in our cohort, which was different from the previous report [Citation15,Citation16]. These results might be related to our limited population. However, JAK2V617F mutation allele burden was higher in patients who suffered from vascular events, which was similar to the previous report [Citation19]. Furthermore, the young group and the old group showed different results with regard to JAK2V617F mutation allele between PV and PMF, but similar results to PV and ET, which might result from the fact that PV and ET were different expressions of a genotypic/phenotypic continuum [Citation9].
JAK2V617F mutation would have an influence on blood parameters in MPN, and in ET patients, PLT was lower in JAK2V617F mutated patients [Citation9], conversely, RBC counts and WBC counts were much higher in JAK2V617F positive patients based on Chinese population [Citation11], which was similar to our findings. Moreover, JAK2V617F mutation allele burden might be corrected with the change of the degrees of blood parameters, and in PV patients, a negative statistically significant correlation between the JAK2V617F mutation allele burden and PLT count was observed [Citation26] and no statistically significant correlation was found in the study performed by Edahiro et al. [Citation27], in addition, JAK2V617F allele burden was correlated with WBC counts and RBC counts in PV patients based on Chinese population [Citation11]. However, the present data indicated that PLT was positively correlated with JAK2V617F mutation allele burden in PV patients, suggesting that the relationship between blood parameters and JAK2V617F mutation allele burden remained inconsistent. Further studies are needed in a large size of samples.
Increased WBC counts, RBC counts, and PLT counts led to blood hyperviscosity and quantitative/qualitative abnormalities of blood cells [Citation28,Citation29], while, activated platelets and neutrophils could affect the hemostatic balance by reducing numerous inhibitors of coagulation, including protein S, protein C, and tissue factor pathway inhibitor, as well as coagulation factors [Citation14]. In fact, in the current study, on the one hand, some ET patients were present with vascular events, and on the other hand, it was also observed that PT-INR was higher in ET and PMF patients with JAK2V617F mutation, which suggested that, at least to some degree, lower levels of one or more of FII, FV, FVII, and FX might exist in MPN patients with JAK2V617F mutation. The mechanism might be related to the present findings that activated platelets and neutrophils might determine both the hypercoagulable state and the decrease in the coagulation protein levels [Citation30]. Additionally, it was found that APTT in ET patients were correlated with JAK2V617F mutation allele burden, whereas no correlation could be detected with PT-INR. This apparent discrepancy might result from the fact that these two parameters represent different coagulation pathways. However, when analyzed with PV and PMF patients, the significant relationship disappeared, which might result from the imbalanced distribution of JAK2V617F mutation allele burden, higher burden, and lower burden located in different diseases or the limited population of PV and PMF patients, and this phenomena might depict that JAK2V617F mutation allele burden was higher in ET patients with vascular events than in ET patients without vascular events.
However, several limitations in the current study need to be acknowledged. First, the study was uncontrolled and retrospective in nature. Second, our sample size was relatively small. Third, data of other molecular mutations, such as CALR and MPL, were not available.
In conclusion, the present results indicated that JAK2V617F mutation allele burden might alter the coagulation function and might at least in part account for the increased vascular events risk associated with these disorders, but further studies are required to validate the association and expand to other molecular mutations.
Acknowledgements
We thank all the medical and nursing staff in our hematology department for their valuable cooperation.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
SDX and LHH assisted the patient, performed data collection, analyzed the data, wrote the manuscript and approved the final manuscript as submitted. LFP, JRL, XR, JXX, and MC wrote the manuscript. YYD, MML, DDY, CZ, and HPW performed molecular assays. DJZ and ZMZ assisted the patient.
Additional information
Funding
References
- Barbui T, Finazzi G, Falanga A. Myeloproliferative neoplasms and thrombosis. Blood. 2013;122(13):2176–2184. doi: 10.1182/blood-2013-03-460154
- Campbell PJ, Green AR. The myeloproliferative disorders. N Engl J Med. 2006;355(23):2452–2466. doi: 10.1056/NEJMra063728
- Marchioli R, Finazzi G, Landolfi R, et al. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J Clin Oncol. 2005;23(10):2224–2232. doi: 10.1200/JCO.2005.07.062
- Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365(9464):1054–1061. doi: 10.1016/S0140-6736(05)74230-6
- Klampfl T, Gisslinger H, Harutyunyan AS, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013;369(25):2379–2390. doi: 10.1056/NEJMoa1311347
- Rumi E, Pietra D, Guglielmelli P, et al. Acquired copy-neutral loss of heterozygosity of chromosome 1p as a molecular event associated with marrow fibrosis in MPL-mutated myeloproliferative neoplasms. Blood. 2013;121(21):4388–4395. doi: 10.1182/blood-2013-02-486050
- Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352(17):1779–1790. doi: 10.1056/NEJMoa051113
- James C, Ugo V, Le Couedic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434(7037):1144–1148. doi: 10.1038/nature03546
- Campbell PJ, Scott LM, Buck G, et al. Definition of subtypes of essential thrombocythaemia and relation to polycythaemia vera based on JAK2 V617F mutation status: a prospective study. Lancet. 2005;366(9501):1945–1953. doi: 10.1016/S0140-6736(05)67785-9
- Antonioli E, Guglielmelli P, Pancrazzi A, et al. Clinical implications of the JAK2 V617F mutation in essential thrombocythemia. Leukemia. 2005;19(10):1847–1849. doi: 10.1038/sj.leu.2403902
- Zhou J, Ye Y, Zeng S, et al. Impact of JAK2 V617F mutation on hemogram variation in patients with non-reactive elevated platelet counts. PLoS One. 2013;8(2):e57856. doi: 10.1371/journal.pone.0057856
- Arellano-Rodrigo E, Alvarez-Larran A, Reverter JC, et al. Automated assessment of the neutrophil and platelet activation status in patients with essential thrombocythemia. Platelets. 2012;23(5):336–343. doi: 10.3109/09537104.2011.630112
- Arellano-Rodrigo E, Alvarez-Larran A, Reverter JC, et al. Platelet turnover, coagulation factors, and soluble markers of platelet and endothelial activation in essential thrombocythemia: relationship with thrombosis occurrence and JAK2 V617F allele burden. Am J Hematol. 2009;84(2):102–108. doi: 10.1002/ajh.21338
- Marchetti M, Castoldi E, Spronk HM, et al. Thrombin generation and activated protein C resistance in patients with essential thrombocythemia and polycythemia vera. Blood. 2008;112(10):4061–4068. doi: 10.1182/blood-2008-06-164087
- Hexner EO. JAK2 V617F: implications for thrombosis in myeloproliferative diseases. Curr Opin Hematol. 2007;14(5):450–454. doi: 10.1097/MOH.0b013e3282861d1b
- Vannucchi AM, Antonioli E, Guglielmelli P, et al. Prospective identification of high-risk polycythemia vera patients based on JAK2(V617F) allele burden. Leukemia. 2007;21(9):1952–1959. doi: 10.1038/sj.leu.2404854
- Barbui T, Finazzi G, Carobbio A, et al. Development and validation of an international prognostic score of thrombosis in world health organization-essential thrombocythemia (IPSET-thrombosis). Blood. 2012;120(26):5128–5133; quiz 5252. doi: 10.1182/blood-2012-07-444067
- Barbui T, Falanga A. Molecular biomarkers of thrombosis in myeloproliferative neoplasms. Thromb Res. 2016;140(Suppl. 1):S71–S75. doi: 10.1016/S0049-3848(16)30102-5
- Borowczyk M, Wojtaszewska M, Lewandowski K, et al. The JAK2 V617F mutational status and allele burden may be related with the risk of venous thromboembolic events in patients with Philadelphia-negative myeloproliferative neoplasms. Thromb Res. 2015;135(2):272–280. doi: 10.1016/j.thromres.2014.11.006
- Arber DA, Orazi A, Hasserjian R. et al. The 2016 revision to the world health organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. doi: 10.1182/blood-2016-03-643544
- Latagliata R, Stagno F, Annunziata M, et al. Frontline dasatinib treatment in a “real-life” cohort of patients older than 65 years with chronic Myeloid Leukemia. Neoplasia. 2016;18(9):536–540. doi: 10.1016/j.neo.2016.07.005
- Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7(4):387–397. doi: 10.1016/j.ccr.2005.03.023
- Zhang SP, Li H, Lai RS. Detection of JAK2 V617F mutation increases the diagnosis of myeloproliferative neoplasms. Oncol Lett. 2015;9(2):735–738.
- Passamonti F, Rumi E, Pietra D, et al. Relation between JAK2 (V617F) mutation status, granulocyte activation, and constitutive mobilization of CD34+ cells into peripheral blood in myeloproliferative disorders. Blood. 2006;107(9):3676–3682. doi: 10.1182/blood-2005-09-3826
- Vannucchi AM, Antonioli E, Guglielmelli P, et al. Clinical correlates of JAK2V617F presence or allele burden in myeloproliferative neoplasms: a critical reappraisal. Leukemia. 2008;22(7):1299–1307. doi: 10.1038/leu.2008.113
- Fantasia F, Di Capua EN, Cenfra N, et al. A highly specific q-RT-PCR assay to address the relevance of the JAK2WT and JAK2V617F expression levels and control genes in Ph-negative myeloproliferative neoplasms. Ann Hematol. 2014;93(4):609–616. doi: 10.1007/s00277-013-1920-0
- Edahiro Y, Morishita S, Takahashi K, et al. JAK2V617F mutation status and allele burden in classical Ph-negative myeloproliferative neoplasms in Japan. Int J Hematol. 2014;99(5):625–634. doi: 10.1007/s12185-014-1567-1
- Elliott MA, Tefferi A. Thrombosis and haemorrhage in polycythaemia vera and essential thrombocythaemia. Br J Haematol. 2005;128(3):275–290. doi: 10.1111/j.1365-2141.2004.05277.x
- Carobbio A, Finazzi G, Guerini V, et al. Leukocytosis is a risk factor for thrombosis in essential thrombocythemia: interaction with treatment, standard risk factors, and Jak2 mutation status. Blood. 2007;109(6):2310–2313. doi: 10.1182/blood-2006-09-046342
- Falanga A, Marchetti M, Evangelista V, et al. Polymorphonuclear leukocyte activation and hemostasis in patients with essential thrombocythemia and polycythemia vera. Blood. 2000;96(13):4261–4266.