ABSTRACT
Objectives and methods: Splenic marginal zone lymphoma (SMZL) is a rare non-Hodgkin lymphoma. We sought to identify prognostic factors and define outcomes in a cohort of 64 patients with SMZL who were treated at two large academic medical centers in North America in the rituximab era.
Results: Over a median follow-up of 37.8 (range 6–167.1) months, Kaplan–Meier estimate of median OS was 156.3 months and median PFS was 52.9 months. On univariate analysis, baseline hemoglobin <12 g/dl was associated with inferior OS (p = 0.045). High-risk FLIPI score was associated with inferior PFS when compared with intermediate/low risk (p = 0.05) and marginally significant with regard to OS (p = 0.056). Splenectomy was not predictive of OS or PFS (p = 0.563 and 0.937, respectively). Transformation to diffuse large B-cell lymphoma occurred in four (6.3%) patients during the observation period. OS was comparable to contemporaneous cohorts of patients with extranodal and nodal marginal lymphomas and FLIPI score was highly predictive for inferior PFS and OS when all three cohorts were analyzed together.
Conclusion: Outcomes of SMZL, in our series, were excellent, with a median OS of >13 years. Low hemoglobin and high-risk FLIPI were associated with inferior outcomes.
Introduction
Marginal zone lymphoma (MZL) is a relatively rare non-Hodgkin B-cell lymphoma that is thought to arise from post-germinal center B-cells. It is further classified into three subtypes: splenic, extranodal, and nodal MZL. Splenic marginal zone lymphoma (SMZL) accounts for <1% of all non-Hodgkin lymphomas [Citation1]. Patients with splenic MZL typically present with splenomegaly, autoimmune cytopenias, and variable presence of villous lymphocytes in the peripheral blood in the absence of peripheral lymphadenopathy. Malignant cells stain was positive on immunohistochemistry for CD20 and negative for CD5 and CD10.
MZLs have been widely shown to arise in the context of chronic inflammatory states. In the case of SMZL, studies have suggested an association between hepatics C virus (HCV) and Kaposi sarcoma/human herpes virus-8 infection (HHV-8) [Citation2,Citation3]. Additionally, spontaneous regression of SMZL was reported after the treatment of HCV with interferon [Citation4]. In most cases of SMZL, no specific etiologic factor is identified.
Treatment of SMZL typically involves a combination of immunotherapy, cytotoxic chemotherapy, and/or surgery, depending on the clinical presentation. Rituximab has gained popularity as the first-line agent given high response rate and excellent tolerance [Citation5,Citation6]. Splenomegaly may be indicated in patients with symptomatic splenomegaly and localized disease [Citation7], and it was, historically, the preferred first-line therapy in the pre-rituximab era. Management decisions (including watchful waiting) are informed by the presence or absence of a combination of symptoms, location and extent of extra-splenic disease, patient co-morbidities, the need for diagnostic splenic tissue (including when transformation in the spleen is clinically suspected) and the presence of co-existing HCV. Because of the absence of phase 3 data, there can be no first-line standard or consensus other than to say most patients can initially be managed by either watch and wait, single-agent rituximab, or splenectomy.
Previous SMZL case series reported included significant numbers of patients treated prior to the widespread use of rituximab and consequently may not reflect current medical practice. We sought to identify prognostic factors and define outcomes of patients with SMZL who were treated at two large academic medical centers in North America in the rituximab era and to compare outcomes to contemporaneous cohort of patients with extranodal/nodal MZL treated at the same institutions.
Methods
Patient selection
We identified 485 patients with MZL who were treated at University Hospitals Seidman Cancer Center and the Taussig Cancer Institute of the Cleveland Clinic, which together comprise the Case Comprehensive Cancer Center. Diagnosis of MZL was confirmed by expert hematopathologists based on characteristic morphologic appearance, positive immunohistochemical staining for CD20, and negative staining for CD5 and CD10. We excluded cases with detectable serum IgM kappa given overlapping features with lymphoplasmacytic lymphoma, and patients with no follow-up, such as those seen only once at either institution for initial consultation. We identified 64 patients with SMZL based on the site of involvement and sparing of peripheral lymph nodes. Patients with mild lymphadenopathy (<2 cm), but whose disease predominantly involved the spleen, were classified as splenic MZL. In addition, there were 56 patients that we identified as having nodal MZL, 211 patients as extranodal MZL, and 22 patients with typical MZL features, but who were unable to further classified. These patients have been reported separately [Citation8].
Data collection
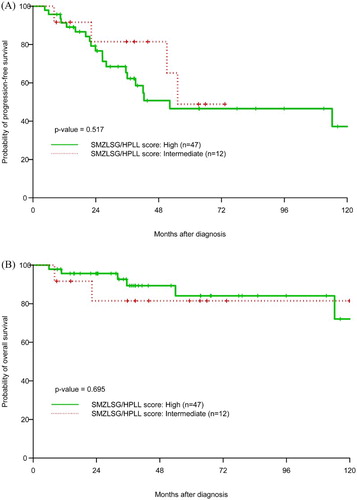
Data points collected from the review of the electronic medical record included patient demographics; site of SMZL involvement; age at diagnosis; number of involved lymph nodes at diagnosis; stage; baseline hemoglobin (Hb), platelets, and serum lactate dehydrogenase (LDH); bone marrow involvement; H. pylori status; hepatitis C infection status; presence of monoclonal protein in the serum or urine; transformation to diffuse large B-cell lymphoma (DLBCL); treatment; and response to therapy. Staging was performed by the Ann Arbor staging system [Citation9,Citation10] and Follicular Lymphoma International Prognostic Index (FLIPI) was calculated using baseline values collected at the time of diagnosis [Citation11]. SMZLSG/HPLL score was calculated at the time of diagnosis as follows: PI = 0.02 × baseline hb (g/l) + 0.006 * baseline plt (109/l) – LDH (1 elevated, 0 normal) – extrahilar lymphadenopathy (1 present, 0 absent) with score defined as ‘low risk’ with PI ≥ 2.6; ‘intermediate risk’ with PI ≥ 0.9 and <2.6; and ‘high risk’ with PI < 0.9 [Citation12].
Statistical analysis
Overall survival (OS) was measured from the date of diagnosis to the date of death and censored at the date of last follow-up for survivors. The progression free survival (PFS) was measured from the date of diagnosis to the date of disease progression or the date of death, whichever occurred earlier, and censored at the date of last follow-up for those alive without progression. Survivor distribution was estimated using Kaplan–Meier methods and difference of OS, PFS between/among groups was examined by log-rank [Citation13]. The effect of continuous variables including FLIPI on survival (OS, PFS) was estimated using the Cox model [Citation14]. Factors (i.e. age, stage, baseline Hb, serum LDH, number of nodal sites) as part of FLIPI were further evaluated using multivariable Cox regression. All tests are two-sided and p-value ≤ 0.05 was considered statistically significant.
Results
Patient demographics and clinical characteristics
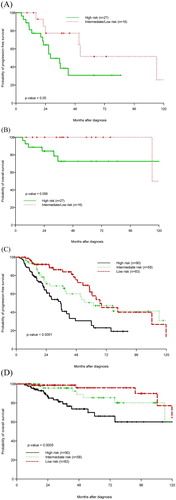
Selected patient demographics and clinical characteristics are summarized in . Sixty-four patients with SMZL were identified. The median age at diagnosis was 67 (range 41–88) years and 43 (67%) patients were ≥60 years of age at the time of diagnosis. On initial presentation, eight (12.5%) patients were classified as early stage (enlarged spleen and clonal B-cell involvement of peripheral blood but negative morphologic involvement of bone marrow on biopsy) and 56 (87.5%) were classified as advanced stage (with histological involvement of bone marrow on biopsy). Serum LDH was above the upper limit of normal in 23 (35.9%) patients, Hb was <12 g/dl in 37 (57.8%) patients, B symptoms were present in 15 (23.4%) patients, positive HCV serology was documented in one (1.6%) patient, and bone marrow involvement was present in 56 (87.5%) patients. There were eight (12.5%) patients in whom >4 lymph nodes were involved but who had predominantly splenic disease characterized by massive splenomegaly (with largest dimension >20 cm). FLIPI classified two (2%) patients as low risk, 14 (21.9%) patients as intermediate risk, and 27 (42.2%) patients and high risk.
Table 1. Patient demographics and clinical characteristics.
Treatment
Initial treatment was rituximab monotherapy in 34 (53.1%) patients; 12 (18.8%) underwent splenectomy, 11 (17.2%) received rituximab plus cytotoxic chemotherapy, two (3.1%) underwent radiation therapy to the spleen, one (1.6%) received cytotoxic chemotherapy, one (1.6%) patient underwent antiviral therapy for hepatitis C infection, and three (4.7%) patients were observed without treatment. A total of 19 (30%) patients underwent splenectomy at any point throughout their treatment course. In the 34 patients who received single-agent rituximab as initial treatment, 11 (32%) had a complete remission (CR), 15 (44%) had a partial remission, and five (15%) had stable disease with overall response rate (ORR) of 76%. In the 11 patients who received rituximab and cytotoxic chemotherapy, four (35%) had CR, five (45%) had PR with ORR of 90%. Fifteen patients (23%) went on to receive 2 years of rituximab maintenance after upfront treatment with rituximab or rituximab and cytotoxic chemotherapy.
Of the 11 patients who underwent splenectomy as the initial treatment, six received no further treatment and five went on to receive cytotoxic chemotherapy +/− rituximab. One patient was treated for HCV infection with pegylated interferon and ribavirin, achieved a partial response, and underwent no further treatment. Two (3.1%) patients underwent allogenic hematopoietic cell transplant, one of whom underwent transplant from a matched unrelated donor following transformation to DLBCL and was alive at the time of last follow-up, 2 months after transplantation. The other patient underwent transplant from a matched sibling donor and had a complicated post-transplant course, including acute graft versus host disease (GVHD), involving the upper GI tract, suspected seizure 8 days post-transplant, cardiac arrest 9 days post-transplant, and died 2 months following transplantation.
The majority of patients (n = 50, 78.1%) initiated treatment at the time of diagnosis and the median time from diagnosis to first treatment was 28 days. Many patients underwent multiple treatment regimens, with 24 (37.5%) patients undergoing two or more lines of therapy and 11 (7.2%) undergoing three or more lines of therapy.
Outcomes and prognostic factors
Over a median follow-up of 37.8 months (range 6–167.1), Kaplan–Meier estimate of median OS was 156.3 months and median PFS was 52.9 months (). At 38 months (median follow-up), the PFS and OS were 66.1 and 88.8%, respectively. On univariate analysis, baseline Hb <12 g/dl was associated with inferior OS (p = 0.045). This association was also demonstrated on univariate Cox analysis (HR = 0.73, 95% CI 0.56–0.95, p = 0.02). High-risk FLIPI score was associated with inferior PFS when compared to intermediate/low risk (p = 0.05). As far as OS, the predictive value for high-risk FLIPI score for worse outcomes did not reach statistical significance (p = 0.056, (a,b)). We were able to calculate SMZLSG/HPLL score in 59 patients: 47 had high scores and 12 had intermediate scores. The SMZLSG/HPLL score, however, was not predictive of PFS and OS in our SMZL cohort ((a,b)). Splenectomy was not predictive of OS or PFS (p = 0.563 and 0.937, respectively). Transformation to DLBCL occurred in four (6.3%) patients during the observation period. Of the four patients whose disease transformed, three presented with advanced stage disease. For the 44 patients who were treated with rituximab +/− chemotherapy, 15 (34%) went on to receive 2 years of maintenance with rituximab and 29 (66%) were observed without maintenance. The median OS/PFS was not reached (OS > 50%)/42.4 months vs. 156.3/42.1 months in both groups, respectively. The difference in outcomes in both groups was not statistically significant (p = 0.215).
Figure 1. Kaplan–Meier estimation of (A) OS and (B) PFS with 95% CI for SMZL and PFS (C) and OS (D) in comparison to contemporaneous cohorts of extranodal and nodal MZL.
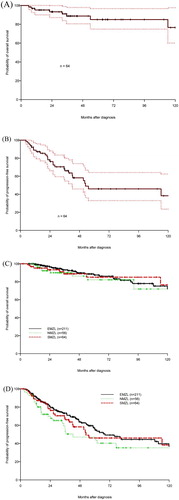
Figure 2. Kaplan–Meier estimation of PFS and OS by FLIPI score for splenic MZL cohort (A, B) and entire MZL cohort (C, D).
The outcomes of this cohort of 64 patients identified with SMZL were compared to two other contemporaneous cohorts with extranodal MZL (211 patients) and nodal MZL (56 patients). The extranodal and nodal MZL cohorts were reported separately [Citation15,Citation16]. There was no significant difference in OS or PFS between the three subtypes ((c,d)). When the three cohorts were analyzed together, the FLIPI scores was highly predictive of PFS and OS ((c,d)).
Discussion
Several previous reports have described the excellent prognosis of patients with SMZL [Citation17–19]. Our series reports the excellent prognosis of such patients with median OS in excess of 10 years. Although most patients had advanced disease at presentation with onset in the 7th decade of their lives, OS in our SMZL cohort was comparable to a contemporaneous cohort of patients with extranodal and splenic MZL; all cohorts had a median OS of excess of 10 years.
Previous studies have identified a link between HCV infection and MZL, and subsequent resolution of the lymphoma with treatment [Citation2,Citation4]. Hermine et al. found regression of seven of nine patients with HCV treatment. In our series, one HCV patient was treated with PEG interferon and ribavirin which resulted in decreased marrow involvement by bone marrow biopsy and improvement in blood counts following HCV treatment. Transformation occurred in a minority (n = 4, 6.3%) of patients in this series, similar to what was reported in prior case series [Citation18–20]. For only 4.7% of patients in our series, the initial decision was for observation without treatment which is in contrast to recent data from Italy showing that 30% of patients with SMZL in their 100 patient case series remained on observation for 10 years [Citation21]. That discrepancy may be readily explained by the fact that our data are likely skewed for more aggressive disease. Such skew is typical for case series reported from tertiary academic medical centers in the United States. It is highly likely that many patients with SMZL who had asymptomatic disease, which was accidentally discovered on imaging or laboratory studies, were managed in local community oncologist office and never traveled to our centers for consultation.
It is hard to make definitive recommendation about initial therapy in patients with SMZL who need treatment in the absence of randomized trials. Splenectomy has been the more popular initial treatment strategy in Europe vs. rituximab (+/− chemotherapy) in the United States. Comparing reports from different centers and study groups is difficult because of the heterogeneity of disease risk as well as significant overlap with nodal MZL cases. We confirm the results reported by other groups that initial treatment with single-agent rituximab is well tolerated and is associated with excellent outcomes. The 10-year OS in our series was 76%; which is almost identical to OS observed in 58 patients treated with single-agent rituximab from Greece [Citation22]. We compare this to only 61% OS observed in 107 patients with SMZL case series from British Columbia [Citation18] and 67% in 100 patients case series from France [Citation7]. Historically, splenectomy was the standard treatment for SMZL; however, it is associated with significant surgical and infectious morbidity. Our results suggest that single-agent rituximab is at least comparable to splenectomy for the initial treatment of SMZL and may be the preferred approach where the morbidity of splenectomy can be avoided.
The FLIPI is widely used in predicting prognosis in multiple lymphoma subtypes. In addition, two SMZL-specific prognostic scoring systems have been developed; the Intergruppo Italiano Linfomi (IIL) system, which uses low Hb, elevated serum LDH, and low serum albumin; and the HPLL system developed by the SMZL Study group (SMZLSG), which uses low Hb, low platelet count, elevated serum LDH, and extrahilar lymphadenopathy as predictors of prognosis [Citation12,Citation23]. In our series, we found that high-risk FLIPI score was associated with inferior survival when compared to intermediate/low-risk scores. In addition, we found Hb <12 g/dl was associated with inferior survival. The SMZLSG/HPLL score was not predictive for outcomes in our series. That discrepancy may be readily explained by the fact that our data are likely skewed for more aggressive disease. Such skew is typical for case series reported from tertiary academic medical centers in the United States. In our series, there were no patients with ‘low risk’ disease (according to SMZLSG/HPLL score) compared to 51–57% in the initial SMZLSG report [Citation12]. We, hence, conclude that the FLIPI score is more predictive for outcomes in cohorts with predominantly more aggressive cases, which is more typical for cases seen in tertiary academic medical centers in the United States compared to their counterparts in Europe.
When we combined SMZL cases with extranodal and nodal cases as one cohort ((c,d)), the FLIPI score remained highly predictive of both PFS and OS. This information is useful, especially in cases where there is significant overlap between MZL entities. Our contemporaneous combined MZL cohort analysis, in contrast to prior reports describing splenic and other MZL cohorts separately, illustrates that in the rituximab era, FLIPI score carries prognostic significance regardless of initial sites of presentation (nodal/extranodal vs. splenic).
Conclusions
In summary, outcomes of SMZL are excellent, with a median OS of 13 years. In our series, low Hb and high-risk FLIPI scores were associated with inferior outcomes. Rituximab as the initial treatment of SMZL was associated with excellent outcomes in our series.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Adam G. Starr was a medical student at the time of manuscript preparation and now a radiology resident at the University of Colorado Hospital.
Paolo F. Caimi is a clinical hematologist, the director of lymphoma program, and an assistant professor of medicine at Case Western Reserve University and UH Case Medical Center.
PingFu Fu is an associate professor at the Department of Epidemiology and Biostatistics at Case Western Reserve University and UH Case Medical Center.
Mira R. Massoud was a research volunteer at UH Case Medical Center and now an internal medicine resident at St Elizabeth’s Medical Center in Brighton, Massachusetts.
Howard Meyerson is an associate professor of pathology and the director of hematopathology and flow cytometry at Case Western Reserve University and UH Case Medical Center.
Eric D. Hsi is the section head of hematopathology at Cleveland Clinic Foundation and a professor of pathology at Case Western Reserve University Lerner College of Medicine.
David B. Mansur is the division chief, Radiation Oncology, UH Rainbow Babies and Children’s Hospital and an associate professor of Radiation Oncology and Pediatrics at Case Western Reserve University and UH Case Medical Center.
Sheen Cherian is an associate staff in the Department of Radiation Oncology at Cleveland Clinic and an associate professor of Radiation Oncology at Case Western Reserve University Lerner College of Medicine.
Brenda W. Cooper is a clinical hematologist, the clinical director of stem cell transplant program, and a professor of medicine at Case Western Reserve University and UH Case Medical Center.
Marcos J.G. De Lima is a clinical hematologist, the section head of hematological malignancies and the director of stem cell transplant program, and a professor of medicine at Case Western Reserve University and UH Case Medical Center.
Hillard M. Lazarus is a clinical hematologist, the director of Novel Cell Therapy, and a tenured professor of medicine at Case Western Reserve University and UH Case Medical Center.
Stanton L. Gerson is a physician/scientist, the director of Case Comprehensive Cancer Center, and a professor of medicine (distinguished university professor) at Case Western Reserve University and UH Case Medical Center. He is also the founding director of the National Center for Regenerative Medicine.
Deepa Jagadeesh is a clinical hematologist at Cleveland Clinic and an assistant professor of medicine at Case Western Reserve University Lerner College of Medicine.
Mitchell R. Smith is a clinical hematologist and the director of the Lymphoid Malignancy Program at Cleveland Clinic Foundation and a professor of medicine at Case Western Reserve University Lerner College of Medicine.
Robert M. Dean is a clinical hematologist at Cleveland Clinic and an associate professor of medicine at Case Western Reserve University Lerner College of Medicine.
Brad L. Pohlman is a clinical hematologist and the vice chair of Operations Taussig Cancer Institute at Cleveland Clinic Foundation and a professor of medicine at Case Western Reserve University Lerner College of Medicine.
Brian T. Hill is a clinical hematologist at Cleveland Clinic and an associate professor of medicine at Case Western Reserve University Lerner College of Medicine.
Basem M. William is a clinical hematologist and was an assistant professor of medicine at Case Western Reserve University and UH Case Medical Center at the time of manuscript preparation. Now Dr Basem M. William is an assistant professor of internal medicine and director of cutaneous lymphoma multidisciplinary program at The Ohio State University Comprehensive Cancer Center.
ORCID
Sheen Cherian http://orcid.org/0000-0002-7210-0997
Mitchell R. Smith http://orcid.org/0000-0003-1428-8765
References
- Liu L, Wang H, Chen Y, et al. Splenic marginal zone lymphoma: a population-based study on the 2001–2008 incidence and survival in the United States. Leuk Lymphoma. 2013;54(7):1380–1386. doi: 10.3109/10428194.2012.743655
- Zuckerman E, Zuckerman T, Levine AM, et al. Hepatitis C virus infection in patients with B-cell non-Hodgkin lymphoma. Ann Intern Med. 1997;127(6):423–428. doi: 10.7326/0003-4819-127-6-199709150-00002
- Benavente Y, Mbisa G, Labo N, et al. Antibodies against lytic and latent Kaposi's sarcoma-associated herpes virus antigens and lymphoma in the European EpiLymph case-control study. Br J Cancer. 2011;105(11):1768–1771. doi: 10.1038/bjc.2011.392
- Hermine O, Lefrere F, Bronowicki JP, et al. Regression of splenic lymphoma with villous lymphocytes after treatment of hepatitis C virus infection. N Engl J Med. 2002;347(2):89–94. doi: 10.1056/NEJMoa013376
- Tsimberidou AM, Catovsky D, Schlette E, et al. Outcomes in patients with splenic marginal zone lymphoma and marginal zone lymphoma treated with rituximab with or without chemotherapy or chemotherapy alone. Cancer. 2006;107(1):125–135. doi: 10.1002/cncr.21931
- Else M, Marin-Niebla A, de la Cruz F, et al. Rituximab, used alone or in combination, is superior to other treatment modalities in splenic marginal zone lymphoma. Br J Haematol. 2012;159(3):322–328. doi: 10.1111/bjh.12036
- Lenglet J, Traulle C, Mounier N, et al. Long-term follow-up analysis of 100 patients with splenic marginal zone lymphoma treated with splenectomy as first-line treatment. Leuk Lymphoma. 2014;55(8):1854–1860. doi: 10.3109/10428194.2013.861067
- Starr AG, Caimi PF, Fu P, et al. Dual institution experience of extranodal marginal zone lymphoma reveals excellent long-term outcomes. Br J Haematol. 2016;173(3):404–412. doi: 10.1111/bjh.13975
- Lister TA, Crowther D, Sutcliffe SB, et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin's disease: Cotswolds meeting. J Clin Oncol. 1989;7(11):1630–1636.
- Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059–3068. doi: 10.1200/JCO.2013.54.8800
- Solal-Celigny P, Roy P, Colombat P, et al. Follicular lymphoma international prognostic index. Blood. 2004;104(5):1258–1265. doi: 10.1182/blood-2003-12-4434
- Montalban C, Abraira V, Arcaini L, et al. Risk stratification for Splenic Marginal Zone Lymphoma based on haemoglobin concentration, platelet count, high lactate dehydrogenase level and extrahilar lymphadenopathy: development and validation on 593 cases. Br J Haematol. 2012;159(2):164–171. doi: 10.1111/bjh.12011
- Kaplan EL, Meier P. Nonparametric-Estimation from Incomplete Observations. J Am Stat Assoc. 1958;53(282):457–481. doi: 10.1080/01621459.1958.10501452
- Cox DR. Regression models and life-tables. J R Stat Soc B. 1972;34(2):187–220.
- Starr AG, Caimi PF, Fu P, et al. Dual institution experience of nodal marginal zone lymphoma reveals excellent long-term outcomes in the rituximab era. Br J Haematol. 2016;175(2):275–280. doi: 10.1111/bjh.14228
- Starr AG, Caimi PF, Fu P, et al. Dual institution experience of extranodal marginal zone lymphoma reveals excellent long-term outcomes. Br J Haematol. 2016;173(3):404–412. doi: 10.1111/bjh.13975
- Thieblemont C, Felman P, Berger F, et al. Treatment of splenic marginal zone B-cell lymphoma: an analysis of 81 patients. Clin Lymphoma. 2002;3(1):41–47. doi: 10.3816/CLM.2002.n.010
- Xing KH, Kahlon A, Skinnider BF, et al. Outcomes in splenic marginal zone lymphoma: analysis of 107 patients treated in British Columbia. Br J Haematol. 2015;169(4):520–527. doi: 10.1111/bjh.13320
- Parry-Jones N, Matutes E, Gruszka-Westwood AM, et al. Prognostic features of splenic lymphoma with villous lymphocytes: a report on 129 patients. Br J Haematol. 2003;120(5):759–764. doi: 10.1046/j.1365-2141.2003.04165.x
- Iannitto E, Ambrosetti A, Ammatuna E, et al. Splenic marginal zone lymphoma with or without villous lymphocytes. Hematologic findings and outcomes in a series of 57 patients. Cancer. 2004;101(9):2050–2057. doi: 10.1002/cncr.20596
- Perrone S, D'Elia GM, Annechini G, et al. Splenic marginal zone lymphoma: Prognostic factors, role of watch and wait policy, and other therapeutic approaches in the rituximab era. Leukemia Res. 2016;44:53–60. doi: 10.1016/j.leukres.2016.03.005
- Kalpadakis C, Pangalis GA, Angelopoulou MK, et al. Treatment of splenic marginal zone lymphoma with rituximab monotherapy: progress report and comparison with splenectomy. Oncologist. 2013;18(2):190–197. doi: 10.1634/theoncologist.2012-0251
- Arcaini L, Lazzarino M, Colombo N, et al. Splenic marginal zone lymphoma: a prognostic model for clinical use. Blood. 2006;107(12):4643–4649. doi: 10.1182/blood-2005-11-4659