ABSTRACT
Objective: Until recently, imatinib was the standard first-line treatment in chronic myeloid leukemia (CML). The inclusion of nilotinib and dasatinib as first-line options in CML raised a debate on treatment selection. The aim of our study was to analyze predictive parameters for imatinib response as the first-line treatment of CML patients.
Methods: The study included 168 consecutive patients with chronic phase Philadelphia-positive CML who were diagnosed and treated with Imatinib 400 mg once daily at a single university hospital. Numerous parameters were analyzed in terms of imatinib response including comorbidities as well as occurrence of second malignancies.
Results: After the median follow-up of 87 months in 61 patients (36.3%), the imatinib failure was verified. Cox regression analysis identified hepatomegaly (p = 0.001), leukocytosis ≥ 100 × 109/l (p = 0.001), blood blasts ≥ 1% (p = 0.002), and the presence of additional cytogenetic aberrations (p = 0.002) as predictors of Imatinib failure. Based on these findings, a new prognostic model was developed according to which imatinib failure had 17% (8/47) of patients in low risk, 34.9% (30/86) of patients in intermediate risk, and 76.7% (23/30) of patients in high-risk group (HR = 3.973, 95% CI for HR 2.237–7.053, p < 0.001).
Conclusion: The new score allows better selection of patients who are suitable for treatment with imatinib and may guideline the clinical decision for front-line treatment of CML.
Introduction
Chronic myeloid leukemia (CML) is a clonal myeloproliferative disorder distinctly characterized by the presence of the Philadelphia chromosome, which results from a reciprocal translocation between chromosomes 9 and 22 [t(9;22)], leading to the development of constitutively active tyrosine kinase enzyme that promotes uncontrolled cell proliferation and reduced apoptosis [Citation1].
Development of tyrosine kinase inhibitor (TKI) has significantly changed natural course of disease and increased 10-year overall survival from 10–20% to 80–90% [Citation2]. The first-approved TKI for chronic phase (CP) of CML was imatinib [Citation3]. It was the only treatment option in the front-line setting for almost a decade. In 2013, according to the European LeukemiaNet (ELN) recommendations second-generation TKI, nilotinib and dasatinib were approved as alternative front-line options [Citation4]. Second-generation TKI has been demonstrated to induce deeper and faster responses compared to imatinib. However, none of three TKIs has been shown to have a clear survival advantage, so they all represent reasonable options [Citation5]. Hematologists are facing with the challenge of making decision which TKI to choose upfront for achieving the best possible response. Currently accepted prognostic scores in CML are Sokal, Hasford, and EUTOS scores [Citation6–8]. These scores are calculated based on the combination of clinical and laboratory characteristics at the time of CML diagnosis. Sokal score was created in 1984 for chemotherapy-treated [Citation6], Hasford score in 1998 for interferon-alpha treated [Citation7], and EUTOS score in 2011 for imatinib-treated CML patients [Citation8]. Thereby, Sokal and Hasford scores were established using overall survival as endpoint, whereas the EUTOS score used complete cytogenetic response (CCyR) at 18 months. However, prediction of EUTOS score in imatinib-treated CML patients has not been confirmed by three independent studies [Citation9–11]. There is little evidence suggesting that current risk scoring systems may be helpful in tailoring TKI therapy [Citation12].
According to the current ELN recommendations, the goal for optimal response represents achievement of earlier CCyR at 6 months of TKI treatment, which is associated with best long-term outcome and survival [Citation4]. In IRIS study, proportion of patients developed resistance or progression of disease, dominantly in the first 3 years of treatment and, by the end of 8 years, only 55% of the patients remained on imatinib [Citation13]. The early identification of patients’ expecting poor outcome is crucial for offering an alternative TKI regimen. Therefore, the aim of our study was to define prognostic factors for a prediction of imatinib response.
Patients and methods
The study was conducted on 168 consecutive patients with CP of Ph+CML who were diagnosed and treated at the single university hospital (the Clinic for Hematology, Clinical Center of Serbia) from December 2000 to January 2015. All patients were treated with imatinib 400 mg orally once daily. Waiting for the approval of imatinib, the majority of patients shortly received cytoreductive treatment with hydroxyurea. The patients’ response details were recorded during the treatment period as per the latest ELN recommendations [Citation4]. Study was complied with the Declaration of Helsinki, approved by the local human investigations committee, and performed in accordance with the legal requirements of the country. Informed consent was obtained from all individual participants included in the study.
Data were extracted, double-checked, and evaluated from the medical records. Following data were analyzed: demographic characteristics (age, gender); liver and spleen size below the costal margins (in cm); blood counts; eosinophils, basophiles and blasts in peripheral blood; lactate dehydrogenase in peripheral blood; a conventional bone marrow cytogenetic (chromosome banding) analysis at least on 20 metaphases; therapy, duration of therapy, cytogenetic responses, survival, and outcome. Data were analyzed in terms of treatment response to imatinib. Additionally, we analyzed the influence of comorbidities and the occurrence of second malignancies on the response to imatinib. These variables were available in 97–100% of the analyzed patients. In all patients, three prognostic scores (Sokal, Hasford, and EUTOS) were calculated at the time of diagnosis [Citation6–8].
Comorbidities were assessed by a comprehensive review of the written medical records by the date when the diagnosis was made. A single investigator assigned all comorbidity scores. The three following comorbidity indices were analyzed in 165 patients: (1) Adult Comorbity Evaluation-27 (ACE-27) [Citation14]; (2) The Hematopoietic Cell Transplantation Comorbidity Index (HCT-CI) [Citation15]; and (3) The Cumulative Illness Rating Scale (CIRS) [Citation16] (Supplemental material).
Treatment failure was defined according to the latest ELN recommendations (4): no complete hematologic response (CHR) and no cytogenetic response (CyR) at 3 months (Ph+ > 95%); < major CyR (Ph+ > 35%) at 6 months, < CCyR at 12 months, loss of CHR or CCyR or progression to accelerated phase (AP) or blast crisis (BC), occurrence of clonal cytogenetic abnormalities in Ph+ cells (ACA/Ph+), permanent discontinuation of imatinib due to toxicity and death due to disease activity. The term CP stands for stable disease, while AP and BC define progression of CML.
Statistical analysis
Quantitative variables are expressed as mean values with standard deviations, or as medians with interquartile ranges (for data with no Gaussian distribution). Categorical data are presented as absolute numbers with percentages. The normal distribution of each variable was tested by the Kolmogorov–Smirnov’s test. The Student’s t -test and Mann– Whitney U-test were used to assess differences in quantitative variables between the groups. Categorical variables were analyzed using the chi-square test. Cox proportional hazard models were conducted with the duration of imatinib therapy as the time scale. The effects of potential predictors on imatinib failure were determined using hazard ratios (HRs) presented with 95% confidence intervals (CIs). Among variables considered for model development, missing values were rare. Variables were evaluated in univariate Cox regression analysis, while the model was developed using a stepwise multivariate Cox regression analysis. A risk score was developed based on regression coefficients from the final multivariate model. To examine differences in survival free of imatinib failure according to the previously proposed and newly developed risk score, Kaplan–Meier curves were constructed and groups were compared using the Log–Rank test. Statistical analysis was performed using the SPSS statistical software (SPSS for Windows, release 21.0, SPSS, Chicago, IL, U.S.A.). In all tests, p value < 0.05 was considered to be statistically significant.
Results
In our study group of 168 consecutive patients with CP of Ph+ CML, the mean age at the time of diagnosis was 48 ± 14.4 years (range: 18–74 years) with 87.5% of patients younger than 65 years. More than a half of the group were man (54%). The overall survival at 5 and 10 years was 97 and 91%, respectively. During the follow-up, seven deaths occurred (4.2%) and the causes of death were as follows: comorbidities in four patients during CCyR and progression of disease in three patients.
Previous therapy received 88% of patients (149/168) mainly cytoreductive treatment with hydroxyurea (88% of patients) with the median duration of exposure of 4 months (IQR 4). The use of previous therapy and the type of drug were not found to be statistically significant in the achievement of CCyR during the treatment with imatinib.
The median duration of exposure to imatinib 400 mg once daily was 46 months (IQR 65) (range: 6–138 months). The median time for CHR was 3 weeks (IQR 3) (range 1–12 weeks). The median time for achievement of CCyR was 6 months (IQR 0) (range 3–24 months). One-hundred and eight patients (64.3%) achieved CCyR at 6 months, while 126 patients at 12 months (75%).
Overall response to imatinib treatment was as follows: 131 patients (78%) achieved CCyR, 14 patients (8.3%) majorCyR, 4 patients (2.4%) minorCyR, 16 patients (9.5%) had no cytogenetic response, 2 patients (1.2%) had hepatic toxicity verified by liver biopsy in the first six months of imatinib treatment and one patient (0.6%) was lost from follow-up.
After the achievement of CCyR on treatment with imatinib 400 mg once daily, 25 patients (19%) had a progression of disease by losing CCyR or development of AP/BP. The median time to progression was 24 months (IQR 38) (range 12–102 months). Currently used prognostic scores including Sokal (HR 0.954; 95%Cl for HR 0.590–1.543; p = 0.848), Hasford (HR 1.049; 95%Cl for HR 0.589–1.867; p 0.871), and EUTOS (HR 1.186; 95% Cl for HR 0.696–2.020; p = 0.531) were not found to be statistically significant in the prediction of progression in patients with CML treated with imatinib.
Twelve patients (7.1%), during the follow-up, developed additional cytogenetic abnormalities (ACAs). Distribution of ACAs was as follows: seven patients developed ACAs in Ph-negative cells, three patients in Ph-positive cells, and two patients in both type of cells. Of these 12 patients, 6 patients achieved CCyR, 1 patient majorCCyR, 1 patient minor CCyR, and 4 patients had no cytogenetic response. The most frequent aberrations detected were trisomy 8 (six patients), monosomy 7 (two patients), and lack of Y chromosome (two patients).
In the present study, during the median follow-up of 87 months (range: 78–98 months) in 61 patients (36.3%), the imatinib failure was verified. Failure rates of imatinib treatment, according to features, are given in . All three prognostic scores (Sokal, Hasford, and EUTOS), age including age >65 years, gender, hemoglobin level, leukocyte and platelet count, eosinophils and basophils in peripheral blood and splenomegaly were not found to be statistically significant for the imatinib failure ((A–C)). Cox regression analysis identified next predictive factors associated with failure to imatinib treatment in the standard dosage: hepatomegaly (p = 0.001), leukocytosis ≥100 × 109/l (p = 0.001), blasts in peripheral blood ≥1% (p = 0.002), and development of ACAs during the follow-up (p = 0.002). Accordingly, we assigned risk scores based on HR to hepatomegaly (HR = 4.089 (95%Cl for HR 1.831–9.134); 2 points), leukocytosis ≥100 × 109/l (HR = 3.158 (95%Cl for HR 1.616–6.172); 1 point), blasts in peripheral blood ≥1% (HR = 2.912 (95%Cl for HR 1.473–5.756); 1 point), and the presence of ACAs (HR = 11.110 (95%Cl for HR 2.372–52.046); 2 points). A final 3-tiered prognostic model named Imatinib-Failure (IMA-FAIL) was thus developed, as low (score 0), intermediate (score 1–3), and high risk (score ≥4) (). According to the new prognostic model, imatinib failure had 17% (8/47) of patients in low-, 34.9% (30/86) of patients in intermediate-, and 76.7% (23/30) of patients in high-risk group (HR = 3.973, 95% CI for HR 2.237–7.053, p < 0.001).
Figure 1. Kaplan–Meier curve for (A) Sokal score; (B) Hasford score and (C) EUTOS score in prediction of imatinib failure in patients with CML.
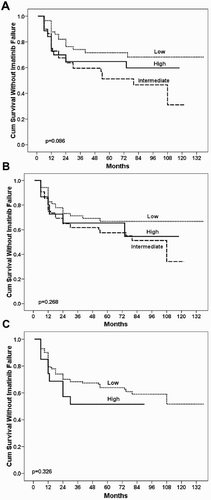
Figure 2. Kaplan–Meier curve for IMA-FAIL score in prediction of imatinib failure in patients with CML.
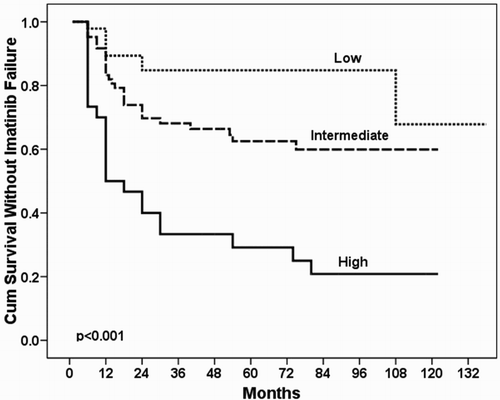
Table 1. Failure rates of imatinib treatment according to features.
In addition, comorbidities analyzed by three comorbidities scores (ACE 27, HCI-CI, and SCIRS) as well occurrence of second malignancy were not predictors for imatinib failure.
Discussion
In the present study, we analyzed retrospective data on a cohort of consecutive CML patients from everyday practice and assessed the influence of many parameters to imatinib response during follow-up. Our findings identified four independent risk factors for imatinib failure in patients with CML: hepatomegaly, leukocytosis ≥100 × 109/l, presence of blasts in peripheral blood, and ACAs. Based on these findings, we made a new prognostic model named IMA-FAIL, which clearly improves identification of high-risk patients for imatinib failure. The absence of these factors is associated with the achievement of stable CCyR. The advantage of this score is that it is dynamic and can be changed during treatment due to the development of ACAs, which may indicate treatment failure. At the time of CML diagnosis, if the patient has first three unfavorable parameters, it already belongs to the high-risk group.
EUTOS registry and analysis carried out in 2904 CML patients identified next percentage of patients in high-risk group: 24.7% by Sokal score, 10.8% by Hasford score, and 11.8% by EUTOS score [Citation17]. Application of these scores in our group of patients showed 27.3% of patients in high-risk group by Sokal, 10.4% by Hasford, and 12.5% by EUTOS. Our results about percentage of patients in high-risk group are similar to the findings of EUTOS registry. In addition, we compared the influence of three available scoring systems on overall response to imatinib treatment without finding a significant importance in the assessment of response ((A–C)). Using a new prognostic model IMA-FAIL, 76.7% of patients were identified in the high-risk group (). These results showed a better prediction of a new score.
Before TKI era, enlarged liver was found to be an unfavorable prognostic parameter associated with shorter survival [Citation18]. It was a part of survival prognostic model based on multivariate analysis. Enlarged liver was found in 24% of our patients. Liver, as the site of extramedullary hematopoiesis, can be the source of more abnormal clones or multipotent hematopoietic progenitors that have the mechanisms of resistance to the present therapy [Citation19,Citation20].
The presence of blasts in peripheral blood is known as an unfavorable prognostic parameter and a part of Sokal and Hasford score. Recently developed ELTS score identified the presence of blood blasts besides the age, platelet count, and spleen size as a parameter associated with increased risk of dying due to CML [Citation21].
Occurrence of ACAs in Ph-+cells (ACA/Ph+) during the treatment with imatinib indicates a clonal cytogenetic evolution and defines TKI failure [Citation4]. The presence of ACA/Ph+ is associated with shorter overall survival in patients treated with imatinib but not with dasatinib or nilotinib [Citation22,Citation23]. ACAs in Ph- cells occur in 5% to 10% of patients and, in the absence of dysplasia, do not seem to have an unfavorable influence on outcome [Citation24]. The exception is abnormalities of chromosome 7 (monosomy 7 and del(7q)), where some studies and case reports indicate a risk of myelodysplasia and acute leukemia (8,28). In our study, 7.7% of patients developed ACAs in Ph-cells during follow-up, while two of them had monosomy 7 and did not respond to imatinib 400 mg once daily. Six patients in our cohort developed trisomy 8 which belongs to part of major route of clonal evolution in CML. Besides clonal evolution within the Ph+ clone, the occurrence of a clone without a Ph translocation but a trisomy 8 was observed in five cases in this cohort. Interestingly, only one of these five patients achieved stable CCyR and continued treatment with imatinib 400 mg daily, other two patients continued nilotinib due to inferior response to imatinib and achieved CCyR, one patient achieved CCyR with escalated imatinib dose of 800 mg, while one patient was transplanted. The development of Ph-negative clones seems to be more frequent in patients treated with imatinib than has been observed during other therapy used regimens and it could be the first sign of genomic instability before the occurrence of Ph translocation or other ACAs of the major route of clonal evolution [Citation25].
Age is one of the parameters entering the calculation of Sokal and Hasford scores. We did not find the influence of age, on outcome with imatinib treatment, which support a finding of GIMEMA WORKING group [Citation26]. Seventy-five percentage of patients in our cohort at 12 months achieved CCyR, while 78% of the patients in the study of Francis et al. achieved CCyR [Citation27].
According to the ELN recommendations from 2013, one of the three TKIs including imatinib, nilotinib, and dasatinib can be used in the first-line treatment of CML [Citation4]. According to this, there is a need for reliable prognostic scoring system and risk stratification to predict treatment response with each of mentioned TKIs. Optimal response is associated with the best long-term outcome and quality of life for a patient. The limitation of our study is the lack of external validation for IMA-FAIL model due to the absence of adequate database among our collaborators.
In conclusion, our new model, IMA-FAIL, allows estimating which patient is capable of achieving a stable CCyR during the imatinib treatment, without which it is not possible to have an adequate molecular response. Evaluating the imatinib response and selection of patients who will benefit with this treatment should be important in making decisions regarding treatment. In addition, further prospective studies are needed in order to compare all three available TKIs in first-line treatment of CML and to analyze the benefit of each of them in individual patients according to their characteristics.
Supplemental_material.doc
Download MS Word (25.5 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Danijela Lekovic, MD, PhD, is a clinical assistant of Internal Medicine and Hematology at Faculty of Medicine, University of Belgrade. She graduated at Faculty of Medicine, University of Belgrade in 2003. She has been working at the Clinic for Hematology, Clinical Center Serbia since 2005. She obtained her master's degree in 2009. She specialized in Internal medicine in 2012. She obtained her PhD in 2016 on the title ‘Importance of angiogenesis in pathogenesis and therapy of BCR-ABL negative myeloproliferative neoplasms’. Her main scientific fields are myeloproliferative neoplasms and hemostatic disorders. She has authored 22 articles published in international and national journals. She won three travel grants of European Hematology Association with poster presentations. She attended specialized training in International Haemophilia Training Centre (IHTC) (Manchester Haemophilia Comprehensive Care Centre, Manchester Royal Infirmary) from August 3 until September 12, 2015, which she had received as fellowship by the World Federation of Hemophilia. She is a member of Research Grant concerning the pathogenesis of malignant hemopathies, in the Serbian Ministry of Education, Science and Technological Development.
Mirjana Gotic, MD, PhD, is a professor of Internal Medicine and Hematology at Faculty of Medicine, University of Belgrade, the Head of the Polyclinic-Diagnostic Department in the Clinic for Hematology, Clinical Center of Serbia, Belgrade, a member of the Academy of Medical Sciences of Serbian Medical Society, and a member of the Editorial Board of the Serbian Archives of Medicine journal. The main scientific field of interest in the last two decades is myeloproliferative neoplasms particularly essential thrombocythemia. She is a member of the Central European Myeloproliferative Neoplasms Organization. She is an investigator in Research Grant concerning the pathogenesis of malignant hemopathies, in the Serbian Ministry of Education, Science and Technological Development. She has participated in more than 50 articles published in international journals from PubMed Library of Medicine.
Natasa Milic, MD, PhD, is an associate professor and the Head of Department for Medical Statistics and Informatics at Belgrade University School of Medicine. She is also research collaborator at Mayo Clinic, Rochester, U.S.A. Her primary research interests are in the statistical design and analysis of clinical studies. Her most recent work involves the use of innovative techniques for data visualization in biomedical research. She is passionate about improving education in medical statistics for researchers.
Biljana Zivojinovic is the head nurse of Outpatient and Diagnostic Department, Clinic for Hematology, Clinical Center of Serbia. She acquired University level Specialist studies in Nursing at Faculty of Medicine, University of Belgrade. She is responsible for the treatment and follow-up of patients with CML and other MPNs. As a study nurse, she participated in several clinical trials in hematological malignancies. Her field of interest includes relationship with patients and their compliance to treatment.
Jelica Jovanovic graduated in Molecular Biology and Physiology in 1995 and finished specialization in Genetics in 2006 in Faculty of Biology, Belgrade, Serbia. From 1997 to 2000, she worked in Genetic laboratory, Mother and Child Health Institute and soon after, from 2000, in Laboratory for cytogenetic and molecular genetics, Clinic of Hematology, Clinical Center of Serbia. She performs conventional cytogenetic, FISH, RT-PCR, and RQ-PCR analyses. Conventional cytogenetic analysis includes: acute leukemia, myelodysplastic syndromes, myeloproliferative neoplasms, and chronic myeloid leukemia. FISH analysis is performed in multiple myeloma and chronic lymphocytic leukemia. RT-PCR analysis is applied in APL, ALL, and CML for the detection of recurrent fusion rearrangements (PML-RARA and BCR-ABL), while RQ-PCR analysis is used for molecular monitoring of CML patients treated with tyrosine kinase inhibitors.
Natasa Colovic graduated in Medical Faculty of the University of Belgrade in 1998. She has been working at the Clinic of Hematology Clinical Center Serbia since 2000. She was the topper in the Exam from Internal Medicine in 2003. She obtained the Master’s degree in 2001 on ‘Clinico-pathologic and immunophenotypic features of chronic lymphocytic leukemia’. She obtained her PhD in 2006 on ‘The significance of FLT3 mutation in acute myeloid leukemia and correlation with cytologic, cytogenetic and immunophenotypic features’. She attended ‘Postgraduate Athens Leukemia Lymphoma Course’, 35th Annual Course Advances in Haematology on Imperial College of Medicine, Hammersmith Hospital, London, and almost all EHA Congresses. She is a member of scientific project ‘Molecular and genetic markers of malignant transformation of haemopoietic stem cell’ financed by the Ministry of Science Republic of Serbia. She has been a member of Serbian Medical Association from 1999, American Society of Hematology since 2008, European Hematology Association since 2010. She has been a lecturer in Internal medicine and Hematology at Faculty of medicine, University of Belgrade since 2015. She has published 130 articles on medline.
Violeta Milosevic graduated on Medical Faculty, University of Belgrade in 2000. She has been working at the Clinic for Hematology, Clinical Center Serbia since 2003. She was the topper in Exam from Internal Medicine. She obtained Master’s degree in 2008. She has been a subspecialist of hematology since 2013. She is pursuing her PhD from CML. She has been a member of Serbian Medical Association from 2003.
Andrija Bogdanovic is an associate professor of Internal medicine and Hematology at Faculty of Medicine, University of Belgrade. He is also a senior consultant in the field of hematology and the chief of laboratory diagnostic Unit of Clinic of Hematology, Clinical Center of Serbia in Belgrade. His fields of interest are diagnostic hematology, various myeloid malignancies (particularly AML, CML, MDS, and MPNs), and also cellular biology of neoplastic blood disorders (apoptosis, proliferation, cellular interactions). He is a member of the Board of national advisors of the International CML Foundation, an active member of WP4 (CML) of the European LeukemiaNet, and the president of the Serbian CML group. Therefore, he is an expert in CML field in Serbia and region. He has authored more than 70 articles published in international and national journals.
ORCID
Danijela Lekovic http://orcid.org/0000-0002-6194-8298
Mirjana Gotic http://orcid.org/0000-0002-8590-0328
References
- Quintás-Cardama A, Cortes J. Molecular biology of bcr-abl1-positive chronic myeloid leukemia. Blood. 2009;113(8):1619–1630. doi: 10.1182/blood-2008-03-144790
- Huang X, Cortes J, Kantarjian H. Estimations of the increasing prevalence and plateau prevalence of chronic myeloid leukemia in the era of tyrosine kinase inhibitor therapy. Cancer. 2012;118(12):3123–3127. doi: 10.1002/cncr.26679
- O’Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004. doi: 10.1056/NEJMoa022457
- Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122(6):872–884. doi: 10.1182/blood-2013-05-501569
- Yilmaz M, Abaza Y, Jabbour E. Selecting the best frontline treatment in chronic myeloid leukemia. Curr Hematol Malig Rep. 2015;10(2):145–157. doi: 10.1007/s11899-015-0254-5
- Sokal JE, Cox EB, Baccarani M, et al. Prognostic discrimination in “good-risk” chronic granulocytic leukemia. Blood. 1984;63(4):789–799.
- Hasford J, Pfirrmann M, Hehlmann R, et al. A new prognostic score for survival of patients with chronic myeloid leukemia treated with interferon alfa. Writing Committee for the Collaborative CML Prognostic Factors Project Group. J Natl Cancer Inst. 1998;90(11):850–858. doi: 10.1093/jnci/90.11.850
- Hasford J, Baccarani M, Hoffmann V, et al. Predicting complete cytogenetic response and subsequent progression-free survival in 2060 patients with CML on imatinib treatment: the EUTOS score. Blood. 2011;21;118(3):686–692. doi: 10.1182/blood-2010-12-319038
- Yamamoto E, Fujisawa S, Hagihara M, et al. European Treatment and Outcome Study score does not predict imatinib treatment response and outcome in chronic myeloid leukemia patients. Cancer Sci. 2014;105(1):105–109. doi: 10.1111/cas.12321
- Jabbour E, Cortes J, Nazha A, et al. EUTOS score is not predictive for survival and outcome in patients with early chronic phase chronic myeloid leukemia treated with tyrosine kinase inhibitors: a single institution experience. Blood. 2012;119(19):4524–4526. doi: 10.1182/blood-2011-10-388967
- Eliasson L, Clifford S, Barber N, et al. Exploring chronic myeloid leukemia patients’ reasons for not adhering to the oral anticancer drug imatinib as prescribed. Leuk Res. 2011;35(5):626–630. doi: 10.1016/j.leukres.2010.10.017
- Cortes J, Kantarjian H. How I treat newly diagnosed chronic phase CML. Blood. 2012;120(7):1390–1397. doi: 10.1182/blood-2012-03-378919
- Deininger M. International randomized study of interferon Vs STI571 (IRIS) 8-year follow up: sustained survival and low risk for progression or events in patients with newly diagnosed Chronic Myeloid Leukemia in Chronic Phase (CML-CP) Treated with Imatinib. ASH 2009; Abstract 1126. 2009.
- Piccirillo JF, Costas I, Claybour P, et al. The measurement of comorbidity by cancer registries. J Registry Manag. 2003;30:8–14.
- Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–2919. doi: 10.1182/blood-2005-05-2004
- Linn BS, Linn MW, Gurel L. Cumulative Illness Rating Scale. J Am Geriatr Soc. 1968;16:622–626. doi: 10.1111/j.1532-5415.1968.tb02103.x
- Hoffmann VS, Baccarani M, Hasford J, et al. The EUTOS population-based registry: incidence and clinical characteristics of 2904 CML patients in 20 European Countries. Leukemia. 2015;29(6):1336–1343. doi: 10.1038/leu.2015.73
- Cervantes F, Rozman C. A multivariate analysis of prognostic factors in chronic myeloid leukemia. Blood. 1982;60(6):1298–1304.
- Chiu SC, Liu HH, Chen CL, et al. Extramedullary hematopoiesis (EMH) in laboratory animals: offering an insight into stem cell research. Cell Transplant. 2015;24(3):349–366. doi: 10.3727/096368915X686850
- Baccarani M, Zaccaria A, Bagnara GP, et al. The relevance of extramedullary hemopoiesis to the staging of chronic myeloid leukemia. Boll Ist Sieroter Milan. 1978;57(3):257–270.
- Pfirrmann M, Baccarani M, Saussele S, et al. Prognosis of long term survival considering disease-specific death in patients with chronic myeloid leukemia. Leukemia. 2016;30:48–56. doi: 10.1038/leu.2015.261
- Cortes JE, Talpaz M, Giles F, et al. Prognostic significance of cytogenetic clonal evolution in patients with chronic myelogenous leukemia on imatinib mesylate therapy. Blood. 2003;101(10):3794–3800. doi: 10.1182/blood-2002-09-2790
- Milojkovic D, Apperley JF, Gerrard G, Responses to second-line tyrosine kinase inhibitors are durable: an intention-to-treat analysis in chronic myeloid leukemia patients. Blood. 2012;119(8):1838–1843. doi: 10.1182/blood-2011-10-383000
- Lee SE, Choi SY, Bang JH, et al. The long-term clinical implications of clonal chromosomal abnormalities in newly diagnosed chronic phase chronic myeloid leukemia patients treated with imatinib mesylate. Cancer Genet. 2012;205(11):563–571. doi: 10.1016/j.cancergen.2012.09.003
- Schoch C, Haferlach T, Kern W, et al. Occurrence of additional chromosome aberrations in chronic myeloid leukemia patients treated with imatinib mesylate. Leukemia. 2003;17(2):461–463. doi: 10.1038/sj.leu.2402813
- Gugliotta G, Castagnetti F, Palandri F, et al. Gruppo Italiano Malattie Ematologiche dell’Adulto CML Working Party. Frontline imatinib treatment of chronic myeloid leukemia: no impact of age on outcome, a survey by the GIMEMA CML working party. Blood. 2011;117(21):5591–5599. doi: 10.1182/blood-2010-12-324228
- Francis J, Dubashi B, Sundaram R, et al. Influence of Sokal, Hasford, EUTOS scores and pharmacogenetic factors on the complete cytogenetic response at 1 year in chronic myeloid leukemia patients treated with imatinib. Med Oncol. 2015;32(8):213. doi: 10.1007/s12032-015-0665-0
