ABSTRACT
Background: Double-unit cord blood transplantation (CBT) can be used to overcome the limitation of single-unit CBT with low cell content for adults and larger adolescents. However, whether double-unit CBT is superior to single-unit CBT remains controversial.
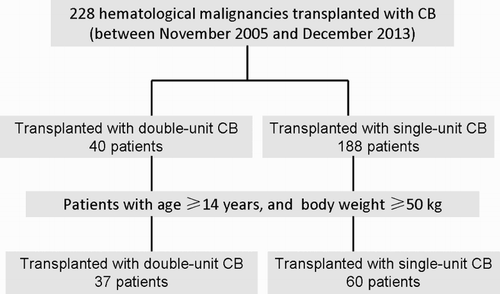
Methods: We reviewed the medical records of 228 consecutive hematological malignancies who received CBT between November 2005 and December 2013. Ninety-seven eligible patients met the criteria (age ≥14 years and body weight ≥50kg) and were enrolled in this study.
Results: The incidence of myeloid engraftment in the double-unit CBT group was significantly lower that in the single-unit CBT group (89.2 vs. 96.7%) (p = 0.026), and the incidence of platelet engraftment in the double-unit CBT group was slightly lower (70.3 vs. 86.7%) (p = 0.057). The 5-year transplant-related mortality rate was significantly higher in the double-unit CBT group when compared with that of the single-unit CBT group [54.1 vs. 33.3%, p = 0.026]. The 5-year probabilities of overall survival, disease-free survival and graft-versus-host disease (GVHD) -free/relapse-free survival in the double-unit CBT group were significantly lower than that of the single-unit CBT group (37.8 vs. 56.7%, p = 0.037; 32.4 vs. 55.0%, p = 0.017; 24.3 vs. 50.0%, p = 0.006). The incidences of GVHD and relapse were similar.
Conclusions: For adolescent and adult hematological malignancies with heavier body weight (≥50kg), double-unit CBT has an inferior clinical outcome when compared with single-unit CBT having a sufficient cell dose. Double-unit CBT should only reserve for patients who need an urgent transplant but lacking of a related or unrelated donor and without an adequately dosed single CB.
Background
Unrelated cord blood transplantation (CBT) is an important alternative transplant modality for the treatment of hematological malignancies, especially for patients lacking a matched sibling or matched unrelated donor. Double-unit CBT (transplantation of two partially HLA-matched CB units) has been increasingly used to overcome the limitation of single-unit CBT with low cell content for adults and larger adolescents [Citation1]. The data from the University of Minnesota had shown that double-unit CBT was associated with high rate of engraftment, low rate of relapse and promising survival outcomes [Citation2]. Labopin et al. [Citation3] indicated that double-unit CBT was more cost-effective than single CBT, with better outcomes, including quality-adjusted life-years for adults with acute leukemia in first complete remission. However, whether double-unit CBT was superior to single-unit CBT remained controversial, and several clinical studies did not show any survival superiority of a double-unit CBT when compared with a single-unit CBT [Citation4–8].
In China, about more than 5000 patients received allogeneic hematopoietic stem cell transplantation (HSCT) each year, and haploidentical HSCT was very popular (more than 30%) nowadays; however, only 100–150 cases of CBT were performed annually, and the experiences of double-unit CBT were also lacked. Our center is the largest CBT center and harbors the vast majority of the registered CBT cases in China, and in this study, we retrospectively perform a clinical comparison to evaluate the safety and efficacy of the double-unit CBT compared with single-unit CBT for the treatment of adolescent and adult hematological malignancies with heavier body weight (≥50 kg).
Patients and methods
Patient eligibility
From November 2005 to December 2013, a total of 228 consecutive hematological malignancies patients received CBT, which included 40 patients receiving double-unit CBT and 188 patients receiving single-unit CBT. All of these patients had no HLA-identical sibling donor. Eligible patients in the present study were at least 14 years of age and also at least 50 kg of body weight; a total of 37 recipients of double-unit CBT and 60 recipients of single-unit CBT met the criteria and were enrolled in this study (). The median age at transplant was 24 years (range, 14–51) in the double-unit CBT group and 23 years (range, 15–46) in the single-unit CBT group (p = 0.31), and the median body weight in the corresponding group was 65 kg (range, 50–90) and 60 kg (range, 50–100), respectively (p = 0.25). The two transplant cohorts were well balanced with respect to the underlying disease, disease risk at first diagnosis, disease stage in transplant, and cytomegalovirus serology before transplant ().
Table 1. Patients’ and transplant characteristics.
Cord blood selection
CB selection depended on the donor-recipient HLA matching, and counts of total nucleated cells (TNC) and CD34 cells. HLA-A and HLA-B were assessed at low resolution antigen level and HLA-DRB1 was assessed at high-resolution allele level. In single-unit CBT group, at least four of six HLA loci should be met, and the CB units contained cryopreserved TNC with minimum dose of 3 × 107/kg and/or CD34+ cells with minimum count of 1.2 × 105/kg of the recipient weight. In double-unit CBT group, all patients had no enough TNC and CD34 cell dose of a single CB unit. Two CB units required 4–6/6 HLA-matching both to the patient and each other, and the combined CB units should contain at least 3.5 × 107 TNC/kg and 2 × 105 CD34+ cells/kg, respectively. Therefore, the median dose of infused TNC was 4.53 (range, 3.27–8.33) × 107/kg for the double-unit CBT group and 3.29 (range, 2.42–6.76) × 107/kg for the single-unit CBT group (p < 0.001), and the dose of infused CD34 cells was 2.37 (range, 1.29–8.78) × 105/kg and 1.78 (range, 1.05–4.95) × 105/kg, respectively (p = 0.011) ().
Table 2. Cord blood characteristics.
Conditionings and GVHD prophylaxis
In the double-unit CBT group, 29 patients (78.4%) received myeloablative total body irradiation (TBI, total 12 Gy, four fractions) (d-7, d-6), cyclophosphamide (CY, 60 mg/kg daily for 2 days) (d-3, d-2), and high-dose cytarabine (HDAC, 2.0 g/m2 every 12 hours for 2 days) (d-5, d-4)] (TBICY + HDAC); one patient received myeloablative busulfan (BU, 0.8 mg/kg every 6 hours for 4 days) (d-7∼ -4), CY (60 mg/kg daily for 2 days) (d-3, d-2), and HDAC (2.0 g/m2 every 12 hours for 2 days) (d-9, d-8) (BUCY2 + HDAC). G-CSF was given with 5 μg/kg daily by subcutaneous injection one day prior to HDAC with 3 days. The other seven patients received reduced intensity conditioning [fludarabine (30∼40 mg/m2 daily for 4 days), busulfan (BU) (0.8 mg/kg every 6 hours for 2 days), low-dose TBI (3 Gy in one fraction), antithymocyte globulin (2.5 mg/kg daily for 3 days)].
All patients in the single-unit CBT group received myeloablative conditioning. Fifty-seven patients (95.0%) received myeloablative TBICY + HDAC, and 3 patients (5.0%) received myeloablative busulfan (0.8 mg/kg every 6 h for 4 days) (d-7∼ -4), and CY (60 mg/kg daily for 2 days) (d-3, d-2)], and fludarabine (Flu, 30 mg/m2 daily for 4 days) (d-8∼ -5) (BUCY2 + Flu). All CBT patients received cyclosporine (CSA) and mycophenolate mofetil (MMF) for GVHD prophylaxis as previously described [Citation9–11].
Definitions and statistical analyses
PCR analysis of short tandem repeat sequences (STR-PCR) was performed for the chimerism analysis on peripheral blood (PB) weekly (at the 7th, 14th, 21st day after transplantation), then on bone marrow monthly. Neutrophil engraftment was defined by an absolute neutrophil count exceeded 0.5 × 109/l at any time after transplantation on the first of 3 consecutive days, and platelet engraftment was defined by an platelet count exceeded 20 × 109/l on the first day of 7 consecutive days without transfusion. Primary graft failure was determined based on a profound, persistent pancytopenia and marrow hypoplasia without donor-derived cells within 42 days post-transplant, or reconstitution with autologous cells. Transplant-related mortality (TRM) was defined as death due to any complication unrelated to the underlying disease following transplant. Relapse was defined by the morphologic recurrence of hematologic malignant disease in the PB, bone marrow, or extramedullary area after transplantation. Diagnosis and clinical grading of acute GVHD and chronic GVHD were conducted according to the previous report [Citation12,Citation13]. Overall survival (OS) was defined as the time between cord blood infusion and death due to any cause. Disease-free survival (DFS) was defined as the time from transplant to disease relapse or death. GVHD-free/relapse-free survival (GRFS) was defined as time interval from transplant to severe aGVHD (grade 3–4 aGVHD), extensive cGVHD or chronic GVHD requiring systemic treatment, relapse, or death, whichever occurred first [Citation14].
Continuous variables such as age, body weight, days of neutrophil and platelet engraftment, total nucleated-cell dose, and CD34 cell dose were measured using Mann–Whitney U test, and categorical variables such as sex, underlying disease, disease risk at first diagnosis, disease stage in transplant, cytomegalovirus (CMV) serology prior to transplant, conditioning regimens, HLA match, ABO compatibility were measured using χ2 test. Cumulative-incidence function method was performed to estimate the probabilities of engraftment, GVHD, TRM, and relapse with considering competing risks (engraftment and aGVHD, TRM and relapse) with gray test. Death of any cause was the end point of OS, and relapse or death was the end point of DFS; severe aGVHD (grade 3–4 aGVHD), extensive cGVHD or chronic GVHD requiring systemic treatment, relapse, or death were the end points of GRFS. Kaplan–Meier method was performed to estimate the probabilities of OS, LFS, and GRFS with log-rank test. Statistical analyses were conducted using R statistical software (R Foundation for Statistical Computing, Vienna, Austria). Differences with р < 0.05 were considered significant.
Results
Engraftment
There were six patients having primary graft failure which confirmed by STR-PCR, including four patients in the double-unit CBT group and two patients in the single-unit CBT group. The cumulative incidence of myeloid engraftment at 42 days was 89.2% (95%CI, 72.7–95.7%) in the double-unit CBT group, which significantly lower that in the single-unit CBT group [96.7% (95%CI, 86.9–99.2%)] (p = 0.026) ((a)). Eleven recipients in the double-unit CBT group and 8 recipients in the single-unit CBT group did not obtain platelet recovery on day 100. The cumulative incidence of platelet engraftment at 100 days was 70.3% (95%CI, 51.2–81.9) in the double-unit CBT group, which slightly lower than that in the single-unit CBT group [86.7% (95%CI, 74.6–93.0)] (p = 0.057) ((b)). The median time to neutrophil engraftment was 22 days (range, 14–65) in the double-unit CBT group and 19 days (range, 12–42) in the single-unit CBT group (p = 0.079). Platelet engraftment was achieved at a median time of 43 days (range, 21–115) in the double-unit CBT group and 41 days (range, 18–75) in the single-unit CBT group (p = 0.51).
Figure 2. Neutrophil and platelet engraftment. The cumulative incidence of myeloid engraftment at 42 days was 89.2% (95%CI, 72.7–95.7%) in the double-unit CBT group, and 96.7% (95%CI, 86.9–99.2%) in the single-unit CBT group (p = 0.026) (a). The cumulative incidence of platelet engraftment at 100 days was 70.3% (95%CI, 51.2–81.9) in the double-unit CBT group, and 86.7% (95%CI, 74.6–93.0)] in the single-unit CBT group (p = 0.057) (b).
![Figure 2. Neutrophil and platelet engraftment. The cumulative incidence of myeloid engraftment at 42 days was 89.2% (95%CI, 72.7–95.7%) in the double-unit CBT group, and 96.7% (95%CI, 86.9–99.2%) in the single-unit CBT group (p = 0.026) (a). The cumulative incidence of platelet engraftment at 100 days was 70.3% (95%CI, 51.2–81.9) in the double-unit CBT group, and 86.7% (95%CI, 74.6–93.0)] in the single-unit CBT group (p = 0.057) (b).](/cms/asset/39d2b106-1279-4c33-b50b-59d372eb73bf/yhem_a_1361078_f0002_c.jpg)
GVHD
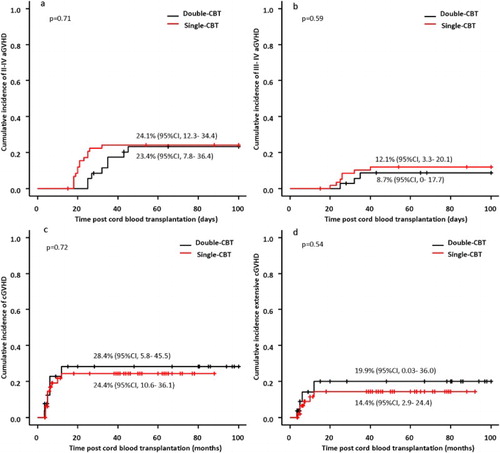
The incidence of aGVHD or cGVHD was similar between the two groups. In the double-unit CBT group, 8 of the 33 evaluable patients with stable engraftment developed grade II to IV aGVHD, and 3 patients developed grade III to IV aGVHD. In the single-unit CBT group, 14 of the 58 evaluable patients with stable engraftment developed grade II to IV aGVHD, and 7 patients developed grade III to IV aGVHD. The cumulative incidences of grade II to IV aGVHD at day 100 in the double-unit CBT and the single-unit CBT groups were 23.4% (95%CI, 7.8–36.4) and 24.1% (95%CI, 12.3–34.4), respectively (p = 0.71) ((a)). The cumulative incidences of grade III to IV aGVHD in the corresponding group were 8.7% (95%CI, 0–17.7) and 12.1% (95%CI, 3.3–20.1), respectively (p = 0.59) ((b)). Among patients who survived more than 100 days after transplantation, 6 of 27 evaluable patients developed cGVHD and 3 patients developed extensive cGVHD in the double-unit CBT group, and 11 of 52 evaluable patients developed cGVHD and 6 patients developed extensive cGVHD in the single-unit CBT group. The cumulative incidences of cGVHD and extensive cGVHD at 5 year were 28.4% (95%CI, 5.8–45.5) and 19.9% (95%CI, 0.03–36.0) in the double-unit CBT group, and 24.4% (95%CI, 10.6–36.1) and 14.4% (95%CI, 2.9–24.4) in the single-unit CBT group, with no differences between the two groups (p = 0.72, 0.54) ((c,d)).
Figure 3. Acute GVHD and chronic GVHD. The cumulative incidences of grade II to IV aGVHD at day 100 in the double-unit CBT and the single-unit CBT groups were 23.4% (95%CI, 7.8–36.4) and 24.1% (95%CI, 12.3–34.4), respectively (p = 0.71) (a). The cumulative incidences of grade III to IV aGVHD in the corresponding group were 8.7% (95%CI, 0–17.7) and 12.1% (95%CI, 3.3–20.1), respectively (p = 0.59) (b). The cumulative incidences of cGVHD and extensive cGVHD at 5 year were 28.4% (95%CI, 5.8–45.5) and 19.9% (95%CI, 0.03–36.0) in the double-unit CBT group, and 24.4% (95%CI, 10.6–36.1) and 14.4% (95%CI, 2.9–24.4) in the single-unit CBT group, with no differences between the two groups (p = 0.72, 0.54) (c) and (d).
TRM and relapse
Causes of death were shown in . Death from treatment-related complications occurred in 21 of 37 recipients of the double-unit CB, and in 19 of 60 recipients of the single-unit CB. The 5-year cumulative incidence of TRM was significantly higher in the double-unit CBT group when compared with that of the single-unit CBT group [54.1% (95%CI, 36.5–68.7) versus 33.3% (95%CI, 21.7–45.4), p = 0.026] ((a)). Five patients in the double-unit CBT group and seven patients in the single-unit CBT group experienced leukemia relapse after transplantation, and the incidence of relapse was similar between the two groups: the 5-year relapse was 13.5% (95%CI, 4.8–26.8) in the double-unit CBT group and 11.7% (95%CI, 5.1–21.3) in the single-unit CBT group (p = 0.82) ((b)).
Figure 4. TRM and relapse. The 5-year cumulative incidence of TRM was significantly higher in the double-unit CBT group when compared with that of the single-unit CBT group [54.1% (95%CI, 36.5–68.7) vs. 33.3% (95%CI, 21.7–45.4), p = 0.026] (a). The 5-year relapse was 13.5% (95%CI, 4.8–26.8) in the double-unit CBT group and 11.7% (95%CI, 5.1–21.3) in the single-unit CBT group (p = 0.82) (b).
![Figure 4. TRM and relapse. The 5-year cumulative incidence of TRM was significantly higher in the double-unit CBT group when compared with that of the single-unit CBT group [54.1% (95%CI, 36.5–68.7) vs. 33.3% (95%CI, 21.7–45.4), p = 0.026] (a). The 5-year relapse was 13.5% (95%CI, 4.8–26.8) in the double-unit CBT group and 11.7% (95%CI, 5.1–21.3) in the single-unit CBT group (p = 0.82) (b).](/cms/asset/d84d3a88-91de-4ea7-97c7-c6d488e6aea3/yhem_a_1361078_f0004_c.jpg)
Table 3. Causes of death.
In the double-unit CBT group, the 5-year cumulative incidence of TRM was 57.1% (95%CI, 13.1–85.7) in patients with reduced intensity conditioning and 53.3% (95%CI, 33.7–69.5) in patients with myeloablative conditioning (p = 0.56) (Supplemental Figure 1). The 5-year relapse rate was 14.3% (95%CI, 0.3–53.1) in patients with reduced intensity conditioning and 13.3% (95%CI, 4.0–28.3) in patients with myeloablative conditioning (p = 0.98) (Supplemental Figure 1).
Survival
The median follow-up time among survivors was 85 months (range, 48–130) for patients in the double-unit CBT group and 52 months (range, 38–102) for patients in the single-unit CBT group. The 5-year probability of OS was 37.8% (95%CI, 22.6–53.0) in the double-unit CBT group, which significantly lower than that of the single-unit CBT group [56.7% (95%CI, 43.2–68.1)] (p = 0.037) ((a)). The 5-year probability of DFS in the double-unit CBT group [32.4% (95%CI, 18.2–47.5)] was significantly decreased compared with that in the single-unit CBT group [55.0% (95%CI, 41.6–66.5)] (p = 0.017) ((b)). The 5-year probability of GRFS was 24.3% (95%CI, 12.1–38.8) among double-unit recipients, which significantly lower than that among single-unit recipients [50.0% (95%CI, 36.8–61.8)] (p = 0.006) ((c)).
Figure 5. Survival. The 5-year probability of OS was 37.8% (95%CI, 22.6–53.0) in the double-unit CBT group, which significantly lower than that of the single-unit CBT group [56.7% (95%CI, 43.2–68.1)] (p = 0.037) (a). The 5-year probability of DFS in the double-unit CBT group [32.4% (95%CI, 18.2–47.5)] was significantly decreased compared with that in the single-unit CBT group [55.0% (95%CI, 41.6–66.5)] (p = 0.017) (b). The 5-year probability of GRFS was 24.3% (95%CI, 12.1–38.8) among double-unit recipients, which significantly lower than that among single-unit recipients [50.0% (95%CI, 36.8–61.8)] (p = 0.006) (c).
![Figure 5. Survival. The 5-year probability of OS was 37.8% (95%CI, 22.6–53.0) in the double-unit CBT group, which significantly lower than that of the single-unit CBT group [56.7% (95%CI, 43.2–68.1)] (p = 0.037) (a). The 5-year probability of DFS in the double-unit CBT group [32.4% (95%CI, 18.2–47.5)] was significantly decreased compared with that in the single-unit CBT group [55.0% (95%CI, 41.6–66.5)] (p = 0.017) (b). The 5-year probability of GRFS was 24.3% (95%CI, 12.1–38.8) among double-unit recipients, which significantly lower than that among single-unit recipients [50.0% (95%CI, 36.8–61.8)] (p = 0.006) (c).](/cms/asset/438fd4e9-3002-41be-9ef5-d2e5e70e9af4/yhem_a_1361078_f0005_c.jpg)
In the double-unit CBT group, the 5-year probability of OS was 28.6% (95%CI, 4.1–61.2) in patients with reduced intensity conditioning and 40.0% (95%CI, 22.8–56.7) in patients with myeloablative conditioning (p = 0.39) (Supplemental Figure 2). The 5-year probability of DFS was similar between the two groups: 28.6% (95%CI, 4.1–61.2) in patients with reduced intensity conditioning and 33.3% (95%CI, 17.5–50.0) in patients with myeloablative conditioning (p = 0.58) (Supplemental Figure 3).
Discussion
In the present study, we found that, for adolescent and adult hematological malignancies with heavier body weight (≥50 kg), double-unit CBT was associated with a lower engraftment rate, a higher TRM, a comparable incidence of GVHD and relapse, and a lower long-term survival than single-unit CBT, even double-unit CB had a higher combined TNC and CD34 cell doses. Our study demonstrated that the higher numbers of hematopoietic cells in double units of CB could not provide improved outcomes after transplantation, and this finding was contrary to that of Labopin’s study [Citation3]. Wagner et al. [Citation6] first conducted a multicenter perspective and randomized clinical trial comparing single- versus double-unit CBT among children and adolescents with hematologic cancer, and found that single-unit CBT (n = 113) was associated with better platelet recovery and a lower risk of GVHD than double-unit CBT (n = 111), but survival rates were similar. Michel et al. [Citation7] also performed a multicenter French randomized study in children and young adults with acute leukemia or MDS, and concluded that a double-unit strategy proved ineffective at improving the outcome of unrelated CBT when a single cord has an adequate cell dose, including similar hematologic recovery, comparable incidence of relapse, TRM, and DFS, but more frequently extensive cGVHD after double-unit CBT. Other previous retrospective studies [Citation4,Citation5,Citation8] had the similar findings that were in accordance with that in these two randomized clinical trials.
For double CB selection, more rigorous randomized clinical trials [Citation6,Citation7] required that the dominant unit had to contain at least 2.5 × 107 TNC/kg of the recipient weight, and 1.5 × 107 TNC/kg for the secondary unit. The dominant unit in the double-unit CBT cohort might have a higher TNC and/or CD34 cell doses than the single-unit CBT cohort in these clinical trials. However, in real-world clinical practice, we cannot get such an adequate cell dose for patients receiving double-unit CBT. We demand that the combined CB units contain at least 3.5 × 107 TNC/kg and 2 × 105 CD34+ cells/kg, regardless of the cell doses in each unit. So in this study, each unit in the double-unit CBT group had a lower TNC and/or CD34 cell doses than the single-unit CBT group, although the combined TNC and CD34 cell doses was higher in the double-unit CBT group.
Our study indicated that double-unit CBT had a higher incidence of primary graft failure than single-unit CBT. This led to the lower rates and longer time of neutrophil and platelet recovery in patients of double-unit CBT. Although no reports illustrated double-unit CBT was associated with the higher probability of graft failure, Tsang et al. [Citation5] found that the median-time of neutrophil and platelet engraftment were both delayed among children transplanted with double-unit CB even with significantly higher TNC and CD34+ cell doses. Wagner’s [Citation6] first randomized comparison also demonstrated that double-unit CBT had a significantly lower incidence of platelet engraftment than single-unit CBT (65 vs. 76%, p = 0.04), corresponding with a longer time of platelet recovery in recipients of double-unit CB (84 vs. 58 days). We speculated that two factors might be related to the delayed engraftment after double-unit CBT. Owing to the fact that only one CB unit contributed to the long-term engraftment after double CBT, the lower TNC and/or CD34 cell doses in the dominant unit might contribute to the delayed hematopoietic recovery. On the other hand, the effect of ‘graft versus graft’ in the double-unit CBT should be concerned. Because of the nature of the HSC niche which the size is limited [Citation15], two units would competitively bind to the specific area of the HSC niche, and we hypothesized that the capacity of the competition of the nondominant unit CB would interfere with the progress of the hematopoiesis of the dominant unit one. Many published data could help in predicting which of the infused units would finally dominate, such as the viability of CD34 cells [Citation16], PB CD3 chimerism [Citation17], and the order of the infusion [Citation18].
Compared to single CBT, double CBT resulted in a higher risk of TRM in this study, directly leading to the decreased long-term survival (OS, DFS, and GRFS). Most deaths after double-unit CBT were attributed to infection and primary graft failure which occurred within the first 6 months. Prolonged neutropenia or graft failure would increase the recipient’s vulnerability to bacterial or fungal infections and contribute to the higher TRM. On the other hand, double CBT was associated with delayed immune reconstitution, leading to an increased risk of opportunistic infections (especially viral infections). Labopin et al. [Citation3] indicated that 71% of recipients after double CBT experienced cytomegalovirus reactivation at day +100, which significantly higher than those after single CBT (37%) (p = 0.01). Although the recovery of T-cells, B-cells, and NK-cells were similar between the single-unit CBT and double-unit CBT, the quantitative recovery of absolute lymphocyte count (including the T- and B-cell subsets) and viral-specific CD4+ and CD8+ T cells were profound delayed until 9–12 months after double-unit CBT with increased infections morbidity and mortality [Citation19].
The current study showed that the incidences of GVHD (including aGVHD and cGVHD) were similar between two groups, and this finding was accordance with the previous retrospective studies. However, two prospective and randomized data indicated that double CBT was associated with increased probabilities of GVHD. Wagner et al. [Citation6] reported that the cumulative incidences of grade III- IV aGVHD and extensive cGVHD were higher after double CBT than that after single CBT (23 vs. 13%, p = 0.02; 15 vs. 9%, p = 0.05), and Michel et al. [Citation7] found that the incidence of extensive cGVHD was more frequently occurred in patients after double-unit CBT than those after single-unit CBT (31.9 vs. 14.7%) (p = 0.02). The data from the GVHD Working Group of the Japan Society for Hematopoietic Cell Transplantation [Citation20] showed that acute and chronic GVHD were both associated with a lower rate of relapse after unrelated CBT, which could be further explained as the development of GVHD would be accompanied by the potent GVL effect. Although the incidence of extensive cGVHD was increased in the above two randomized clinical studies, the investigators did not find any differences of relapse between the double-unit and the single-unit arms. However, Michel et al. [Citation7] revealed that double-unit CBT in patients receiving TBI without ATG had a significantly lower relapse risk when compared with single-unit CBT, which indicated that transplant conditioning was an important contributing factor for relapse after transplantation. Our relatively lower relapse rate both in double-unit and the single-unit arms (13.5 and 11.7%) might be correlated with TBI-based myelablative conditioning, GVHD prophylaxis without ATG, and HLA disparity, etc.
In summary, this comparison suggests that, for adolescent and adult hematological malignancies with heavier body weight (≥50 kg), double-unit CBT has an inferior clinical outcomes when compared with single-unit CBT having a sufficient cell dose (TNC ≥ 3 × 107/kg and/ or CD34+ cells ≥ 1.2 × 105/kg). We recommend that double-unit CBT should only reserve for patients who need an urgent transplant but lacking of a related or unrelated donor and without an adequately dosed single CB, and should be conducted in an experienced transplant center. Owing to the fact that the median body weight in both cohorts is lower when compared with that of patients in Europe and America, so the conclusion of this study is more suitable for Asian patients who received unrelated CBT. On the other hand, there are some inherent limitations to this study. First, the nature of this study is a non-prospective and non-randomized study in a single center, and the sample size in each group is small. Second, enrolled patients consist of a heterogeneous disease entity, and we cannot evaluate potential differences between two cohorts in terms of disease subgroups (e.g. acute myeloid leukemia, acute lymphoblastic leukemia, chronic myeloid leukemia) due to the small number of patients. Third, the transplant conditioning is not uniform between the two groups, such as the fact that more patients in the double-unit CBT group received reduced intensity conditioning. A large-scale randomized, double-blind clinical trial in multicenter might solve these limitations.
Supplementary_material.zip
Download Zip (137.2 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Sideri A, Neokleous N, Brunet De La Grange P, et al. An overview of the progress on double umbilical cord blood transplantation. Haematologica. 2011;96(8):1213–1220. doi: 10.3324/haematol.2010.038836
- Verneris MR, Brunstein CG, Barker J, et al. Relapse risk after umbilical cord blood transplantation: enhanced graft-versus-leukemia effect in recipients of 2 units. Blood. 200;114(19):4293–4299. doi: 10.1182/blood-2009-05-220525
- Labopin M, Ruggeri A, Gorin NC, et al. Eurocord and Société Française de Greffe de Moelle et de Thérapie Cellulaire (SFGM-TC). Cost-effectiveness and clinical outcomes of double versus single cord blood transplantation in adults with acute leukemia in France. Haematologica. 2014;99(3):535–540. doi: 10.3324/haematol.2013.092254
- Bradstock K, Hertzberg M, Kerridge I, et al. Single versus double unrelated umbilical cord blood units for allogeneic transplantation in adults with advanced haematological malignancies: a retrospective comparison of outcomes. Intern Med J. 2009;39(11):744–751. doi: 10.1111/j.1445-5994.2008.01825.x
- Tsang KS, Leung AW, Lee V, et al. Indiscernible benefit of double-unit umbilical cord blood transplantation in children: a single-center experience from Hong Kong. Cell Transplant. 2016;25(7):1277–1286. doi: 10.3727/096368915X689631
- Wagner JE, Jr, Eapen M, Carter S, et al. Blood and marrow transplant clinical trials network. One-unit versus two-unit cord-blood transplantation for hematologic cancers. N Engl J Med. 2014;371(18):1685–1694. doi: 10.1056/NEJMoa1405584
- Michel G, Galambrun C, Sirvent A, et al. Single- vs double-unit cord blood transplantation for children and young adults with acute leukemia or myelodysplastic syndrome. Blood. 2016;127(26):3450–3457. doi: 10.1182/blood-2016-01-694349
- Scaradavou A, Brunstein CG, Eapen M, et al. Double unit grafts successfully extend the application of umbilical cord blood transplantation in adults with acute leukemia. Blood. 2013;121(5):752–758. doi: 10.1182/blood-2012-08-449108
- Zheng C, Zhu X, Tang B, et al. The impact of pre-transplant minimal residual disease on outcome of intensified myeloablative cord blood transplant for acute myeloid leukemia in first or second complete remission. Leuk Lymphoma. 2016;57(6):1398–1405. doi: 10.3109/10428194.2015.1102241
- Zheng C, Zhu X, Tang B, et al. Comparative analysis of unrelated cord blood transplantation and HLA-matched sibling hematopoietic stem cell transplantation in children with high-risk or advanced acute leukemia. Ann Hematol. 2015;94(3):473–480. doi: 10.1007/s00277-014-2213-y
- Zheng C, Tang B, Yao W, et al. Comparison of unrelated cord blood transplantation and HLA-matched sibling hematopoietic stem cell transplantation for patients with chronic myeloid leukemia in advanced stage. Biol Blood Marrow Transplant. 2013;19(12):1708–1712. doi: 10.1016/j.bbmt.2013.09.008
- Dignan FL, Clark A, Amrolia P, et al. Haemato-oncology task force of British committee for standards in haematology; British society for blood and marrow transplantation. diagnosis and management of acute graft-versus-host disease. Br J Haematol. 2012;158:30–45. doi: 10.1111/j.1365-2141.2012.09129.x
- Dignan FL, Amrolia P, Clark A, et al. Haemato-oncology task force of British committee for standards in haematology; British society for blood and marrow transplantation. diagnosis and management of chronic graft-versus-host disease. Br J Haematol2012;158:46–61. doi: 10.1111/j.1365-2141.2012.09128.x
- Holtan SG, DeFor TE, Lazaryan A, et al. Composite end point of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood. 2015;125:1333–1338. doi: 10.1182/blood-2014-10-609032
- Haspel RL, Ballen KK. Double cord blood transplants: filling a niche? Stem Cell Rev. 2006;2(2):81–86.
- Scaradavou A, Smith KM, Hawke R, et al. Cord blood units with low CD34+ cell viability have a low probability of engraftment after double unit transplantation. Biol Blood Marrow Transplant. 2010;16(4):500–508. doi: 10.1016/j.bbmt.2009.11.013
- Newell LF, Milano F, Nicoud IB, et al. Early CD3 peripheral blood chimerism predicts the long-term engrafting unit following myeloablative double-cord blood transplantation. Biol Blood Marrow Transplant. 2012;18(8):1243–1249. doi: 10.1016/j.bbmt.2012.01.014
- Ramirez P, Wagner JE, DeFor TE, et al. Factors predicting single-unit predominance after double umbilical cord blood transplantation. Bone Marrow Transplant. 2012;47(6):799–803. doi: 10.1038/bmt.2011.184
- Saliba RM, Rezvani K, Leen A, et al. General and virus-specific immune cell reconstitution after double cord blood transplantation. Biol Blood Marrow Transplant. 2015;21(7):1284–1290. doi: 10.1016/j.bbmt.2015.02.017
- Kanda J, Morishima Y, Terakura S, et al. Impact of graft-versus-host disease on outcomes after unrelated cord blood transplantation. Leukemia. 2017;31(3):663–668. doi: 10.1038/leu.2016.288