ABSTRACT
Objectives: We performed molecular analysis to evaluate clinical implications of a rare nucleotide change, particularly when co-inherited with other known mutations in the globin clusters, in order to conduct an appropriate genetic counselling.
Methods: Complete blood cell counts and high-performance liquid chromatography were the routine first level analysis for patients referred to our Hospital Center in Palermo to undergo the screening test for haemoglobinopathies. Sequencing analysis was the selected method for the phenotypic characterization, especially in case of new or very rare mutations in globin genes.
Results: We report data of a rare single nucleotide variation at position −56 relative to transcription initiation site (NM_000518.4(HBB):c.−106G > C), identified in ten patients of Italian origin during the screening programme of the ‘Sicilian population’. It was found in simple heterozygosity (n = 8), in association with beta haemoglobin variant Hb S (n = 1) and in heterozygosity with beta-thalassaemic allele IVS-I-1 G->A [(HBB):c.92 + 1G > A] and ααα anti3.7 rearrangement (n = 1).
Discussion: Heterozygous subjects for this substitution showed normal haematological and electrophoretic features. Heterozygotes for this mutation and other defect in globin genes showed the classical phenotype of a healthy carrier, therefore it can be considered a benign variant that does not alter the production and function of haemoglobin.
Conclusion: This is another example of rare or new nucleotide variations whose identification and characterization is crucial in order to carry out appropriate genetic counselling to a potential risk couple.
Introduction
Haemoglobinopathies are the commonest recessive monogenic disorders worldwide [Citation1]. About 2033 haemoglobin mutations, either affect the synthesis of globin chains (thalassaemias) or alter the structure and properties of haemoglobin (haemoglobin variants), are currently described and available in the Ithanet database [Citation2–4].
Particularly, beta-thalassaemia is a common autosomal recessive disorder in the Mediterranean area and in Sicily displays a high level of clinical and molecular heterogeneity [Citation5]. At the molecular level, 1082 single nucleotide substitutions and small deletions or insertions of β-globin gene (HBB) of different severity are described [Citation6].
Novel mutations are still occasionally being described, especially when evaluating milder phenotypes. Characterization of rare mutations is important for genetic counselling in couples at risk of generating an affected foetus.
In this report we describe the phenotype of individuals with a rare single nucleotide variation, NM_000518.4(HBB):c.−106G > C, found in heterozygous state and in co-inheritance with other mutations in globin genes. It is located several nucleotides downstream to the highly conserved CACCC box (position −90 to −86), which is a known binding site for transcription factors stimulatory protein 1 and erythroid Krüppel-like factor, and it is critical for developmentally regulated high-level expression of HBB gene [Citation7–9].
Position −56 is not a conserved nucleotide and this region of the promoter is devoid of mutations that are known β-thalassaemic alleles.
It was reported in HbVar database (http://globin.bx.psu.edu/hbvar/menu.html) as a ‘rare variant’ of Moroccan origin, found in association with an alpha-thal 3.7 kb (type I) deletion alpha-2. The phenotype is superimposable to that of a simple carrier of alpha+ thalassaemia [Citation10].
This molecular defect is reported in different databases. In dbSNP Short Genetic Variation (https://www.ncbi.nlm.nih.gov/projects/SNP/) it is reported (rs63750681) with clinical significance ‘with uncertain significance allele’ and MAF/MinorAlleleCount: G = 0.0002/1(1000 Genomes) and G = 0.0003/8 (TOPMED); in the international database ClinVar (rs63750681-https:// www.ncbi.nlm.nih.gov/clinvar/variation/) with ‘uncertain clinical significance’, based on a unique submission (LabCorp Variant Classification Summary – May 2015) and an allele frequency GMAF 0.00020 (G); the Ensemble database reported it as an intron variant with a MAF: < 0.01 (G) (http://www.ensembl.org/Homo_sapiens/Gene/Summary?db=core;g=ENSG00000244734;r=11:5225464-5229395) and, finally, in the Human Gene Mutation Database (http://www.hgmd.cf.ac.uk/ac/all.php) it is reported as a beta-thalassaemia allele (accession number: CR082014) based on a single reference.
Particularly, this nucleotide variation was described in an Algerian woman who was compound heterozygous for the G > C substitution at position −56 as well as the IVS-I-1 G > A β°-thalassaemia mutation. She showed a β-thalassaemia intermedia phenotype [Citation11]. Recently, this defect was observed in a Tunisian girl who was homozygous for the G > C substitution at position −56. She showed a moderate microcytic anaemia, low MCHC, normal ferritin and a borderline value for the HbA2 (3.9%) with normal HbF. Authors reported that the role of this mutation in the expression of the β-globin gene remains uncertain to delineate its responsibility in causing β-thalassaemia phenotype [Citation12].
We performed molecular analysis to evaluate clinical implications of this rare nucleotide change, particularly co-inherited with other known mutations in the globin clusters, in order to conduct an appropriate genetic counselling. In this paper we present the data of eight subjects with simple heterozygosity for HBB:c.−106G > C and two cases of co-inheritance of this nucleotide substitution with other mutations in globin genes. This nucleotide substitution was found in association with an HBB haemoglobin variant and in compound heterozygosity with a β-thalassaemic allele and a rearrangement in α-globin genes cluster.
Patients and methods
The study was approved by the Committee for Ethics of Villa Sofia-Cervello Hospital and performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. Informed consent was obtained from all subjects prior to their inclusion in the study and peripheral venous blood samples were collected in EDTA tubes.
First level analysis
Complete blood cell counts were performed using automatic cell counters (ACT diff Beckman Coulter, Fullerton, CA, USA), while haemoglobin fractions were determined by high-performance liquid chromatography (HPLC) using G8 Tosoh Corporation Shiba, Minato-ku Tokyo, Japan, according to the procedures provided by manufacturer. Serum ferritin levels were measured using a Kriptor immunofluorescent assay (Brahams, Hennigsdorf/Berlin, Germany).
Second level analysis
Genomic DNA was extracted from peripheral blood leukocytes by a modified salting out protocol [Citation13].
The β-globin gene was investigated by sequencing analysis of two amplified fragments, the first of 956 bp from −204 nucleotides to the CAP site (5′-agagatatatcttagagggaggg-3′) to IVS II nucleotide 257 (5′-acaatgataaggcattaagtataatag-3′) and the second of 1121 bp from IVS II nt 361 (5′-ctattatacttaatgccttaacattgt-3′) to + 1849 nucleotides from the Cap site in the 3′ to the Poly-A site(5′-acaatgataaggcattaagtataatag-3′). DNA sequencing reactions were performed on amplified PCR fragments using the same forward and reverse primers as for PCR.
Exo-Sap reaction [United States Biochemical (USB) Corporation, Cleveland, OH, USA] and Dye terminator Kit V3.1 (Applied BioSystems, Foster City, CA, USA) were used; capillary electrophoresis was performed on an ABI PRISMTM 3130 xl automated sequencer (Applied BioSystems). ααα anti 3.7 and ααα anti 4.2 arrangements were investigated by GAP-PCR as previously described protocol [Citation14].
We compared the haematological parameters for each categories (HBB: c.−106G > C in heterozygous state and in co-inheritance with other defects in the globin genes) on the base of 35837 subjects who underwent to screening test for haemoglobinopathies in our Centre from 2003 to now, with or without molecular analysis of globin genes depending on phenotype, and on the base of published data. Particularly, we referred to our data of prevalence of haemoglobinopathies in Sicily, our retrospective study on a cohort of 10205 subjects (8875 carriers of beta-globin gene defects and 1330 carriers of a variant haemoglobin of beta-globin gene) [Citation15] and our retrospective study on a cohort of 3825 subjects carriers of α-thalassaemia and rearrangements in alpha globin genes cluster (Passarello C, personal communication).
Results
A rare nucleotide substitution in the HBB gene, the nucleotide −56 relative to transcription initiation site (NM_000518.4(HBB):c.−106G > C), was identified in ten patients of Italian origin (, cases I-X), ().
Figure 1. DNA sequence analysis (with forward and reverse primers) of PCR-amplified fragments corresponding to G > C substitution at the nucleotide −56 in the promoter region of HBB gene. The arrow indicates the nucleotide substitution at the heterozygous state.
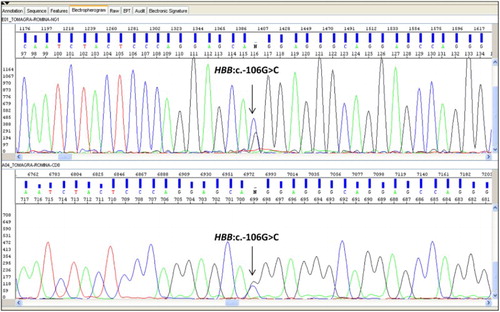
Table 1. Haematological and molecular data of the ten cases of Italian origin with HBB:c.−106G > C.
This nucleotide substitution was found in simple heterozygosity (n = 8), in association with HBB haemoglobin variant HbS [beta 6(A3) Glu > Val (HBB:c.20A > T)] (n = 1) and in compound heterozygosity with beta-thalassaemic allele IVS-I-1 G->A [NM_000518.4 (HBB):c.92 + 1G > A] and ααα anti3.7 rearrangement (n = 1).
i) HBB:c.−106G > C in heterozygous state
Normal or borderline red cell indices, morphology and plasma ferritin levels were observed in eight subjects of Italian origin. HbA2 and HbF resulted within normal or borderline range without abnormal haemoglobin peaks. The HBB:c.−106G > C was identified by direct sequencing of the HBB gene. Molecular analysis excluded any other globin genes mutation. In case VII, the initially observed increased HbA2 value was due to hyperthyroidism problems and HbA2 value normalized after two years of therapy for thyroid disease. In case VIII moderate microcytosis, low MCV and MCH were due to iron deficiency. Additionally in both of these cases molecular analysis excluded any other globin genes mutations (). Furthermore, case III was the father of case II and they were related to case X discussed later (paragraph iii).
ii) HBB:c.−106G > C in heterozygous state co-inherited with HBB haemoglobin variant
One case (patient IX) of Italian origin was observed in association with the HBB haemoglobin variant Hb S beta 6(A3) Glu > Val(HBB:c.20A > T). HPLC revealed an abnormal peak in S window (retention time = 4.30÷4.70 min) and haematological indices were normal. Haematological and molecular findings are summarized in . The observed phenotype was superimposable to that of simple healthy carrier of this variant. This is supported by our data on 958 subjects carrier of HBB:c.20A > T in heterozygous state (on a cohort of 1330 carriers of a variant haemoglobin of beta-globin gene) that pointed out normal haematological indices, an average HbA2 value of (3.8 ± 0.2)% and an average abnormal peak in S window of (38.5 ± 2.5)%.
iii) Compound heterozygotes for HBB: c.−106G > C and other mutations in globin genes
One case of Italian origin (patient X) was observed in compound heterozygosity with the beta-thalassaemic allele IVS-I-1 G->A [NM_000518.4 (HBB):c.92 + 1G > A] and ααα anti3.7 rearrangement. The subject displayed marked microcytosis, low MCV and MCH, an increased level of HbA2 and a low level of haemoglobin. She occasionally needs blood transfusions. The haematological indices and molecular findings are summarized in . The phenotype was superimposable to that of a compound carrier for β°−thalassaemia and ααα anti3.7 rearrangement. This is supported by our data on 58 subjects with the same compound heterozygosity (on a cohort of 3825 subjects carrier of alpha-thalassaemia and rearrangements in alpha globin genes cluster) that pointed out an average MCV value of (59 ± 4)fL, an average MCH value of (19 ± 2)pg, an average Hb value of (10.5 ± 3.0) g/dl and an average HbA2 value of (5.6 ± 0.3)% (Passarello C, personal communication). The patient was the daughter of case III and the sister of case II (). Molecular analysis on her mother (wife of case III and mother of case II and case X) pointed out the ααα anti3.7 rearrangement and the beta-thalassemic allele IVS-I-1 G->A [NM_000518.4 (HBB):c.92 + 1G > A] without the rare nucleotide substitution HBB: c.−106G > C. Consequently, case X inherited the ααα anti3.7 rearrangement and the beta-thalassaemic allele IVS-I-1 G->A of maternal origin and the nucleotide substitution HBB: c.−106G > C of paternal origin. Therefore, the two above alterations in HBB gene were inherited in trans. The mother’s phenotype was superimposable to that of the daughter, with marked microcytosis, low MCV (67 fL) and MCH (20.6 pg), an increased level of HbA2 (5%) and low level of haemoglobin (9 g/dl).
Figure 2. Pedigree of Case X’s family [I-1: father (case III); I-2: mother; II-1: brother (case II); II-2: propositus (case X)]. Black half solid symbols indicate heterozygosity for HBB:c.92 + 1G > A; grey half solid symbols indicates heterozygosity for αααanti 3.7 arrangement; striped half solid symbols indicate heterozygosity for HBB:c.−106G > C; black and grey solid symbol indicates double heterozygosity for HBB:c.92 + 1G > A and ααα anti 3.7 arrangement. Black, grey and striped symbols indicate compound heterozygosity for HBB:c.92 + 1G > A, ααα anti 3.7 arrangement and HBB:c.−106G > C. The propositus is indicated by an arrow.
![Figure 2. Pedigree of Case X’s family [I-1: father (case III); I-2: mother; II-1: brother (case II); II-2: propositus (case X)]. Black half solid symbols indicate heterozygosity for HBB:c.92 + 1G > A; grey half solid symbols indicates heterozygosity for αααanti 3.7 arrangement; striped half solid symbols indicate heterozygosity for HBB:c.−106G > C; black and grey solid symbol indicates double heterozygosity for HBB:c.92 + 1G > A and ααα anti 3.7 arrangement. Black, grey and striped symbols indicate compound heterozygosity for HBB:c.92 + 1G > A, ααα anti 3.7 arrangement and HBB:c.−106G > C. The propositus is indicated by an arrow.](/cms/asset/f381d7a3-8bf7-4825-912e-19f78d6ebf07/yhem_a_1403737_f0002_b.gif)
Discussion
National and regional antenatal screening programmes are now well established in many endemic countries as Sicily and also in no-endemic countries because of the growing incidence of immigrant carriers. The widespread adoption of HPLC has much improved the accuracy of screening through more accurate measurement of HbA2 and Hb F together with numerous PCR-based techniques used for the molecular diagnosis of carrier mutations.
The promoter of the HBB gene contains sequence motifs that are highly conserved during evolution, including two CACCC boxes at nt −105 to −101 and −90 to −86 from the Cap site, the CCAAT box at −76 to −72 and the TATA box at −32 to −26 [Citation16].
Unknown or very rare mutations detected within the promoter region have to be considered in order to understand the possible consequences in transcription. Sequencing analysis is the election method to obtain complete data on gene structure and to correlate specific phenotypic expression with mutations, especially in case of new or very rare mutations in globin genes.
The previously reported data on HBB:c.−106G > C substitution in literature or in international databases are limited and do not elucidate the real clinical significance of this nucleotide substitution, neither in co-heredity with other mutations in globin genes.
In this paper we report a significant number of HBB:c.−106G > C substitution in heterozygous state and in co-heredity with known mutations in the globin clusters which supports the characterization of the clinical significance of this nucleotide substitution and the conduction of an appropriate genetic counselling for couples presumed to be at risk.
Based on our data, this rare substitution can be considered a benign variant. This is supported by a comparative analysis for the haematological parameters for each categories (HBB: c.−106G > C in heterozygous state and in compound heterozygosity with other defects in the β-globin genes).
Eight patients in heterozygous state showed normal haematological and electrophoretic values so it is impossible to distinguish it from a non-carrier subject. The phenotype of patient with compound heterozygosity for HBB: c.−106G > C and Hb S was not associated with phenotypic variations respect to the phenotype of the simple βS-allele carrier. The co-inheritance of HBB: c.−106G > C, the beta-thalassaemic allele IVS-I-1 G->A [NM_000518.4 (HBB):c.92 + 1G > A] and ααα anti3.7 rearrangement, observed in one patient, showed the same phenotype of the compound heterozygosity for β0-thalassaemia and ααα anti3.7 rearrangement.
Conclusion
In conclusion the HBB:c.−106G > C variation was found:
In compound heterozygous state with the IVSI-1 mutation in one beta-thalassaemia intermedia patient (12)
In homozygous state in one patient with borderline HbA2 but confusing haematological indices (13)
In heterozygous state in eight cases presented with normal or borderline parameters (this manuscript)
In compound heterozygosity with HbS in one patient with a typical HbS carrier phenotype (this manuscript)
In compound heterozygosity with the IVSI-1 and double heterozygosity with triplicate alpha globin gene in one patient with a phenotype typical of a double heterozygote for a beta zero thalassaemia mutation and a triplicated alpha globin gene arrangement (this manuscript)
The data presented in this paper as well as the data presented by other groups show that the variant in some cases can be characterized as a silent beta-thal mutation while in other cases as a benign variant. Although the latter is the most convincing, more cases in which the variant will be found in compound heterozygosity with beta-thal mutations should be evaluated in order to definitively determine the significance of this variant. It can be therefore concluded that, with the current data, the significance of this variant is uncertain.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Weatherall DJ. The inherited diseases of hemoglobin are an emerging global health burden. Blood. 2010;115(22):4331–4336. doi: 10.1182/blood-2010-01-251348
- Giardine B, Borg J, Viennas E, et al. Updates of the HbVar database of human hemoglobin variants and thalassemia mutations. Nucleic Acids Res. 2014;42(Database issue):D1063–D1069. doi: 10.1093/nar/gkt911
- Weatherall DJ, Clegg JB. The thalassaemia syndromes. 4th ed.Oxford: Blackwell Scientific Publication; p. 770–780.
- http://www.ithanet.eu
- Giambona A, Vinciguerra M, Cannata M, et al. The genetic heterogeneity of β-globin gene defects in Sicily reflects the historic population migrations of the island. Blood Cells Mol Dis. 2011;46(4):282–287. doi: 10.1016/j.bcmd.2011.01.006
- Kountouris P, Lederer CW, Fanis P, et al. Ithagenes: An interactive database for haemoglobin variations and epidemiology. PLoS One. 2014;9(7):1–10. doi: 10.1371/journal.pone.0103020
- Feng WC, Southwood CM, Bieker JJ. Analyses of beta-thalassemia mutant DNA interactions with erythroid Krüppel-like factor (EKLF), an erythroid cell-specific transcription factor. J Biol Chem. 1994;269(2):1493–1500.
- Nuez B, Michalovich D, Bygrave A, et al. Defective haematopoiesis in fetal liver resulting from inactivation of the EKLF gene. Nature. 1995;375(6529):316–318. doi: 10.1038/375316a0
- Perkins AC, Sharpe AH, Orkin SH. Lethal beta-thalassaemia in mice lacking the erythroid CACCC-transcription factor EKLF. Nature. 1995;375(6529):318–322. doi: 10.1038/375318a0
- Giordano PC. Personal communication. (http://globin.bx.psu.edu)
- Agouti I, Bennani M, Nezri M, et al. Beta-thalassemia intermedia due to two novel mutations in the promoter region of the beta-globin gene. Eur J Haematol. 2008;80(4):346–350. doi: 10.1111/j.1600-0609.2007.01017.x
- Douzi K, Moumni I, Zorai A, et al. Two new β+ -thalassemia mutation [β −56 (G → C); HBBc. −106 G → C] and [β −83 (G → A); HBBc. −133 G → A] described among the Tunisian population. Am J Hum Biol. 2015;27(5):716–719. doi: 10.1002/ajhb.22695
- Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16(3):1215. doi: 10.1093/nar/16.3.1215
- Foglietta E, Deidda G, Graziani B, et al. Detection of α-globin gene disorders by a simple PCR methodology. Haematologica. 1996;81(5):387–396.
- Giambona A, Damiani G, Vinciguerra M, et al. Incidence of haemoglobinopathies in Sicily: the impact of screening and prenatal diagnosis. Int J Clin Pract. 2015;69(10):1129–1138. doi: 10.1111/ijcp.12628
- Cao A, Galanello R. β−thalassemia. Genet Med. 2010;12(2):61–76. doi: 10.1097/GIM.0b013e3181cd68ed
