ABSTRACT
Background: Acute promyelocytic leukemia (APL) is a rare form of acute myelogenous leukemia (AML). Survival rates exceed 80% in developed countries. Successful treatments rely on all-trans retinoic acid with anthracycline-based chemotherapy. Availability of modern care and public knowledge play important roles in pediatric APL survival.
Method: A cytogenetic diagnosis of APL was confirmed in 30 (14.5%) out of 207 children consecutively diagnosed with de novo AML between January 2005 and December 2012 at nine cancer care centers in Saudi Arabia. Patients were treated based on the standard protocol used by the center following the PETHEMA or the C9710 treatment protocols. We modeled 5-year overall survival (OS), event-free survival (EFS) and cumulative incidence of relapse (CIR) vs. treatment and potential covariates of age at diagnosis, involvement of central nervous system (CNS), and white blood cell (WBC) levels.
Results: The median age was 10.4 years with a male:female ratio of 1.9. WBC was 10 × 109/l or greater in 57% and CNS involvement was confirmed in 13%. OS, EFS, and CIR were 74 ± 12%, 55 ± 19%, and, 36 ± 17% respectively. No significant difference was found by treatment protocol. WBC levels were significantly prognostic for all negative events, but treatment with C9710 significantly ameliorated negative WBC effects. Overall outcomes were comparable to those reported in developed countries.
Conclusions: Access to modern care is likely to be a critical factor in successful and comparable outcomes of childhood APL across the globe. In the present study, utilizing a cytarabine-containing protocol improved outcome of high-risk pediatric patients with APL.
1. Introduction
Acute promyelocytic leukemia (APL) is a rare form of acute myelogenous leukemia (AML) particularly in children, accounting for only 5–7% of pediatric AML [Citation1]. However, variation in incidence is reported among children in different geographic areas or across various racial/ethnic groups [Citation1,Citation2]. The incidence of childhood APL in Saudi Arabia has not been reported.
APL is classified as type M3 under the French-American-British classification and by translocation between chromosomes 15 and 17, t(15:17), by the World Health Organization. With currently available treatments, survival rates have reached over 70% worldwide [Citation3–12], and over 80% in some regions [Citation3,Citation5,Citation6,Citation8–12]. The most distinct prognostic feature is association of elevated white blood cell (WBC) count with poor overall outcomes [Citation5,Citation7,Citation10–15]. Early treatments included chemotherapy, such as anthracycline plus cytarabine. Greater understanding of unique molecular features of APL lead to successful introduction of all-trans retinoic acid (ATRA) to induce primary remission, followed by chemotherapy [Citation12]. It was ultimately shown by the Programa Espãnol de Tratamientos en Hematología (PETHEMA) that eliminating all non-anthracycline agents, including cytarabine, did not reduce efficacy of treatment [Citation16]. However, with the current cumulative anthracycline doses, cardiac toxicity remains a real threat for children undergoing treatment for APL [Citation17]. Improved outcomes have been found by substituting arsenic trioxide (ATO) for anthracyclines [Citation3,Citation6,Citation8,Citation9,Citation13,Citation18]. In addition, cytarabine's leucopenic effects have led to its re-adoption to address poor outcomes associated with elevated WBC in protocols such as North American Leukemia Intergroup Protocol C9710, which combines ATO and cytarabine with daunorubicin [Citation8,Citation12,Citation15]. C9710 has shown improved outcomes in terms of overall survival (OS) and event-free survival (EFS).
Outcomes of APL in developing countries are reported to be inferior to those reported from developed countries [Citation19,Citation20]. Recent report of improvements in Latin America has suggested that this may be strongly related to delay of care [Citation19]. As it is a high-income and resource-rich developing country that provides free access to cancer care, we hypothesize that outcomes in Saudi Arabia will be similar to outcomes reported in countries with widely accessible modern cancer care. In addition, the present retrospective study compares efficacy of PETHEMA vs. C9710 in children aged 14 years or less in Saudi Arabia.
2. Methods
2.1. Patients
The study was conducted at nine centers in Saudi Arabia and funded by the Sanad Children's Cancer Support Association. The study was approved by the hospital ethics review committee of each participating institution and was conducted in accordance with the Declaration of Helsinki [Citation21]. Children aged 14 years or younger consecutively diagnosed with APL between January 2005 and December 2012 in any of the participating centers who also had genetic confirmation of the t(15;17)/PML-RARA fusion were eligible. Patients with a clinical diagnosis of APL but no genetic confirmation were excluded from the analysis. Data were collected using Research Electronic Data Capture (REDCap) tools hosted and stored in a secure Microsoft SQL database at SAPHOS central office [Citation22]. Data collected included: age, gender, WBC count at diagnosis, central nervous system (CNS) status, immunophenotype, genetic abnormalities, treatment protocol, response, events, and vital status at last follow up date.
2.2. Study design
The study was a retrospective multicenter collaborative study. Our primary objective was to describe clinical characteristics and survival outcomes of children with APL, treated according to two standard APL treatment protocols, specifically ‘PETHEMA’ [Citation10] and ‘C9710’ [Citation8]. Briefly, in the PETHEMA regimen we utilized idarubicin and ATRA without cytarabine for remission induction, anthracycline with ATRA during consolidation, and ATRA, methotrexate and mercaptopurine during maintenance. While in the standard arm of the C9710 regimen, ATRA in conjunction with standard-dose cytarabine and daunorubicin was used for remission induction followed by two cycles of ATRA and daunorubicin for consolidation and intermittent ATRA plus mercaptopurine and methotrexate chemotherapy during maintenance. The ATRA dose used was 25 mg/m2/day in both protocols, and the cumulative anthracycline equivalent dose ranged from 650 to 750 mg/m2 in the PETHEMA protocol and 400 mg/m2 in the C9710 protocol. Subjects were assigned to treatments based on the standard protocol used in each center. Events of remission, relapse, and death were recorded.
Treatment of relapsed APL was not standardized among centers. However, all centers used ATO-based therapy (with or without all-trans-retinoic acid) at a dose of 0.15 mg/kg/day intravenous infusion until second complete remission (CR2) or a maximum of 60 days. Patients who achieved CR2 either continued to receive further courses of ATO at the same dose for 5 weeks on a daily basis on 5 days each week with 2–4-week interval rest in between courses or were offered allogeneic hematopoietic stem cell transplant from a matched-related donor.
2.3. Statistical methods
We chose 5-year OS, defined as survival from the date of diagnosis to the last follow up date or death from any cause; 5-year EFS, defined as survival from the date of diagnosis to the last follow up date or the occurrence of the first event (relapse, refractory APL, or death from any cause); 5-year cumulative incidence of relapse (CIR), and early death (death up to 14 days after diagnosis) as our outcomes of interest. We used Cox proportional hazards models when possible to test outcome associations with protocol or covariates. In some cases, Cox models could not be run because no recipients of a treatment suffered the event in question (specifically death or early death) during the study period. In those cases, we used rank tests of the Kaplan–Meier estimate. We repeated models with a random intercept for study centers. We tested proportional hazards assumptions for all Cox models. Since survival analysis defines ‘events’ as negative outcomes, such as death, and right-censors subjects who did not suffer an ‘event’, OS and EFS are inversions of events counted. All statistics were performed with the R statistical environment [Citation23].
3. Results
3.1. Patient demographics
Patient demographics and baseline characteristics are summarized in . A total of 207 children were diagnosed with de novo AML and had complete data. Of these, 32 (15%) had a clinical diagnosis of APL. Of the 32 enrolled patients with APL, 30 had the requisite t(15;17) molecular abnormality. The remaining two patients were excluded. Male gender was predominant (77%) among study subjects. Age at diagnosis was less than10 years in 47% of subjects and the median age at diagnosis was 10.4 years. The WBC was greater or equal to 10 × 109/l in 57% of patients. Thus, 57% of patients in our cohort were high risk based on WBC at diagnosis.
Table 1. Demographic and clinical characteristics of study subjects.
3.2. Study outcomes and treatment responses
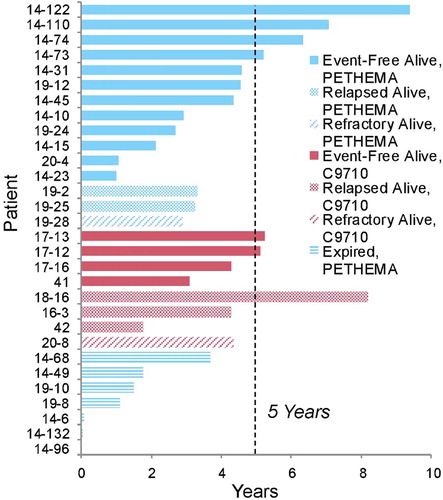
The 5-year OS and EFS were 74 ± 12% and 55 ± 19%, respectively. Complete remission was achieved in 92.8% of evaluable patients. CIR was 36 ± 17%, and early deaths were 7 ± 5%. Two subjects failed to achieve a complete remission. All study subjects’ outcomes are presented in . A total of seven patients died during the study. All deaths occurred before 4 years from the date of diagnosis. Of the surviving patients five suffered a relapse event, of which three were treated with the C9710 and two with the PETHEMA protocol. A total of eight patients relapsed, of which five attained CR2. Of those, three were treated with matched-related hematopoietic stem cell transplant and the remainder continued on ATO therapy only. Of the relapsed patients, five were alive and off therapy in CR2 at the last follow up date and three died on therapy not in CR2.
Figure 1. Patient disposition by time across entire study. Patients are grouped by treatment Protocol (PETHEMA vs. C9710) and outcome (EFS, death, relapse, or survival to last follow up date). Participation is shown from day of diagnosis to last day of follow up for the patient. Time of study participation is shown in years. Vertical dashed line indicates 5-year threshold for each patient from time of diagnosis.
3.3. Protocol had no independent significant primary effect on outcomes of interest
Outcomes are summarized in . While C9710 treatment was associated with no deaths and no early deaths ((A,B)), neither outcome was significant by Kaplan–Meir estimate (p = 0.066 and 0.388, respectively). Odds ratio of OS associated with C9710 over PETHEMA was 8.226, and for early death associated with C9710 was 0.482. Owing to zero counts, a pseudocount of 0.5 was added to each cell to estimate OR for OS and early death. Relapse and EFS were tested by univariate Cox models. No discernable difference was found for relapse ((C)): OR = 1.067, p = 0.930. C9710 favored EFS, OR 1.476 ((E)), but this was not significant (p = 0.563). Potential random effect of study center was assessed by anova of fixed-effect only models vs. models with random intercepts by study center. No models had significant study center effects.
Figure 2. Effects of treatment Protocols on four outcome measures as independent effects and in interaction with WBC. (A, C, E) Independent effects of treatment Protocol on outcome measures of (A) OS, (C) Relapse, and (E) EFS were modeled by Kaplan–Meier followed by G-ρ (OS) or Cox proportional hazards model (Relapse and EFS). Odds ratios and p values are reported. (B, D, F) Interaction of WBC counts and treatment Protocol on outcome measures. Effects of interaction between WBC and treatment Protocol on outcome measures of (B) OS, (D) Relapse, and (F) EFS were modeled by Cox proportional hazards model of Outcome ∼ Protocol + WBC + (Protocol × WBC). OS and EFS were modeled vs. their inverses (Death or any ‘event’, defined as death, relapse, or refractory APL, respectively). Survivals are presented according to three WBC values by Protocol, 25th percentile (long-dashed line), median WBC (solid line), and 75th percentile WBC (short-dash line). Odds ratios and p values are reported.
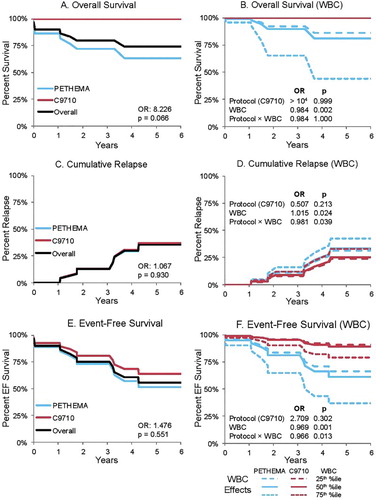
Table 2. Univariate analysis outcomes for protocol.
3.4. Covariate analysis revealed significant interactions between protocol and WBC
Cox models were generated to test Protocol against the covariates of age at diagnosis (AaD), stratified AaD (above or below 10th birthday), WBC count, stratified WBC (above or below 10), whether or not the CNS was involved, and patient gender. Mixed-level models were also built to test random study center effects. No models had significant study center effects. Further analysis was done without study center as a random variable. Of covariates tested, only WBC showed significant (p ≤ 0.05) results as a primary effect or in interaction with Protocol (). Outcomes are summarized in . OS ((B)) showed a significant effect vs. WBC (OR 0.984, p = 0.002), where higher WBC associated with lower OS. No significant interaction occurred between WBC and Protocol for OS. Early death showed no significant associations.
Table 3. Outcomes compared by Protocol and WBC as covariates.
Relapse ((D)) and EFS ((F)) had significant interactions between Protocol and WBC. Higher WBC was significantly associated with higher relapse (OR 1.015, p = 0.024). Interaction between Protocol and WBC was significant, specifically; C9710 reduced the effect of higher WBC (OR 0.981, p = 0.039). Interaction with protocol did not have a significant effect, although C9710 was associated with lower overall relapse (OR 0.507, p = 0.213). Lower WBC was associated with greater rates of EFS (OR 0.969, p = 0.001). In short, WBC appeared to have been a significant independent prognostic factor: Higher WBC significantly associated with greater risk of negative events. Interaction between Protocol and WBC was significant (OR 0.966, p = 0.013), C9710 again ameliorated negative effects of higher WBC. Protocol primary effect on EFS was sizeable but not significant (OR 2.709, p = 0.302). Independence of Protocol and WBC from each other was tested by Spearman's rank correlation, ρ = −0.026 (C9710 = 1, PETHEMA = 0, vs. rank WBC). This was taken to indicate that WBC was not collinear with Protocol. Had Protocol and WBC been found to have collinearity, any interaction found by Cox models would have been attributed to collinear effects.
4. Discussion
We conducted a retrospective study of childhood patients with APL, diagnosed and treated at the major childhood cancer centers in Saudi Arabia. Childhood APL accounted for approximately 15% of all de novo AML cases in children aged 14 years or younger in Saudi Arabia. This frequency is higher than what has been reported from North America and Europe, but lower than what has been reported from Central and South America, China, and low-income countries such as Iraq [Citation1]. The median age at diagnosis in the present study was 10.4 years with male gender predominance and approximately half of patients had a high presenting WBC count. Thus, except for variability in gender frequency in our region, the demographic and clinical features were similar to reports from other regions [Citation1].
We found no independent significant differences between PETHEMA and C9710 for any of our outcome measures. Nevertheless, no patients treated with the C9710 protocol died, vs. seven deaths with the PETHEMA protocol. A similar (and non-significant) effect for C9710 has previously been reported [Citation8]. We lack sufficient mechanistic data to speculate on causes, but it has been noted elsewhere that reducing anthracycline results in reduction of toxic effects of treatment without loss of efficacy against APL [Citation3,Citation18]. While our outcome for OS was non-significant, we suggest this may be due to sample size as Post hoc power analysis supports this likelihood and a hypothetical sample size of 64 was predicted [Citation24]. Owing to the rarity of childhood APL, randomized studies are difficult to conduct and would require multinational collaborative efforts.
On the other hand we did determine that WBC exerted a significant independent effect and elevated WBC levels were prognostic for all adverse effects, including death, which agrees with the consensus in the field [Citation5,Citation7,Citation10–15]. The C9710 protocol may significantly ameliorate this effect. We interpret this as indicating cytarabine (in C9710) may have a beneficial effect in pediatric patients with APL and high WBC.
The early death rate in the present study was 7%, which was similar to the 3–7% early death range reported in pediatric APL by other groups [Citation1]. This reflects the success of early access to modern care for children with APL in Saudi Arabia. Similarly, the CR and OS rates were comparable in the present study to those reported by other groups () [Citation1]. However, EFS rates were lower than what has been published elsewhere, specifically 55% in our study vs. >70% in many others [Citation3,Citation4,Citation6,Citation7,Citation9–12,Citation25,Citation26]. Our CIR was 36%, which was notably higher than that reported in other studies [Citation4,Citation6,Citation10,Citation12,Citation25,Citation26]. Compared to reports by other groups, childhood APL in our study was more frequently associated with high WBC. In the present study, 57% of patients were high risk compared to the 35–48% range reported in other studies [Citation1,Citation11]. Thus the inferior EFS outcomes are likely related to the fact that a relatively higher proportion of patients in the present study were high risk (WBC ≥ 10).
Table 4. Selected review of childhood APL treatment studies.
While approximately half of the subjects were less than 10 years of age at diagnosis, age at diagnosis had no significant impact on any outcomes in the present study. This is in contrast to another report that indicated a relationship between age and poor outcome [Citation14]. The authors of that study suggested their results might be due to possible delays in administration of care in their sample set. Our lack of an age-related finding may be due to differences in availability of care.
The largest Pediatric APL series to date are from Italy (the AIDA 0493 trial) [Citation11], China (comparing four protocols) [Citation4], and the U.S.A. (preliminary report) [Citation25]. In the Italian study, 107 patients had 10 year EFS of 76% and a 10 year OS of 89% [Citation2,Citation11]. The more recent Chinese study of 119 patients reported 3.5 year EFS of 78.5%. The US study of 101 patients reported 2-year OS of 94% and EFS of 92%. PETHEMA pediatric results from two consecutive trials showed 5-year EFS of 77% and 5-year OS of 87% [Citation10]. This was achieved with anthracyclines and ATRA for induction and consolidation without other agents. A smaller French study showed comparable overall results using daunorubicin, cytarabine, and ATRA [Citation12]. Only limited pediatric data from the last North American intergroup trial C9710 have been released at this point. The CR rate, 3-year EFS and 3-year OS for the 57 eligible patients less than 15 years of age on this trial were 89, 62, and 86%, respectively. Only two of these 57 children were treated on the arsenic arm of the trial. There were no statistically significant differences in these figures compared to the adults who were randomized to the standard therapy arm without arsenic [Citation8]. Other studies, including more recent work, show that OS and EFS have not markedly improved, whether the clinical populations were from China [Citation3], Western Europe [Citation5], Korea [Citation7], or North America [Citation8,Citation25]. Our outcome rates were comparable to other reports, indicating that effective availability of modern treatment and population awareness, as has been shown in Latin America [Citation19], that spell the greatest difference in patient survival of APL.
As previously noted, if a pediatric patient with APL receives treatment, the most commonly reported predictor of adverse events is elevated WBC, including in our study. In addition, multiple studies, including our own, have reported greater OS for protocols that reduced anthracycline and included cytarabine. We suggest that sufficient data exists from multiple groups and countries that these practices ought to be more widely adopted. We also suggest that greater international cooperation in a retrospective study of APL treatments would provide far more statistical power to determine significance of differences in outcomes and directly compare, rather than infer, country-specific differences in both treatment preferences and outcomes. Likewise, more international-scale studies would permit more robust design of randomized trials.
Acknowledgments
The authors wish to acknowledge the staffs and KAMC/National Guard-Jeddah, KAMC/National Guard-Riyadh, KSU-Riyadh, KFMC-Riyadh, KFSH-Dammam, KFSHRC-Jeddah, KFSHRC-Riyadh, Military Hospital-Riyadh, and PFOC-Buraidah hospitals for their cooperation with this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Walid Ballourah http://orcid.org/0000-0002-2936-142X
Additional information
Funding
References
- Testi AM, D’Angio M, Locatelli F, et al. Acute promyelocytic leukemia (APL): comparison between children and adults. Mediterr J Hematol Infect Dis. 2014;6(1):e2014032. doi: 10.4084/mjhid.2014.032
- Maule MM, Dama E, Mosso ML, et al. High incidence of acute promyelocytic leukemia in children in northwest Italy, 1980-2003: a report from the childhood cancer registry of piedmont. Leukemia. 2008;22(2):439–441. doi: 10.1038/sj.leu.2404916
- Long ZJ, Hu Y, Li XD, et al. ATO/ATRA/anthracycline-chemotherapy sequential consolidation achieves long-term efficacy in primary acute promyelocytic leukemia. PLoS ONE. 2014;9(8):e104610. doi: 10.1371/journal.pone.0104610
- Li EQ, Xu L, Zhang ZQ, et al. Retrospective analysis of 119 cases of pediatric acute promyelocytic leukemia: comparisons of four treatment regimes. Exp Ther Med. 2012;4(1):93–98. doi: 10.3892/etm.2012.546
- Bally C, Fadlallah J, Leverger G, et al. Outcome of acute promyelocytic leukemia (APL) in children and adolescents: an analysis in two consecutive trials of the European APL Group. J Clin Oncol. 2012;30(14):1641–1646. doi: 10.1200/JCO.2011.38.4560
- Wang H, Hao L, Wang X, et al. Retrospective study of arsenic trioxide for childhood acute promyelocytic leukemia in China: a single-center experience. Int J Hematol. 2010;91(5):820–825. doi: 10.1007/s12185-010-0575-z
- Kim MH, Choi CS, Lee JW, et al. Outcome of childhood acute promyelocytic leukemia treated using a modified AIDA protocol. Korean J Hematol. 2010;45(4):236–241. doi: 10.5045/kjh.2010.45.4.236
- Powell BL, Moser B, Stock W, et al. Arsenic trioxide improves event-free and overall survival for adults with acute promyelocytic leukemia: North American leukemia intergroup study C9710. Blood. 2010;116(19):3751–3757. doi: 10.1182/blood-2010-02-269621
- Zhang L, Zhao H, Zhu X, et al. Retrospective analysis of 65 Chinese children with acute promyelocytic leukemia: a single center experience. Pediatr Blood Cancer. 2008;51(2):210–215. doi: 10.1002/pbc.21510
- Ortega JJ, Madero L, Martin G, et al. Treatment with all- trans retinoic acid and anthracycline monochemotherapy for children with acute promyelocytic leukemia: a multicenter study by the PETHEMA Group. J Clin Oncol. 2005;23(30):7632–7640. doi: 10.1200/JCO.2005.01.3359
- Testi AM, Biondi A, Lo Coco F, et al. GIMEMA-AIEOPAIDA protocol for the treatment of newly diagnosed acute promyelocytic leukemia (APL) in children. Blood. 2005;106(2):447–453. doi: 10.1182/blood-2004-05-1971
- de Botton S, Coiteux V, Chevret S, et al. Outcome of childhood acute promyelocytic leukemia with all-trans-retinoic acid and chemotherapy. J Clin Oncol. 2004;22(8):1404–1412. doi: 10.1200/JCO.2004.09.008
- Testa U, Lo-Coco F. Prognostic factors in acute promyelocytic leukemia: strategies to define high-risk patients. Ann Hematol. 2016;95(5):673–680. doi: 10.1007/s00277-016-2622-1
- Farah RA, Horkos JG, Bustros YD, et al. A multicenter experience from Lebanon in childhood and adolescent acute myeloid leukemia: high rate of early death in childhood acute promyelocytic leukemia. Mediterr J Hematol Infect Dis. 2014;7(1):e2015012. doi: 10.4084/mjhid.2015.012
- Shepshelovich D, Edel Y, Goldvaser H, et al. Pharmacodynamics of cytarabine induced leucopenia: a retrospective cohort study. Br J Clin Pharmacol. 2015;79(4):685–691. doi: 10.1111/bcp.12530
- Sanz MA, Martin G, Rayon C, et al. A modified AIDA protocol with anthracycline-based consolidation results in high antileukemic efficacy and reduced toxicity in newly diagnosed PML/RARalpha-positive acute promyelocytic leukemia. PETHEMA group. Blood. 1999;94(9):3015–3021.
- Montesinos P, Gonzalez JD, Gonzalez J, et al. Therapy-related myeloid neoplasms in patients with acute promyelocytic leukemia treated with all-trans-retinoic acid and anthracycline-based chemotherapy. J Clin Oncol. 2010;28(24):3872–3879. doi: 10.1200/JCO.2010.29.2268
- Cull EH, Altman JK. Contemporary treatment of APL. Curr Hematol Malig Rep. 2014;9(2):193–201. doi: 10.1007/s11899-014-0205-6
- Correa de Araujo Koury L, Ganser A, Berliner N, et al. Treating acute promyelocytic leukaemia in Latin America: lessons from the international consortium on acute leukaemia experience. Br J Haematol. 2017;177(6):979–983. doi: 10.1111/bjh.14589
- Hassan IB, Zaabi MRA, Alam A, et al. Characteristics features and factors influencing early death in acute promyelocytic leukemia; experience from United Arab Emirates (UAE). Int J Hematol. 2017;106(1):90–98. doi: 10.1007/s12185-017-2211-7
- World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053
- Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010
- R Core Team. 2017 29 May 2017. R: a language and environment for statistical computing. R Foundation for Statistical Computing. [cited May 2017]. Available from: https://www.R-project.org/
- Chow S, Shao J HW. Sample size calculations in clinical research. Boca Raton (FL): Chapman & Hall/CRC Biostatistics; 2008.
- Kutny MA, Alonzo TA, Gerbing RB, et al. Results of a phase III trial including arsenic trioxide consolidation for pediatric patients with acute promyelocytic leukemia (APL): a report from the Children’s Oncology Group study AAML0631. Blood. 2015;126:219.
- Imaizumi M, Tawa A, Hanada R, et al. Prospective study of a therapeutic regimen with all-trans retinoic acid and anthracyclines in combination of cytarabine in children with acute promyelocytic leukaemia: the Japanese childhood acute myeloid leukaemia cooperative study. Br J Haematol. 2011;152(1):89–98. doi: 10.1111/j.1365-2141.2010.08332.x
