ABSTRACT
Background: Iron deficiency without anemia has been associated with decreases in functional work capacity and fatigue. The aim of this study was to determine the prevalence of iron deficiency without anemia in a preoperative cohort of orthopedic patients and to determine if iron deficiency is a condition which warrants inclusion into a prehabilitation program prior to surgery.
Methods: One hundred consecutive patients going through preoperative testing for total joint replacement were enrolled in the study. In addition to the standard preoperative complete blood count, a ferritin concentration was also measured before the patients had their surgeries performed. Iron deficiency was defined as a ferritin concentration <50. Patients were then followed for 30 days following their surgical procedure to determine if any complications ensued, or if red blood cell transfusions were required.
Results: The average age of the patients in this study was 65.2 ± 11.8 years and there were 41/100 males. Overall, 16 patients had anemia of which 6 patients also had low ferritin levels. Twenty-two other patients had ferritin levels consistent with iron deficiency but were not anemic. There was no association between iron deficiency and length of stay, complications, and transfusion.
Discussion: No association was found between simple iron deficiency and any perioperative complications. Only a ferritin level was assessed so other measures of iron deficiency may have revealed a higher prevalence of iron deficiency. A study with a larger sample size is warranted in order to further understand iron deficiency in a preoperative setting.
Introduction
There is abundant literature on the correlation between anemic patients undergoing surgery and poorer surgical outcomes relative to those without anemia, however, the prevalence of iron deficiency without anemia in a pre-surgical patient and whether it impacts surgical outcome has not been studied. The aim of this study was to determine the iron status of preoperative patients scheduled for orthopedic procedures and to collect preliminary data on the impact of preoperative iron stores on surgical outcomes. This may be an issue that should be addressed during the preoperative process as part of a prehabilitation program.
Prehabilitation has become a central concept in the redesign of the perioperative process. The concept involves improving an individual’s functional capacity through diet, exercise, and tobacco cessation prior to a scheduled surgical procedure with the goal of improving the overall outcome from the significant physiologic stress of surgery [Citation1,Citation2].
Iron deficiency is the most common micronutrient deficiency in the world [Citation3]. According to the World Health Organization (WHO) and Centers for Disease Control and Prevention (CDC), the importance of iron deficiency even in the absence of anemia has been overlooked and it should be considered a significant and common health problem [Citation4]. Recently, iron deficiency has been associated with poor functional capacity and quality of life in patients with chronic heart failure [Citation5]. Correction of this iron deficiency with intravenous iron has been shown to improve exercise tolerance in these patients [Citation6]. Fixing the iron deficiency also reduced hospitalizations from heart failure decompensation [Citation7].
Iron is needed for many important biochemical functions in the human body. When iron is deficient, it can affect a person’s energy and work capacity, erythropoiesis, and immune function [Citation8–10]. Furthermore, iron deficiency is associated with weakened cell-mediated and innate immunity [Citation11]. Iron deficiency has been shown to increase postoperative infection rates in abdominal surgery [Citation12]. With this background, we hypothesized that iron deficiency could be a factor that impairs a patient’s ability to recover after surgery. Impairment of work capacity could prevent adequate engagement in physical therapy following a joint replacement. Impaired immune function might increase postoperative wound infections, which have a sizable impact on functional status and recovery. Furthermore, blood loss is common in orthopedic surgeries, which could exacerbate an underlying iron deficiency problem.
Iron deficiency without anemia has been associated with delays in the regeneration of red cell mass after blood donation [Citation13]. This delay in red cell mass generation generally takes place when ferritin levels are <50 ng/ml. Likewise, iron deficiency is defined as a ferritin concentration of <50 ng/ml in patients suffering from restless leg syndrome [Citation14,Citation15]. Ferritin levels less than 30 ng/ml were associated with higher risk of heart failure [Citation16]. In surgery, inadequate stores of iron represented by a ferritin < 100 ng/ml, interfere with the bone marrows ability to sustain erythropoiesis after surgery. Munoz defined an absolute iron deficiency as <30 ng/ml and inadequate iron stores as <100 ng/ml in patients at high risk of developing postoperative anemia [Citation17]. Given that no standard has been established of a ferritin level associated with poor surgical outcome, a definition of a ferritin < 50 ng/ml was used for the purposes of this study.
Materials and methods
The patient population for this study was those that were scheduled for a total joint replacement at a single hospital with four orthopedic surgeons. As a routine prior to surgery, these patients were seen in a preoperative evaluation clinic where samples for a complete blood count (CBC) and a metabolic panel were drawn. From the blood drawn for the metabolic panel, a ferritin test was also performed as part of this study. Because the blood had already been drawn and the patient did not need an additional blood draw for this study, the need for informed consent was waived by the University of Pittsburgh’s Quality Improvement Council, a division of the Institutional Review Board.
The data that were collected for this study included the patients’ basic demographic information (American Society of Anesthesiology (ASA) classification, age, gender, height, weight, and reason for surgery) as recorded in their charts during the preoperative visit, along with the results of the ferritin and CBC tests. The patients were then followed for 30 days following surgery to assess their rates of requiring an allogeneic red blood cell (RBC) transfusion, readmission, and any morbidity or mortality.
The CBC was performed on a Beckman Coulter DxH 800 CBC machine (Brea, CA, USA). Ferritin levels were assessed using a two-site immunoenzymatic assay on a Beckman Coulter UniCel DxI 800 machine (Brea, CA, USA).
Data analysis and definition of iron deficiency
A sample size calculation was performed prior to collecting patient samples. Based on an assumed prevalence of iron deficiency without anemia of 4%, the assumed sensitivity and specificity of a ferritin level of 98% [Citation18], a confidence level of 0.95 and a precision of 0.05, the required sample size was estimated to be 92 patients. An additional 10% was added to this sample size in order to account for any surgical cancelations or delays.
The patients were divided into two groups based on their preoperative ferritin level; <50 and ≥50 ng/ml. Descriptive statistics were performed on the demographic data. Unpaired-t-tests were used to compare the data points between groups, while a Fisher’s exact test was used to compare the incidence of transfusion and readmission between these two groups. All statistical testing was performed using Prism version 6.0 for Windows (GraphPad, La Jolla, CA, USA).
Results
Between February through April 2016, data were collected on a total of 105 consecutive patients scheduled to have joint replacement surgery at a single regional referral center. Five patients were censored from the data set either because they did not have surgery, or a ferritin level was not measured. Of the 100 patients in the analysis, there were 41 males and 59 females, and the mean age (years), height (cm), and weight (kg) of these 100 patients were 65.2 ± 11.8 years, 169.2 ± 10.9 cm, 88.9 ± 22.9 kg, respectively. There were 51 total knee arthroplasties, 34 total hip arthroplasties, 8 revision total knee arthroplasties, and 7 revision total hip arthroplasties. Osteoarthritis was the main reason for surgery (n = 76 patients). For revision procedures, there were 11 patients with prosthetic failure and/or intractable pain, and 4 with infected prostheses.
The age, ASA ranking, height, and weight categories were divided into groups by the preoperative ferritin levels of <50 and ≥50 ng/ml (). The mean ferritin levels for the two groups were 26.96 ± 9.88 and 117.20 ± 90.90, respectively (p < 0.0001). The normal range for ferritin in this hospital’s laboratory is 10–282 ng/ml. The mean height of the patients in the ferritin <50 ng/ml group was significantly lower than those in the ferritin ≥50 ng/ml group, most likely because of the greater proportion of females in the former group. When the patients in the <50 ng/ml ferritin group were analyzed by age and gender, there was a steady increase in iron deficiency with advancing age ().
Figure 1. Number of patients who were iron deficient, defined as ferritin concentration <50 ng/ml, stratified by age and sex.
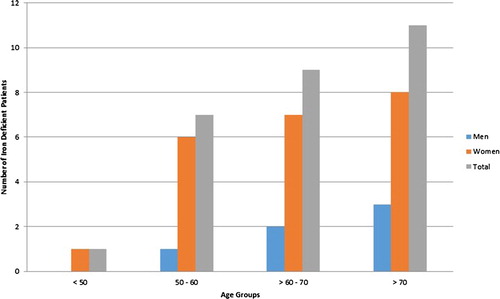
Table 1. Patient demographics of those in the ferritin < 50 ng/ml group and the ferritin ≥ 50 ng/ml group.
When evaluating the CBC data from the two groups, there was a significantly lower hemoglobin (Hgb) concentration and a trend towards a smaller MCV, in the ferritin <50 ng/ml group (). Seventeen patients met the World Health Organization definition of anemia [Citation19] (<12 g/dl for women; <13 g/dl for men). Of these 17 patients, 6 (35%) had ferritin levels less than 50 ng/ml.
Table 2. Laboratory values for the patients in the <50 ng/ml ferritin and ≥50 ng/ml ferritin groups.
There were three patients who were transfused postoperatively. Two of the transfused patients underwent a primary joint replacement and both were anemic prior to their surgery. One of these patients was readmitted to the hospital after discharge because of a pulmonary embolus, from which he ultimately died. The third transfused patient was not anemic preoperatively and underwent a revision total knee replacement, which is a procedure in which the blood loss tends to be greater than in a primary joint replacement. There were 12 patients with some form of postoperative complication. The average ferritin levels for the patients with complications was 68 ± 47 ng/ml, whereas patients without complications had ferritin levels of 96 ± 96 ng/ml (p = 0.21). When comparing non-anemic patients with ferritin < 50 to non-anemic patients with ferritin levels ≥50 ng/ml, there was no difference in complication rates (p = 0.29).
demonstrates the 100 study patients arranged by the four possible combinations of Hgb and ferritin. There were 22/100 (22%) patients who were not anemic but who had low iron stores as manifested by a low ferritin level. Three of these patients (13.6%) had complications following surgery but none required a transfusion, likely owing to their normal preoperative Hgb level. Of note, the patients with iron deficiency and anemia had the highest platelet counts of all four groups.
Table 3. Comparison of iron deficiency anemia, iron deficiency, and non-iron deficient anemia.
Discussion
Data on 100 consecutive patients scheduled for a total joint replacement surgery were assessed to evaluate the prevalence of iron deficiency. A ferritin level of <50 ng/ml was chosen as an indicator of iron deficiency as it has been demonstrated to be a marker of depleted iron stores [Citation20–23]. The prevalence of iron deficiency, based on this definition, was 28% in this cohort of patients with females comprising the majority (78.6%) of the iron deficient patients. Anemia was present in 16/100 (16%) of these patients. Overall, 6/16 (37.5%) of the anemic patients were iron deficient.
The significance of iron deficiency without anemia has been recognized for some time in the blood donor population. Repetitive donation eventually leads to depletion of iron stores and ultimately to iron deficiency anemia [Citation24]. Iron deficiency, by itself, can lead to changes in immune function, energy metabolism, work performance, and neurological function. One of the aims of this study was to determine if iron deficiency without anemia should be addressed preoperatively in the same way that preoperative anemia should be resolved before major surgery. Changes in immune function, energy metabolism, and work performance could all impact the recovery from a total joint replacement.
Though the sample size is small, no evidence of hospital readmission, overall complications or requirement for transfusion was seen in association with preoperative iron deficiency. Complications involving quality of life measures such as the patients’ subjective feelings of energy level and emotional well-being were not evaluated in this study. Previous studies have demonstrated that iron deficiency slows the return of the hemoglobin mass following blood donation [Citation13]. While return of the red cell mass was not evaluated in this study, it could possibly diminish the energy levels necessary to participate in physical therapy. Further work is needed to explore this aspect of iron deficiency in perioperative patients.
From this study, it was noted that iron deficiency was more common in women and in association with aging. Iron deficiency is most commonly associated with pregnancy, menstruation, and in children who are rapidly growing or who are fed cow’s milk. That this association was present was somewhat surprising given that the average age of the patients in this study was 65.2 ± 11.8 years old, so the traditional iron deficient groups were not part of this cohort.
This study has several limitations. First was the use of ferritin concentrations without other measures of iron stores. Ferritin was measured as a surrogate of iron stores because it has been well studied, and because discarded patient samples could be easily used for its measurement. While a low ferritin level typically indicates iron deficiency, ferritin is an acute phase reactant and can be elevated in the presence of iron deficiency when there is concurrent inflammation. Other measures of iron stores, such as total iron binding capacity and transferrin levels, would be necessary to more precisely identify the patients whose anemia was caused by iron deficiency. Given that a high percentage of these patients were having operations because of arthritis, it is very possible that a segment of the patient population that was iron deficient was missed because of an acute phase elevation in the ferritin concentration.
Conclusion
In this population of surgical patients, the prevalence of iron deficiency without anemia was higher than that of iron deficiency anemia. Further questions remain as to whether this is a problem that warrants preoperative screening and treatment.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Carli F, Zavorsky GS. Optimizing functional exercise capacity in the elderly surgical population. Curr Opin Clin Nutr Metab Care. 2005;8:23–32. doi: 10.1097/00075197-200501000-00005
- Gillis C, Li C, Lee L, et al. Prehabilitation versus rehabilitation a randomized control trial in patients undergoing colorectal resection for cancer. Anesthesiology. 2014;121:937–947. doi: 10.1097/ALN.0000000000000393
- Oppenheimer SJ. Iron and its relation to immunity and infectious disease. J Nutr. 2001;131:616S–635S. doi: 10.1093/jn/131.2.616S
- World Health Organization, Centers for Disease Control and Prevention. Assessing the iron status of populations second edition, including literature reviews: report of a joint world health organization/centers for disease control and prevention technical consultation on the assessment of iron status at the population level. 2007;1–108.
- Pozzo J, Fournier P, Delmas C, et al. Absolute iron deficiency without anaemia in patients with chronic systolic heart failure is associated with poorer functional capacity. Arch Cardiovasc Dis. 2017;110:99–105. doi: 10.1016/j.acvd.2016.06.003
- Okonko DO, Grzeslo A, Witkowski T, et al. Effect of intravenous iron sucrose on exercise tolerance in anemic and nonanemic patients with symptomatic chronic heart failure and iron deficiency FERRIC-HF: a randomized, controlled, observer-blinded trial. J Am Coll Cardiol. 2008;51:103–112. doi: 10.1016/j.jacc.2007.09.036
- Manito N, Cerqueiro JM, Comin-Colet J, et al. Consensus document of the Spanish Society of Cardiology and the Spanish Society of Internal Medicine on the diagnosis and treatment of iron deficiency in heart failure. Rev Clin Esp. 2017;217:35–45. doi: 10.1016/j.rce.2016.08.001
- Haas JD, Brownlie IV T. Iron deficiency and reduced work capacity: a critical review of the research to determine a causal relationship. J Nutr. 2001;131:676S–690S. doi: 10.1093/jn/131.2.676S
- Bisbe E, Castillo J, Sàez M, et al. Prevalence of preoperative anemia and hematinic deficiencies in patients scheduled for elective major orthopedic surgery. Transfus Altern Transfus Med. 2008;10:166–173. doi: 10.1111/j.1778-428X.2008.00118.x
- Looker AC, Dallman PR, Carroll MD, et al. Prevalence of iron deficiency in the United States. JAMA. 1997;277:973–976. doi: 10.1001/jama.1997.03540360041028
- Ahluwalia N, Sun J, Krause D, et al. Immune function is impaired in iron-deficient, homebound, older women. Am J Clin Nutr. 2004;79:516–521. doi: 10.1093/ajcn/79.3.516
- Harju E. Empty iron stores as a significant risk factor in abdominal surgery. JPEN J Parenter Enteral Nutr. 1988;12:282–285. doi: 10.1177/0148607188012003282
- Kiss JE, Brambilla D, Glynn SA, et al. Oral iron supplementation after blood donation: a randomized clinical trial. JAMA. 2015;313:575–583. doi: 10.1001/jama.2015.119
- Allen RP, Earley CJ. The role of iron in restless legs syndrome. Mov Disord. 2007;22:S440–S448. doi: 10.1002/mds.21607
- Lee CS, Lee SD, Kang SH, et al. Comparison of the efficacies of oral iron and pramipexole for the treatment of restless legs syndrome patients with low serum ferritin. Eur J Neurol. 2014;21:260–266. doi: 10.1111/ene.12286
- Silvestre OM, Goncalves A, Nadruz Jr. W, et al. Ferritin levels and risk of heart failure-the atherosclerosis risk in communities study. Eur J Heart Fail. 2017;19:340–347. doi: 10.1002/ejhf.701
- Munoz M. Peri-operative correction of non-anaemic iron deficiency. A reply. Anaesthesia. 2017;72:911–912. doi: 10.1111/anae.13947
- Punnonen K, Irjala K, Rajamaki A. Serum transferrin receptor and its ratio to serum ferritin in the diagnosis of iron deficiency. Blood. 1997;89:1052–1057.
- World Health Organization (1994) Indicators and Strategies for Iron Deficiency and Anemia Programmes. Report of the WHO/UNICEF/UNU Consultation. Geneva, Switzerland, 6–10 December, 1993.
- Suominen P, Punnonen K, Rajamaki A, et al. Serum transferrin receptor and transferrin receptor ferritin index identify healthy subjects with subclinical iron deficitis. Blood. 1998;92:2934–2939.
- Guyatt GH, Oxman AD, Ali M, et al. Laboratory diagnosis of iron-deficiency anemia: an overview. J Gen Intern Med. 1992;7:145–153. doi: 10.1007/BF02598003
- Mast AE, Blinder MA, Gronowski AM, et al. Clinical utility of the soluble transferrin receptor and comparison with serum ferritin in several populations. Clin Chem. 1998;44:45–51.
- Kiss JE. Laboratory and genetic assessment of iron deficiency in blood donors. Clin Lab Med. 2015;35:73–91. doi: 10.1016/j.cll.2014.10.011
- Cable RG, Glynn SA, Kiss JE, et al. Iron deficiency in blood donors: the REDS-II donor iron status evaluation (RISE) study. Transfusion. 2012;52:702–711. doi: 10.1111/j.1537-2995.2011.03401.x
