ABSTRACT
Introduction: Hereditary spherocytosis (HS) is the most common congenital hemolytic anemia, characterized by anemia, jaundice, and splenomegaly. The diagnosis of HS relies on symptoms of hemolysis, a family history of HS, and a positive laboratory test which is usually the osmotic fragility test (OFT). We conducted a study to assess the utility of mean corpuscular hemoglobin concentration (MCHC), mean corpuscular volume (MCV), mean sphered cell volume (MSCV), and mean reticulocyte volume (MRV) in the diagnosis of HS and if these are helpful in distinguishing cases of HS from immune hemolytic anemia.
Methods: A total of 102 patients suspected to have HS were enrolled. In addition 10 cases of immune hemolytic anemia (IHA) were included in the study and performance of the above screening tests was evaluated. The diagnosis of HS was based on incubated OFT, eosin 5′-maleimide (EMA) dye binding test, and flowcytometric OFT.
Results: A total of 29 patients were diagnosed as having HS. The sensitivity and specificity for diagnosis HS by MCHC > 35 g/dL was 44.82%, and ΔMCV−MSCV > 10 fL has a sensitivity and specificity of 82.75% and 95.9% for diagnosis of HS. Using an algorithm of ΔMCV−MSCV > 10 fL and ΔMRV−MSCV < 25, for the differentiation of HS from IHA had sensitivity of 68.9% and specificity of 98.8%.
Introduction
Hereditary spherocytosis (HS) is one of the hereditary hemolytic diseases caused by abnormal red cell membrane proteins. The diagnosis of HS depended on the presentation of classic hemolytic symptoms, a positive family history and positive laboratory test [Citation1]. There are many tests described for the diagnosis of HS. Commonly employed tests for the diagnosis are the incubated osmotic fragility test (OFT), the eosin 5 maleimide (EMA) dye binding test and recently described flowcytometric OFT [Citation2,Citation3]. However, none of these tests are able to diagnose 100% patients correctly and all have their own pitfalls. Many of mild cases of HS are missed if only OFT is employed and in addition the test is time consuming, labor intensive, and requires technical expertise [Citation1,Citation2]. The EMA test requires the EMA dye which requires a separate sample and availability of the dye is temperature and storage time sensitive and both EMA and flow cytometric OFT require the flow cytometer which may not be available at all centers [Citation2,Citation3].
Spherocytes are the red cells formed with changes in cellular conformation resulting in its inability to undergo deformability compared to the biconcave red cells. The spherocytes thus formed have a shortened half life owing to the splenic sequestration. HS and autoimune hemolytic anemia (AIHA) are common causes of spherocytosis causing diagnostic difficulty. Spherocytes in HS results due to abnormal/absence of membrane proteins commonly spectrin, ankyrin, band 3, and protein 4.2 resulting in loss of unstable membrane. In AIHA the partial phagocytosis of immunoglobulin bound red cells leads to loss of the red cell membrane leading to the formation of spherocytes. This difference in mechanism of the formation of spherocytes is thought to produce difference in the reticulocyte volume in these two disorders [Citation4,Citation5].
Red cell membrane loss begins at the reticulocyte stage in HS, however in AIHA commonly the peripheral blood red cells are only affected and reticulocytes are spared. Advantage of this difference can be taken and reticulocyte volume can be used to differentiate these two conditions [Citation4,Citation5]. The aim of our study was to evaluate the utility of the Beckman Coulter LH 780 (Beckman Coulter, CA, USA) generated values of mean sphered cell volume (MSCV) and mean reticulocyte volume (MRV) for screening of HS. The secondary objective was to utilize these parameters to differentiate HS from AIHA. Reticulocytes are assessed using the new methylene blue stain on the LH780 series of analysers. This dye precipitates residual RNA and it identified reticulocytes separately from the mature red cells. Following this staining, an acidic hypo-osmotic solution is introduced which causes sphering of red cells and clearing of hemoglobin. These sphered red cells are then categorized as reticulocytes or mature red cells using Volume Conductivity and scatter (VCS) technology. MRV represents the average volume of all reticulocyte events while MSCV represents the average volume of all red cell events including both reticulocytes and mature red cells [Citation4]. In a patient of HS, it has been shown that Δ mean corpuscular volume (MCV)−MSCV values are higher than control patients [Citation4,Citation6]. Da Costa et al. have earlier shown that in HS, the cell surface loss starts at the reticulocyte stage, whereas in AIHA, the cell surface loss is largely restricted to the mature red cells [Citation5]. Hence, ΔMRV−MSCV values in HS are lower than those seen in AIHA [Citation4,Citation5].
Material and methods
After obtaining institutional ethical clearance, a total of 102 patients of suspected HS were enrolled for the study prospectively. In all these patients, Direct Coomb's test was documented as negative. In addition 10 cases of immune hemolytic anemia (IHA) with a positive direct Coombs test were enrolled for the study. None of the patient was transfused in the last three months. Peripheral blood was collected in K2-EDTA vacutainers (Becton Dickinson, San Jose, CA, USA) from patients and controls during diagnostic procedures and processed within 1 hour of collection for blood counts. For incubated OFT, 2 mL of peripheral blood was collected in heparin vacutainers (Becton Dickinson).
All patients underwent clinical and physical evaluation and the following laboratory tests: complete blood counts, blood smear examination, reticulocyte count, assays of bilirubin and direct antiglobulin test, serum lactate dehydrogenase level, and abdominal ultrasonography. All patients underwent incubated OFT, EMA dye binding test, and flow cytometric OFT. All the tests were performed on the day of sample collection except for incubated OFT which was done after 24 h incubation and EMA which was completed within 5 days of sample collection and controls collected on the same day as patient sample were used.
Hematologic parameters were determined on LH-780 (Beckman Coulter). The sample was run in the reticulocyte mode and the value of MSCV was obtained in all patients in addition to the complete blood count and other RBC indices (mean corpuscular hemoglobin concentration (MCHC), MCV and MRV. Difference between MCV and MSCV has been suggested to be a useful screening test for the diagnosis of HS and differentiation for AIHA. These parameters were recorded and the difference between MCV and MSCV termed ΔMCV−MSCV and the difference between MRV and MSCV termed ΔMRV−MSCV were calculated for controls, IHA, and HS patients.
Incubated OFT was performed after incubating the patient's sample at 37°C for 24 h following the technique described in [Citation7]. The EMA dye binding test was performed as per King et al. [Citation8] with some modifications [Citation9]. Twenty five microliters of the EMA dye in a final strength of 0.5 mg/mL was added to 5 µL washed red cells and the solution was kept for 1 h in dark. The excess dye was removed after centrifugation in a bench top centrifuge and cells were washed till the supernatant was clear and cells were suspended in 600 µL of PBS. Mean fluorescence intensity (MFI) was determined for at least 15,000 events in the FITC channel, using a FACS Canto II flowcytometer (Becton Dickinson). Results were expressed as the percent EMA fluorescence reduction (%EMA decrease) of the patient compared with the MFI of five normal controls. A laboratory determined cut-off of> 12.5% fall in EMA fluorescence was taken as positive for HS. Flow cytometric OFT was performed as described by Won and Suh [Citation10]. %Residual red cells (%RRC) were calculated for each patient and five normal controls. A laboratory determined cut-off of < 25.6% %RRC was taken as diagnostic of HS.
HS was diagnosed on the basis of clinical and laboratory signs of chronic hemolysis, the presence of spherocytes on peripheral blood smear, positivity of at least one of the diagnostic tests, family history of hereditary spherocytosis if any, and exclusion of other causes of secondary spherocytosis.
Results
A total of 102 patients underwent screening for HS in the present study. Of these, 29 patients were classified as HS based on the history, clinical, and laboratory findings. These 29 patients belonged to 20 different unrelated families. The median age of patients was 24.5 years and ranged from 17 days to 57 years. There were 55% males and 45% females. Of the patients screened, 32 were normal, 9 represented other hemolytic anemias, and 18 patients were microcytic hypochromic anemia and were equally distributed in iron deficiency anemia and thalassemia trait groups. Rest of the 14 patients were distributed among many disorders such as chronic liver disease, systemic lupus erythematosus, dengue fever, and hypothyroidism. The hematological parameters in various different subgroups have been compared in .
Table 1. Comparison of various parameters in different subgroups of patients.
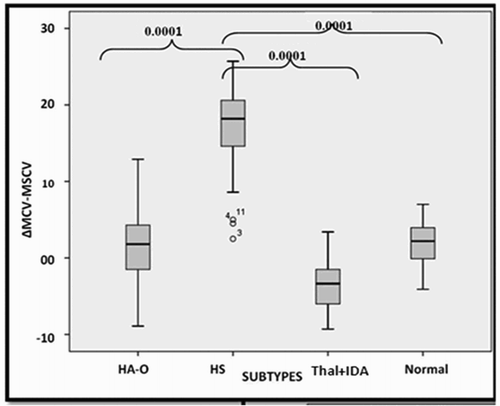
It is clearly evident from that the MRV is significantly different between HS and normal individuals and other hemolytic anemia. ΔMCV−MSCV is also significantly higher in HS compared to normal individuals, other hemolytic anemias and the microcytic hypochromic anemia group (). The ΔMRV−MSCV value is significantly lower in HS when compared with normal individuals.
For further analysis, the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated for MCHC > 35% and ΔMCV−MSCV > 10 fL to differentiate HS from all other patients ().
Table 2. Sensitivity, specificity, PPV and NPV of hematological parameters in predicting the diagnosis of HS.
The sensitivity of ΔMCV−MSCV > 10 fL is almost twice as that of MCHC > 35% for the screening of HS, but the specificity of both the parameters is ∼95%. The accuracy, PPV, and NPV are better for ΔMCV−MSCV > 10 fL than MCHC > 35%. Therefore, where available, the parameter ΔMCV−MSCV > 10 fL is better than MCHC > 35% to identify patients of HS.
It was observed that the majority of the cases of HS (24/29; 82.8%) and those of IHA (5/10; 50%) had an ΔMCV−MSCV > 10 fL, whereas none of the normal individuals (0/32) had a value of ΔMCV−MSCV > 10 fL. () The other significant observation was regarding ΔMRV−MSCV, that the majority of the cases of HS (24/29; 82.8%) had an ΔMRV−MSCV < 25 fL, whereas (3/10; 30%) IHA cases had an ΔMRV−MSCV < 25 fL.
Table 3. Combination of ΔMCV−MSCV > 10 fL and ΔMRV−MSCV < 25 fL in the screening of HS and IHA.
By combining these two parameters and following an algorithm of ΔMCV−MSCV > 10 fL and ΔMRV−MSCV < 25 fL () it was observed that 20/29 cases of HS had both these features and all normal cases lacked these findings. Only 1/10 cases of IHA (10%) had both these features. Using the combination of ΔMCV−MSCV > 10 fL and ΔMRV−MSCV < 25 fL to differentiate HS from IHA, the sensitivity is 68.97% with a high specificity of 98.8%. Although ΔMCV−MSCV > 10 fL has a very good sensitivity and specificity as a screening test of HS; however, the addition of ΔMRV−MSCV < 25 fL to the algorithm further enhanced its specificity, especially in relation to its differentiation from IHA.
Discussion
Hematological parameters are rarely used in the diagnosis of HS due to the lack of sensitivity. However, the newer hematological parameters can overcome these problems and give better results when used as a screening test.
The diagnostic sensitivity and specificity of MCHC > 35% in our study were 44.8% and 94.3% respectively this is comparable to the study by Tao et al. [Citation11] as they reported a sensitivity of 41.1%. Michalis et al. [Citation12] and Eberle et al. [Citation13] have reported higher sensitivities of 70% and 81% respectively. Specificity in all the other three studies [Citation11–13], however, was comparable at ∼90%.
In our study the diagnostic sensitivity of ΔMCV−MSCV > 10 fL was 82.8% which was comparable to other studies. In the studies by Nair et al. [Citation4], Tao et al. [Citation11] and Broseus et al. [Citation6] using MSCV for the diagnosis of HS were 84.2%, 89.28% and 100%, respectively,. The specificity of the use of MSCV for the diagnosis of HS was ∼90% [Citation4,Citation6,Citation11] and is comparable to ∼95.9% obtained in the present study. Broseus et al. [Citation6] used ΔMCV−MSCV > 9.6 fL and Nair et al. [Citation4] used ΔMCV−MSCV > 10 as the positive screening test. Tao et al. [Citation11] used MSCV < MCV as the optimum cut-off to differentiate HS from other individuals.
Though MCHC > 35% gives variable sensitivity [Citation6–8], the use of a newer parameter ΔMCV−MSCV > 10 fL gives consistently higher sensitivity [Citation4,Citation6,Citation11] even better than the diagnostic incubated OFT [Citation14].
Da Costa et al. [Citation5] initially proposed that reticulocytes of a patient of HS have a lower volume due to the shedding of the membrane from the time it was born. However in the case of IHA, the shedding of the red cell membrane is delayed in the peripheral blood and reticulocytes preserve their volume resulting in MRV difference between the two conditions. MRV is another newer parameter which can be used in alone or in conjunction with MSCV to differentiate HS from IHA. So if there is a doubt whether spherocytes in the peripheral smear are due to HS or IHA, the fact that a lower MRV favors HS and higher MRV favors IHA can be considered. MRV used along with MSCV increases the specificity for the diagnosis of HS.
In our study we used the algorithm of ΔMCV−MSCV > 10 fL and MRV−MSCV < 25 fL similar to the one used by Nair et al. [Citation4] and found that though the sensitivity decreases (68.9%), the specificity increases to 98.8% in differentiating HS cases from IHA. Nair et al. [Citation4] reported a sensitivity of 84.2% and specificity of 94.7% for differentiating HS from IHA using similar parameters.
Conclusion
Use of newer screening parameters such as MSCV and MRV significantly enhances the likelihood of diagnosing HS and differentiating HS from IHA. In resource constraints setting availability these parameters can enhance the ability to diagnose HS over and above the cumbersome and labor-intensive OFT and the expensive EMA dye binding test and flowcytometric OFT.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Bolton-Maggs PH, Langer JC, Iolascon A, et al. General haematology task force of the British committee for standards in H. Guidelines for the diagnosis and management of hereditary spherocytosis--2011 update. Br J Haematol. 2012;156:37–49. doi: 10.1111/j.1365-2141.2011.08921.x
- King MJ, Zanella A. Hereditary red cell membrane disorders and laboratory diagnostic testing. Int J Lab Hematol. 2013;35:237–243. doi: 10.1111/ijlh.12070
- Warang P, Gupta M, Kedar P, et al. Flow cytometric osmotic fragility – an effective screening approach for red cell membranopathies. Cytometry B Clin Cytom. 2011;80:186–190. doi: 10.1002/cyto.b.20583
- Nair A, Jain S, Inbakumar D, et al. Mean reticulocyte volume enhances the utility of red cell mean sphered cell volume in differentiating peripheral blood spherocytes of hereditary spherocytosis from other causes. Indian J Pathol Microbiol. 2015;58:307–309. doi: 10.4103/0377-4929.162836
- Da Costa L, Mohandas N, Sorette M, et al. Temporal differences in membrane loss lead to distinct reticulocyte features in hereditary spherocytosis and in immune hemolytic anemia. Blood. 2001;98:2894–2899. doi: 10.1182/blood.V98.10.2894
- Broseus J, Visomblain B, Guy J, et al. Evaluation of mean sphered corpuscular volume for predicting hereditary spherocytosis. Int J Lab Hematol. 2010;32:519–523. doi: 10.1111/j.1751-553X.2009.01216.x
- Roper D, Layton M. Investigation of the hereditary hemolytic anemias: membrane and enzyme abnormalities. 11th ed. In: Bain BJ LM BI, Lewis SM, editors. Philadelphia, PA: Churchill Livingstone Elsevier; 2012. p. 245–254.
- King MJ, Behrens J, Rogers C, et al. Rapid flow cytometric test for the diagnosis of membrane cytoskeleton-associated haemolytic anaemia. Br J Haematol. 2000;111:924–933.
- Hunt L, Greenwood D, Heimpel H, et al. Towards the harmonization of result presentation for the eosin-5’- maleimide (EMA) binding test in the diagnosis of hereditary spherocytosis. Cytometry B Clin Cytom. 2014;88:50–57. doi: 10.1002/cytob.21187
- Won DI, Suh JS. Flow cytometric detection of erythrocyte osmotic fragility. Cytometry B Clin Cytom. 2009;76B:135–141. doi: 10.1002/cyto.b.20448
- Tao YF, Deng ZF, Liao L, et al. Comparison and evaluation of three screening tests of hereditary spherocytosis in Chinese patients. Ann Hematol. 2015;94:747–751. doi: 10.1007/s00277-014-2270-2
- Michalis LA, Cohen AR, Zhao H, et al. Screening for hereditary spherocytosis by use of automated erythrocyte indexes. J Pediatr. 1997;130:957–960. doi: 10.1016/S0022-3476(97)70283-X
- Eberle SE, Sciuccati G, Bonduel M, et al. Erythrocyte indexes in hereditary spherocytosis. Medicina (B Aires). 2007;67:698–700.
- Mariani M, Barcellini W, Vercellati C, et al. Clinical and hematologic features of 300 patients affected by hereditary spherocytosis grouped according to the type of the membrane protein defect. Haematologica. 2008;93:1310–1317. doi: 10.3324/haematol.12546