ABSTRACT
Objectives: Danazol is an attenuated androgen and is used in the treatment of aplastic anemia (AA) in resource constraint settings. We chose to study the role of CD4+ CD25high CD127low FoxP3+ T regulatory cells (T-regs) in the pathophysiology of AA and their response to treatment with Danazol alone or in combination with immunosuppressive treatment (IST).
Methods: T-regs’ percentages of 25 acquired idiopathic AA patients and 25 healthy controls who completed study protocol were analyzed by performing multicolor flowcytometry on peripheral blood samples.
Results: More than one-third (36%) of AA patients in our study received Danazol as monotherapy, whereas less than a third (32%) each received standard doses of IST with equine Anti Thymocyte Globulin (ATG) and Cyclosporine combination, or Cyclosporine and Danazol combination, respectively. Results showed that all AA patients had a significantly lower percentage of T-regs at the time of diagnosis when compared to healthy controls (p < 0.0001), implicating their role in the pathophysiology. On treatment, all 25 patients showed a significant rise in the percentage of T-regs when compared to baseline (p < 0.0001).
Discussion: The rise in T-regs’ percentage was higher in patients treated with Danazol alone when compared to standard IST (ATG with Cyclosporine), or Cyclosporine with Danazol combinations (p = 0.585).
Conclusion: We conclude that Danazol also leads to increase in T-regs in acquired idiopathic AA.
Introduction
Aplastic anemia (AA) is a syndrome consisting of pancytopenia in the peripheral blood (PB) associated with hypocellularity of bone marrow, in the absence of infiltrates with no increase in reticulin. It is characterized by destruction of hematopoietic stem cells by cytotoxic T lymphocytes [Citation1]. A subset of T lymphocytes, CD3+ CD4+ IL-17 producing cytotoxic (Th17) T cells are increased in the PB, whereas another subset of T lymphocytes, CD4+ CD25high CD127low FoxP3+ T regulatory cells (T-regs), are decreased, suggesting the regulation of hematopoiesis by these cells in AA patients [Citation2]. It has been demonstrated that T-regs are decreased or functionally defective at presentation in almost all immune-mediated idiopathic AA patients, and their numbers are likely to increase following immunosuppressive treatment (IST) [Citation3–5]. Hematopoietic response and hematologic recovery after successful IST with Cyclosporine and Anti Thymocyte Globulin (ATG) represent the most powerful evidence that AA is immune mediated [Citation6,Citation7].
The standard treatment of AA consists of allogeneic stem cell transplantation in transplant-eligible patients or IST in non-transplant-eligible patients. Danazol is an attenuated androgen and has been used in the treatment either alone or in combination with IST [Citation1,Citation2]. Although the role and exact mechanism by which Danazol acts in the treatment of AA is unclear. However, it is still used in the treatment of AA in resource constraint settings. The response to Danazol has been postulated to be around 46% in acquired idiopathic AA [Citation8]. It is also postulated that Danazol may increase the marrow function by acting on telomerase in CD34 cells through the estradiol receptor [Citation9].
We studied T-regs in acquired idiopathic AA patients who received treatment with ATG + Cyclosporine, or Cyclosporine + Danazol, or Danazol alone to assess the change in the percentage of T-regs.
Materials and methods
It was a prospective case-control study conducted from January 2013 to May 2014. Forty-six newly diagnosed adult patients (age more than 18 years) of AA were enrolled after obtaining their written informed consent in accordance with the declaration of Helsinki. The diagnosis of AA was made by standard criteria as per International Aplastic Anemia Study Group (IAASG) guidelines (1987). Patients with pancytopenia and having myelodysplasia, myelofibrosis, and malignant infiltration were excluded. AA due to secondary causes, inherited syndromes, and paroxysmal nocturnal hemoglobinuria (PNH) clones were not part of the study group (PNH clones were detected by flowcytometry with sensitivity of 0.1%, using CD15 and CD33 as gating markers for neutrophils and monocytes, and recording fluorescent aerolysin (FLAER) and CD24 dual negative neutrophils and FLAER and CD14 dual negative monocytes). A total of five patients showed the presence of PNH clone size varying from 8% to 80% on neutrophils. The present study was designed to study T-regs in a homogenous group of patients of AA, and hence excluded any patient positive for PNH clone above 0.1%. Age- and sex-matched healthy controls (n = 25; M = 15, F = 10) were selected from the relatives of patients accompanying them and not suffering from any chronic medical disorders.
Multicolor flowcytometry for enumeration of T-regs was performed on PB samples of 46 acquired idiopathic AA patients at the time of diagnosis along with 25 healthy controls. T-regs were calculated as percentage of T lymphocytes for all the patients, along with healthy controls. Sample processing for surface and cytoplasmic markers was performed according to the manufacturer's instructions and the protocols already standardized in Hematology department at this institute [Citation10]. This was achieved by multicolor analysis of CD4+ CD25high CD127low FoxP3+ T-regs in PB of all study patients and controls. Surface staining was done after lysis of RBCs using FACS Lyse solution (BD Biosciences, Franklin Lakes, NJ, U.S.A.). Intra-nuclear FoxP3 staining was used in the panel for specific identification of T-regs and was done using FACS Perm-2 solution (BD Biosciences, U.S.A.), which also fixes the cells simultaneously. The cells were incubated for 10 minutes with BD FACS Perm-2 solution, and further incubated with the flourochrome-conjugated monoclonal antibody to human FoxP3 for 30 minutes at room temperature in the dark.
Data of 25 eligible adult AA (treatment naïve) patients fulfilling the diagnostic criteria and who completed 3 months of treatment as per the study protocol was finally analyzed (). The patients did not have any health insurance and hence patients chose a treatment based on their out-of-pocket expenses [Citation11]. Eight patients received standard IST with equine ATG (40 mg/kg/day for 4 days) and Cyclosporine (5 mg/kg/day) – ‘ATG + CSA’ group. Eight patients received Cyclosporine (5 mg/kg/day) and Danazol (200 mg thrice daily) – ‘CSA + DAN’ group. Nine patients received Danazol alone (200 mg thrice daily) – ‘DAN’ group. T-regs’ enumeration was repeated for AA patients after 3 months of treatment. Clinical response to treatment was recorded as None, Partial or Complete response (Camitta, Citation2000 criteria) [Citation12].
Statistical analysis
All the study AA patients were initially categorized as per the severity. First, comparison of T-regs’ percentage in AA patients and healthy controls were done. Predictors for the change in T-regs were assessed using univariate followed by multivariate model to predict the change in T-regs’ value. Second, changes in percentage of T-regs before and after treatment were compared within the three treatment groups. Percentage of T-regs was used for statistical analysis between different groups, since a change in absolute lymphocyte counts before and after treatment was likely to become a confounding factor for using absolute counts of T-regs for statistical analysis. Statistical analyses were done with the SPSS software version 16.0. Student's paired t test was used to find the significance of difference of the paired samples. Unpaired/independent t test was used to find the significance of difference of the patients versus controls. For qualitative variables, Chi square test was used. For quantitative variables, the unpaired t test was used to compare between the two groups (i.e. patients and controls) and paired t test was used to compare the data within the same group before and after treatment using repeated measures ANOVA (i.e. the same patient at diagnosis and 3 months post treatment). P < 0.05 was considered significant. Mean was expressed as mean ± SD. Descriptive statistics in the form of mean (95% CI), median, and standard deviation were described for variables.
Results
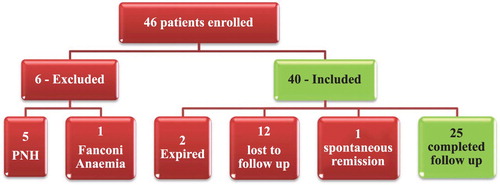
A total of 46 patients of acquired idiopathic AA were enrolled during the study period. Six patients were excluded from the study (one patient diagnosed as Fanconi anemia and five patients had a major PNH clone). Of the 40 patients, 12 patients were lost to follow up and 2 patients died before completing the study protocol. In addition, one patient with non-severe aplastic anemia (NSAA) had spontaneous improvement of hematological parameters, hence was excluded from analysis. Data of 25 patients were finally analyzed in this study (). Baseline characteristics and demographic variables of the study population are shown in .
Table 1. Baseline characteristics of the study population.
The mean T-regs’ percentages were low at the time of diagnosis in all AA patients. There was a significant difference between the mean T-regs in the 25 patients (3.45 ± 1.33%) and the controls (7.90 ± 0.99%) (p < 0.0001).
Following treatment, all patients showed a significant rise in mean T-regs (6.70 ± 1.99%) (p < 0.0001). Thirteen patients showed no response (Non-responders), 11 patients had partial response (PR), and 1 patient had a complete response (CR) (Responders). The mean rise in T-regs was higher in the Responders group than the Non-responders group, as shown in (p = 0.613).
Table 2. Comparison with other studies of T-regs in AA patients and healthy controls.
The comparison of T-regs’ percentage at diagnosis and at 3 months post treatment between ‘ATG + CSA’, ‘CSA + DAN’ and ‘DAN’ patient groups is depicted by the median and range as shown in . The mean rise in T-regs after 3 months of treatment was not significantly different among three groups (p = 0.585) as shown in but did increase significantly in all three groups (p < 0.0001). No significant predictors of change in T-regs’ value were found by univariate analysis. All the three treatment groups, though not formed randomly, were matched for the age (p = 0.725). Relatively more number of females were recruited in the ‘CSA + DAN’ group compared to other two groups. This, however, is unlikely to affect the T-regs’ response, as an independent analysis of gender was not a predictor for the rise in T-regs’ percentage after treatment (p = 0.38).
Figure 2. The Box–whisker plot to show the comparison of T-regs’ percentage at diagnosis and 3 months post treatment in the three groups. (a) ATG + CSA group, (b) CSA + DAN group, and (c) DAN group.
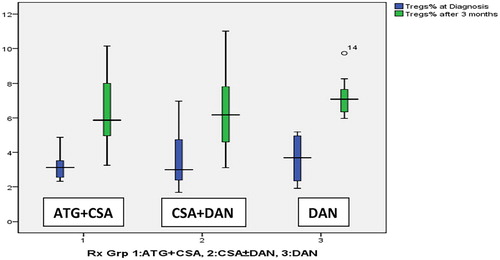
Table 3. Mean T-regs at diagnosis and 3 months post treatment between responders and non-responders.
Discussion
The T-regs’ percentages of T lymphocytes in AA patients at presentation were found to be significantly lower than healthy controls in our study. The results of our study were consistent with other studies demonstrating that T-regs indeed are low in AA patients [Citation3,Citation13–16], as shown in . Few studies worldwide have assessed the T-regs’ response to treatment with IST in AA patients. They have demonstrated that T-regs’ number or percentage increase after treatment with IST, especially ATG and Cyclosporine [Citation17–19]. We found a significant increase in mean T-regs’ percentage after 3 months of treatment in all the 25 AA patients.
In our study, the mean rise in T-regs after 3 months of treatment was higher in the Responders group than the Non-responders group (). Almost half of the AA patients (48%) in our study were ‘Responders’. Increase in T-regs was also noted in patients designated as non-responders who may show an improvement in blood counts after a longer follow-up period, suggesting that the assessment of T-regs at 3 months may identify a group of patients likely to be late responders. Conversely, it may be argued that patients failing to demonstrate a T-regs’ increment at 3 months are unlikely to respond to IST, and thus may be candidates for alternative therapies or stem cell transplantation.
In our study, almost equal numbers of patients received ATG + CSA (32%), CSA + DAN (32%), and DAN alone (36%), and all of them had a rise in T-regs % of T lymphocytes. We found that, after 3 months of treatment of idiopathic AA patients, the mean rise in T-regs was slightly higher after treatment in the DAN group > ATG + CSA group > CSA + DAN group (). Literature is scarce on the effect of Danazol on T-regs in patients with acquired AA. Our data give further credence to a few studies on the therapeutic effect of Danazol/androgens in AA [Citation8,Citation20,Citation21,Citation22] and suggests that possibly attenuated androgens like Danazol may also have an immune-modulatory role in addition to the standard IST.
Table 4. Mean T-regs amongst treatment groups in 25 AA patients.
Though the pathomechanism underlying the response to Danazol is not fully understood, the focus has mostly been directed towards its effect on telomeres [Citation9]. The limitation of our study is that we did not study the telomere lengths of the AA patients due to the non-availability of the facility at our center.
A possible explanation for lower T-regs’ response in ATG group could be that the time period for follow-up evaluation after treatment with ATG was less than 3 months in most of our patients. There was a time gap of 1–2 months between diagnosis and receiving ATG. The response assessment criteria described by Camitta et al. was primarily based on ATG-based IST and patients were assessed at least after a period of 6 months [Citation12]. As the response in this study was assessed at 3 months, and IST responses have been known to occur as late as 6–9 months, a longer duration of follow up may identify a larger population of responders. This is a major limitation of this study.
Conclusion
The rise in T-regs following treatment and its correlation with response indicates their pivotal role in the pathophysiology of AA. Our study demonstrates that Danazol (an attenuated androgen) may have an immune-modulatory potential. Hence, Danazol as a monotherapy or in addition to Cyclosporine or ATG may be a useful adjunct in the treatment of AA.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Young NS, Calado RT, Scheinberg P. Current concepts in the pathophysiology and treatment of aplastic anemia. Blood. 2006;108(8):2509–2519. doi: 10.1182/blood-2006-03-010777
- Shinohara K. The immune-mediated pathogenesis and treatment of aplastic anemia. Bull Yamaguchi Med Sch. 2012;59(1-2):7–13.
- Solomou EE, Rezvani K, Mielke S, et al. Deficient CD4+ CD25+ FOXP3+ T regulatory cells in acquired aplastic anemia. Blood. 2007;110(5):1603–1606. doi: 10.1182/blood-2007-01-066258
- Yan L, Fu R, Liu H, et al. Abnormal quantity and function of regulatory T cells in peripheral blood of patients with severe aplastic anemia. Cell Immunol. 2015;296(2):95–105. doi: 10.1016/j.cellimm.2015.04.001
- Qi W, Ren Y, Fu R, et al. Detection and significance of CD4+ CD25+ CD127dim regulatory T cells in individuals with severe aplastic anemia. Turk J Haematol. 2015;32(3):220–227. doi: 10.4274/tjh.2013.0410
- Malhotra P, Bodh V, Guru Murthy GS, et al. Outcomes of immunosuppressant therapy with lower dose of antithymocyte globulin and cyclosporine in aplastic anemia. Hematology. 2015;20(4):239–244. doi: 10.1179/1607845414Y.0000000196
- Sharma S, Malhotra P, Lal V, et al. Asymptomatic cerebral bleeds in patients with aplastic anemia. Ann Hematol. 2012;91(8):1187–1191. doi: 10.1007/s00277-012-1448-8
- Jaime-Pérez JC, Colunga-Pedraza PR, Gómez-Ramírez CD, et al. Danazol as first-line therapy for aplastic anemia. Ann Hematol. 2011;90(5):523–527. doi: 10.1007/s00277-011-1163-x
- Calado RT, Yewdell WT, Wilkerson KL, et al. Sex hormones up-regulate telomerase activity of normal human hematopoietic cells and restore telomerase activity in carriers of telomerase complex mutations [abstract]. Blood. 2005;106:641a. Abstract 2276. doi: 10.1182/blood-2004-12-4589
- Lad DP, Varma S, Varma N, et al. Regulatory T-cells in B-cell chronic lymphocytic leukemia: their role in disease progression and autoimmune cytopenias. Leuk Lymphoma. 2013;54(5):1012–1019. doi: 10.3109/10428194.2012.728287
- Malhotra P, Gella V, Guru Murthy GS, et al. High incidence of aplastic anemia is linked with lower socioeconomic status of Indian population. J Public Health (Oxf). 2016;38(2):223–228. doi: 10.1093/pubmed/fdv027
- Camitta BM. What is the definition of cure for aplastic anemia? Acta Haematol 2000;103(1):16–18. doi: 10.1159/000040999
- Shi J, Ge M, Lu S, et al. Intrinsic impairment of CD4(+)CD25(+) regulatory T cells in acquired aplastic anemia. Blood. 2012;120(8):1624–1632. doi: 10.1182/blood-2011-11-390708
- Kordasti S, Marsh J, Al-Khan S, et al. Functional characterization of CD4+ T cells in aplastic anemia. Blood. 2012;119(9):2033–2043. doi: 10.1182/blood-2011-08-368308
- de Latour RP, Visconte V, Takaku T, et al. Th17 immune responses contribute to the pathophysiology of aplastic anemia. Blood. 2010;116(20):4175–4184. doi: 10.1182/blood-2010-01-266098
- Wang XG, Luan B, Hu JT. Expression of regulatory T cells and Foxp3 gene in peripheral blood of children with aplastic anemia. Zhongguo Dang Dai ErKeZaZhi. 2010;12(4):241–243.
- Wang XG, Wang M, Liu S, et al. Effect of cyclosporine on regulatory T cells and Foxp3 in the peripheral blood of children with chronic aplastic anemia. Zhongguo Dang Dai ErKeZaZhi. 2011;13(12):936–939.
- Lopez M, Clarkson MR, Albin M, et al. A novel mechanism of action for ATG: induction of CD4+ CD25+ Foxp3+ regulatory T cells. J Am SocNephrol. 2006;17(10):2844–2853.
- Kordasti S, Costantini B, Seidl T, et al. Deep phenotyping of T regs identifies an immune signature for idiopathic aplastic anemia and predicts response to treatment. Blood. 2016;128(9):1193–1205. doi: 10.1182/blood-2016-03-703702
- Kojima S, Hibi S, Kosaka Y, et al. Immunosuppressive therapy using antithymocyte globulin, cyclosporine, and danazol with or without human granulocyte colony-stimulating factor in children with acquired aplastic anemia. Blood. 2000;96(6):2049–2054.
- Bacigalupo A, Chaple M, Hows J, et al. Treatment of aplastic anaemia (AA) with antilymphocyte globulin (ALG) and methylprednisolone (MPred) with or without androgens: a randomized trial from the EBMT SAA working party. Br J Haematol. 1993;83:145–151. doi: 10.1111/j.1365-2141.1993.tb04645.x
- Chuhjo T, Yamazaki H, Omine M, et al. Danazol therapy for aplastic anemia refractory to immunosuppressive therapy. Am J Hematol. 2008;83(5):387–389. doi: 10.1002/ajh.21118