ABSTRACT
Objectives: Multiple studies have shown that the expression level of Homeobox (HOX) transcript antisense RNA (HOTAIR) has a correlation with the diagnosis and prognosis of acute leukemia (AL) and lymphoma. The prognostic significance of HOTAIR on AL and lymphoma has been controversial. Our study thus was performed to further reveal its prognostic value in leukemia and lymphoma.
Methods: By literature searching in some common electronic databases, five studies covering a number of 531 patients were included in this meta-analysis. We extracted useful data to calculate the pooled hazard ratio (HR) and p-value.
Results: The combined HR estimated for overall survival (OS) was 1.87 (95% confidence interval (CI): 1.03–3.47; p = .041) when comparing patients with high HOTAIR with those low. For event/disease/relapse-free survival, the HR was 1.53 (95%CI: 0.58–4.06; p = .39). Subgroup analyses showed that the HR for OS was 2.32 (95%CI: 1.56–3.44; p = .000) in patients with AL and 1.24 (95%CI: 0.21–7.45; p = .817) in lymphoma. Additionally, the Ann-Arbor stage (p = .0009) and the international prognostic index score (p = .0065) were found to be statistically significant between patients with high and low HOTAIR expression. Also, the hemoglobin (HGB) level (p = .008), platelet (PLT) count (p = .001) and blasts in bone marrow (p = .001), but not the French–American–British classification, were found statistically significant.
Discussion: Although our analysis has its limitation, it showed that high expression of HOTAIR had a significantly inferior impact on OS and some clinical parameter of leukemia and lymphoma patients.
Conclusion: HOTAIR played an important prognostic role in leukemia and lymphoma and might serve as a potential target for therapeutic intervention in those patients.
Introduction
Acute leukemia (AL) and lymphoma are the two most common hematological malignancies. AL is a malignant clonal disorder of the hematological stem cells. According to the difference of biological characteristics and clinical features, AL can be divided into two main categories, acute myeloid leukemia (AML) and acute lymphoblastic leukemia, of which AML with a prevalence of 38 cases per 100,000 adults is the more common one [Citation1–3]. Although the treatment of AL has improved markedly in the last few decades, the achievement of complete remission and the obtaining of relapse-free survival (RFS) are still low [Citation4]. Therefore, seeking new indicators to guide treatment and prognosis is necessary.
Lymphoma is a diverse group of malignancies with distinct clinical, histopathological and molecular features. It is reported that more than 250,000 new cases lymphoma per year worldwide, accounting for about 3% of all cancer-related deaths [Citation5]. Lymphoma can be divided into two groups, namely Hodgkin lymphoma and non-Hodgkin lymphoma (NHL), of which NHL is the more common one. Diffuse large B-cell lymphoma (DLBCL) is one of the most life-threatening NHL due to the lack of symptoms in the early period and the lack of efficient therapeutic strategies [Citation6]. Thus, in order to improve the diagnosis and prognosis of these hematological malignancies, there is an urgent requirement to identify novel biomarkers for early detection and prediction of prognosis.
Homeobox (HOX) transcript antisense RNA (HOTAIR), a long intergenic noncoding RNA, interacts with the Polycomb repressive complex 2 (PRC2) to enhance H3K27 trimethylation and finally lead to a decrease in the expression of multiple genes [Citation7–9]. HOTAIR has been found to be described as a potential diagnostic and prognostic biomarker in various cancers, including breast, colorectal, hepatic, gastric and pancreas cancers [Citation10–14]. Multiple studies have shown that the expression level of HOTAIR has a correlation with the diagnosis and prognosis of AL and lymphoma. But the prognostic significance has been controversial. Therefore, to gain full insight into the prognostic value of HOTAIR in patients with leukemia and lymphoma, we performed this meta-analysis.
Materials and methods
Study strategy
A systematic literature search of PubMed, Medline, EMBASE, the Cochrane library and Web of science with the following search terms ‘HOTAIR’ and ‘Leukemia’ or ‘Lymphoma’ was done. The search was restricted to human studies, free articles with the language of English. Relevant papers published between 2007 and 2017 were obtained by two independent reviewers (Y.L. and Z.H.F.). We also reviewed the references for missing information.
Inclusion and exclusion criteria
All of the included studies had to meet the following inclusion criteria: (1) published between 1 January 2007 and 31 July 2017 as original articles; (2) assessed the association between the expression level of HOTAIR and prognosis in leukemia or lymphoma patients; (3) studies provided sufficient data for extraction or calculation of the individual OR, HR and 95%CI. We excluded studies that were published in the abstract form, review articles, case reports, only analyzing pediatric patients or studies with unavailable or incomplete data.
Data extraction and quality assessment
Two reviewers (Y.L. and Z.H.F.) independently extracted data from the articles. Disagreements were resolved by discussion until a consensus was achieved. The following data were extracted: basic information of included records, characteristics of the patients and essential data for systematic review and meta-analysis. When outcomes published in the original articles were only survival curves, the HRs and 95% CIs were calculated by the methods proposed by Parmar et al. [Citation15] and Hotta et al. [Citation16]. If HRs of the univariate analysis and multivariate analyses were reported, the results of the multivariate analysis including other variables should be preferentially considered because they could be more accurate. The methodological quality of prognostic studies was evaluated using the Newcastle–Ottawa–Scale (NOS) tool [Citation17]. This scale has nine items that are classified into the following three major categories: selection (four items), comparability (two items) and outcome (three items). The NOS score has a maximum of nine and those studies ≥7 were considered to be of high quality.
Statistical analysis
All statistical analyses were performed using Stata ver.12 software (college station, TX, U.S.A.). A different effect size was selected for each meta-analysis. For the OS and complete response rate, the HRs or ORs and their 95% CIs were directly extracted from the included studies or indirectly calculated from the reported events, the p-value in the log-rank test or from the published Kaplan–Meier curves [Citation18–20]. Pooled HRs and ORs with 95%CIs were used to evaluate the association between HOTAIR expression level and prognostic features. An observed HR > 1 implied a worse survival for patients with high HOTAIR expression. Conversely, an HR < 1 implied a worse survival for patients with low HOTAIR expression [Citation21]. The point estimate of the HR or OR was considered statistically significant at a level of p < .05 if the 95%CI did not cover the value ‘1’. The statistical heterogeneity of the studies was assessed by the chi-square-based Q-test and quantified with the I2 statistic (I2 = 0–25%; no heterogeneity; I2 = 25–50%; moderate heterogeneity; I2 = 50–75%; large heterogeneity; and I2 = 75–100%; extreme heterogeneity). When the heterogeneity across studies was identified (>50%), the random effects model (the DerSimonian and Laird method) was used, otherwise, the fixed-effect model (the Mantel–Haenszel method) [Citation22]. Furthermore, Begg’s and Egger’s tests were conducted to detect possible publication bias. Asymmetric funnel plots or p < .05 in Egger’s test suggest the existence of publication bias in the incorporated studies [Citation23,Citation24]. A two-tailed p-value less than .05 was considered statistically significant. All the statistical analyses were done by Y.L.
Results
Study selection
As shown in the flow diagram (), our combined search strategy generated 77 studies. After screening the titles and abstracts of these studies, 60 duplicate or irrelevant articles were excluded. Subsequently, the remaining 17 full-text articles were assessed for eligibility, and 12 studies, including 1 without sufficient clinical data, 5 with no relevant outcome and 6 no comparative studies were included. Finally, five [Citation10,Citation25–28] articles with a total of 531 patients were selected according to our inclusion and exclusion criteria.
Study characteristics
Five studies covering a total of 531 patients were included in the meta-analysis. The characteristics are listed in . The five included studies were published between 2007 and 2017 and included 184 high expression of HOTAIR and 347 low expression of HOTAIR. All of the studies are retrospective or prospective design. Four studies [Citation24,Citation26–28] of them were from China, and one study [Citation26] from Korea. Three of the studies focused on AL [Citation24,Citation27,Citation28] and two of them focused on diffuse large B-cell lymphoma [Citation25,Citation26]. We also extracted p-values of the association between HOTAIR and clinicopathological features (). The median overall score of NOS results of the included studies was 7.5 (range 6–9), which indicated that the methodological quality was high (). The NOS scores are listed in .
Table 1. Summary of the data extracted from the five studies included.
Table 2. Summary of the comparison for the p-values of the association between HOTAIR and clinicopathological features.
Table 3. Quality assessment of individual study.
Outcomes of the meta-analysis
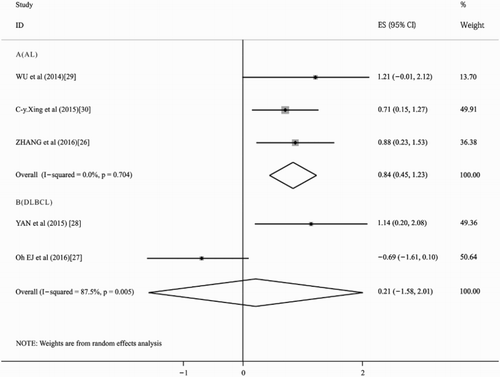
As shown in (a), the summary HR in OS of overall patients was 1.89 (95% CI: 1.03–3.47, p = .041, I2 = 67.1%), which indicated that high expression of HOTAIR was a poor factor for OS of leukemia and lymphoma patients. Because of limited information, we combined event-free survival (EFS) in study [Citation24], RFS in study [Citation27] and disease-free survival (DFS) in study [Citation11] to evaluate the prognosis, the overall HRs were 1.53 (95% CI: 0.58–4.06, p = .39, I2 = 88.9%). The result suggested that high expression of HOTAIR had no significant impact on EFS/RFS/DFS in patients with leukemia or lymphoma (). This is similar to the result of study [Citation24,Citation27]. However, Xing et al. reported that higher expression of HOTAIR predicted favorable factor for DFS in AL. Although the heterogeneity of our meta-analysis was high, the methodological quality of all the included studies were high. This may be due to the fact that our meta-analysis included only three studies, covering a small number of patients and evaluating similar prognostic indicators, such as EFS, RFS and DFS. Therefore, prospective randomized controlled studies covering large numbers and investigating the same prognostic indicator are needed to get a more accurate assessment of the prognosis. Then, different subgroup analyses were also performed. The results of one subgroup analysis showed that in patients with AL [Citation24,Citation27,Citation28], the pooled HR for the OS was 2.32 (95% CI: 1.56–3.44, p = .00, I2 = 0.0%) (), which indicated that high expression of HOTAIR was a poor factor for OS in patients with leukemia. Another subgroup analysis was performed in patients with DLBCL [Citation25,Citation26], the pooled HR for the OS was 1.24 (95%CI: 0.21–7.45; p = .817, I2 = 87.5%) when comparing patients with high expression of HOTAIR with those low expression (). For the limited number of the included studies, we found no significant impact in patients with lymphoma between high expression of HOTAIR with those low expression. In addition, we calculated the overall p-value in age, gender in all the included patients, platelets count, hemoglobin level and bone marrow blasts (%) in patients with AL, Ann-Arbor stage, extra-nodal status and international prognostic index (IPI) score in patients with DLBCL. We found that the high expression of HOTAIR was more frequently found in male patients (p = .0498), but the difference for age (p = .341) between high expression level of HOTAIR was not statistically significant with low expression ones. In patients with DLBCL [Citation25,Citation26], we found that there were Ann Arbor stage (p = .001) and IPI score (p = .007) but not Extra-nodal status (p = .298), which were significantly different between the high expression level of HOTAIR with those low expression ones. In patients with AL [Citation24,Citation27], we found that platelets count, hemoglobin level and bone marrow blasts (%) were all statistically significant between the high expression level of HOTAIR with those low expression ones.
Figure 2. Forest plots of the hazard ratios (HRs) and 95% confidence intervals for overall survival (OS) in leukemia and lymphoma patients (a) and the HRs and 95% confidence intervals for (EFS/DFS/RFS) in leukemia and lymphoma patients (b). The size of the blocks or diamonds represents the weight and the length of the straight line represents the width of 95% CI.
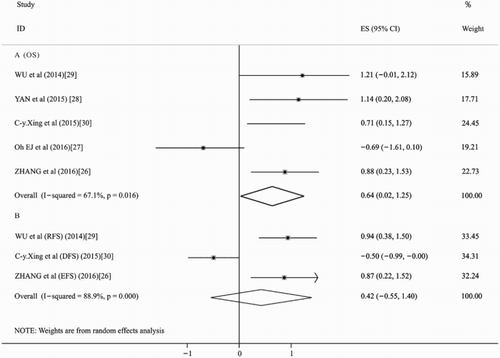
Figure 3 . Forest plots of the hazard ratios (HRs) and 95% confidence intervals for overall survival (OS) in AL patients (a) and the HRs and 95% confidence intervals for OS in DLBCL patients (b). The size of the blocks or diamonds represents the weight and the length of the straight line represents the width of 95%CI.
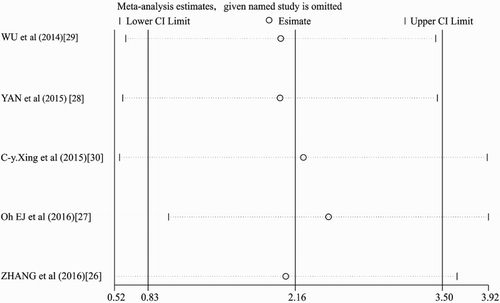
Sensitivity analysis and publication bias
For high heterogeneity of our meta-analysis, we conducted a sensitivity analysis by omitting one study at a time to assess the effect of the study quality on the stability of this meta-analysis. As shown in , no individual study had a predominant influence on the overall HR. Because only a few studies included in the meta-analysis, we could not conduct a publication bias analysis.
Discussion
In recent years, numerous studies have demonstrated that, by chromosome remodeling, transcription and post-transcriptional processing, lncRNAs are involved in cancer progression and metastasis [Citation29]. HOTAIR was the first lncRNA discovered to be involved in carcinogenesis. Many studies have reported that HOTAIR is aberrantly expressed in different types of cancer, including breast cancer, colorectal cancer, laryngeal squamous cell carcinoma and liver cancer [Citation10,Citation30,Citation31]. HOTAIR is a long intergenic noncoding RNA with a length of 2.2 kb in HOXC locus and regulates gene expression by modulating chromatin structures. In this meta-analysis, we have examined the prognostic role of HOTAIR in leukemia and lymphoma and the relation between HOTAIR and some clinicopathological characteristics. Although many studies have analyzed the association of HOTAIR with other types of cancers, we believe that this meta-analysis is the first to investigate the relationship between HOTAIR and hematological malignancy prognosis.
In this meta-analysis, five studies covering a total of 531 patients were included. The results revealed that patients with high HOTAIR expression level were more likely to have a poor OS in patients with leukemia and lymphoma. The combined HR estimated for OS was 1.87 (95%CI: 1.03–3.47; p = .041, I2 = 67.1%). Our results are consistent with a number of studies, which have demonstrated that HOTAIR expression is related to a poor prognosis of many malignancies [Citation32–34]. But for EFS/DFS/RFS, the pooled HR was 1.53(95%CI: 0.58–4.06; p = .39), which indicated that the high HOTAIR expression level did not significantly affect the EFS/DFS/RFS in patients with leukemia and lymphoma. Although the heterogeneity was large (I2 = 67.1% and I2 = 88.9%), the sensitivity analysis indicated the stability of our analysis, which demonstrated that the results of the meta-analysis were reliable. Then, different subgroup analyses were conducted. The results showed the pooled HR for OS was 2.32 (95%CI: 1.56–3.44; p = .000) in AL patients. This is consistent with the findings by Hao and Shao [Citation35] and Garzon et al. [Citation36]. We also found the pooled HR for OS was 1.24 (95%CI: 0.21–7.45; p = .817, I2 = 87.5%) in patients with DLBCL. Although our results integrated data from multiple studies [Citation25,Citation26], which could be more reliable, the heterogeneity of our meta-analysis was extreme (I2 = 87.5%) and the number of studies included in the analysis was limited. Additionally, we analyzed the association between HOTAIR and clinicopathological characteristics. As we know, higher Ann-Arbor stage and IPI score in lymphoma; higher WBC count and blasts in BM, lower PLT and HGB in leukemia are well-known poor prognostic variables. Our study identified that the Ann-Arbor stage (p = .0009) and IPI score (p = .0065) were statistically significant in patients with DLBCL between patients with high and low HOTAIR expression; and the HGB (p = .008), PLT (p = .001) and blasts in BM (p = .001) were statistically significant in AL patients, which may explain why, to a certain degree, the high HOTAIR expression level indicates a poor prognostic impact in patients with leukemia and lymphoma. HOTAIR is aberrantly expressed in a variety of human cancers and it has been suggested that HOTAIR expression may play a useful prognostic role in some tumors. However, there are a limited number of studies examining the implications of HOTAIR expression in hematological malignancies. Therefore, we conducted a systematic review and quantitative meta-analysis to clarify the prognostic value of HOTAIR expression in patients with leukemia and lymphoma. Our analysis had its limitation. First, the analysis was based on observational studies rather than a randomized trial or prospective studies. Second, the analysis, especially the subgroup analysis, covered a small number of leukemia and lymphoma patients. Third, we could not avoid potential heterogeneity and publication bias in the meta-analysis. Beyond the limitation, we found that high expression of HOTAIR was the poor factor for OS of leukemia and lymphoma patients. In addition, we found that the high expression of HOTAIR was more frequently found in male patients. Also, we found that Ann-Arbor stage (p = .001) and IPI score (p = .007) were significantly different between the high expression level of HOTAIR with those low expression ones in DLBCL, and platelet count, hemoglobin level and bone marrow blasts (%) were statistically significant in AL.
Conclusion
Considering the limitations mentioned above, our meta-analysis shows that HOTAIR might be a novel predictive factor for assessing poor prognosis in leukemia and lymphoma. However, before that, prospective randomized controlled studies covering large numbers and investigating the prognostic role in leukemia and lymphoma patients are needed.
Acknowledgements
We thank all patients and clinical investigators who were involved in the studies selected for this meta-analysis.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Yiming Luo http://orcid.org/0000-0001-5467-1318
Additional information
Funding
References
- Asif N, Hassan N. Acute myeloid leukemia amongst adults. J Islamabad Med Dental College. 2013;2:58–63.
- Ahmad F, Mohota R, Sanap S, et al. Molecular evaluation of DNMT3A and IDH1/2 gene mutation: frequency, distribution pattern and associations with additional molecular markers in normal karyotype Indian acute myeloid leukemia patients. Asian Pac J Cancer Prev. 2014;15:1247–1253. doi: https://doi.org/10.7314/APJCP.2014.15.3.1247
- Estey E, Döhner H. Acute myeloid leukaemia. Lancet. 2006;368:1894–1907. doi: https://doi.org/10.1016/S0140-6736(06)69780-8
- Tallman MS, Gilliland DG, Rowe JM. Drug therapy for acute myeloid leukemia. Blood. 2005;106:1154–1163. doi: https://doi.org/10.1182/blood-2005-01-0178
- Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide. IARC CancerBase No. 11; 2013 [cited 2016 April 29]. Available from: http://globocan.iarc.fr/Pages/fact_sheets_population.aspx.
- Shipp MA, Ross KN, Tamayo P, et al. Diffuse large B-cell lymphoma outcome prediction by gene-expression profiling and supervised machine learning. Nat Med. 2002;8:68–74. doi: https://doi.org/10.1038/nm0102-68
- Rinn JL, Kertesz M, Wang JK, et al. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. 2007;129(7):1311–1323. doi: https://doi.org/10.1016/j.cell.2007.05.022
- Woo CJ, Kingston RE. HOTAIR lifts noncoding RNAs to new levels. Cell. 2007;129:1257–1259. doi: https://doi.org/10.1016/j.cell.2007.06.014
- Hajjari M, Salavaty A. HOTAIR: an oncogenic long non-coding RNA in different cancers. Cancer Biol Med. 2015;12(1):1–9.
- Gupta RA, Shah N, Wang KC, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464(7291):1071–1076. doi: https://doi.org/10.1038/nature08975
- Kogo R, Shimamura T, Mimori K, et al. Long noncoding RNA HOTAIR regulates polycomb - dependent chromatin modification and is associated with poor prognosis in colorectal cancers. Cancer Res. 2011;71(20):6320–6326. doi: https://doi.org/10.1158/0008-5472.CAN-11-1021
- Geng YJ, Xie SL, Li Q, et al. Large intervening non-coding RNA HOTAIR is associated with hepatocellular carcinoma progression. J Int Med Res. 2011;39(6):2119–2128. doi: https://doi.org/10.1177/147323001103900608
- Xu ZY, Yu QM, Du YA, et al. Knockdown of long non-coding RNA HOTAIR suppresses tumor invasion and reverses epithelial-mesenchymal transition in gastric cancer. Int J Biol Sci. 2013;9(6):587–597. doi: https://doi.org/10.7150/ijbs.6339
- Kim K, Jutooru I, Chadalapaka G, et al. HOTAIR is a negative prognostic factor and exhibits pro-oncogenic activity in pancreatic cancer. Oncogene. 2013;32(13):1616–1625. doi: https://doi.org/10.1038/onc.2012.193
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–2834. doi: https://doi.org/10.1002/(SICI)1097-0258(19981230)17:24<2815::AID-SIM110>3.0.CO;2-8
- Hotta K, Matsuo K, Ueoka H, et al. Meta-analysis of randomized clinical trials comparing cisplatin to carboplatin in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2004;22:3852–3859. doi: https://doi.org/10.1200/JCO.2004.02.109
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: https://doi.org/10.1007/s10654-010-9491-z
- Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: https://doi.org/10.1186/1745-6215-8-16
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. Br Med J. 2003;327:557–560. doi: https://doi.org/10.1136/bmj.327.7414.557
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: https://doi.org/10.1016/0197-2456(86)90046-2
- Ding X, Wan X, Jiang H, et al. The clinical value of ncRNAs in gastric cancer: a systematic review and meta-analyses. Tumour Biol J Int Soc Oncodev Biol Med. 2015;36:4017–4025. doi: https://doi.org/10.1007/s13277-015-3411-5
- Egge M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. Br Med J. 1997;315:629–634. doi: https://doi.org/10.1136/bmj.315.7109.629
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi: https://doi.org/10.2307/2533446
- Zhang YY, Huang SH, Zhou HR, et al. Role of HOTAIR in the diagnosis and prognosis of acute leukemia. Oncol Rep. 2016;36:3113–3122. doi: https://doi.org/10.3892/or.2016.5147
- Oh EJ, Kim SH, Yang WL, et al. Long non-coding RNA HOTAIR expression in diffuse large B-cell lymphoma: in relation to polycomb repressive complex pathway proteins and H3K27 trimethylation. J Pathol Transl Med. 2016;50:369–376. doi: https://doi.org/10.4132/jptm.2016.06.06
- Yan Y, Han J, Li Z, et al. Elevated RNA expression of long noncoding HOTAIR promotes cell proliferation and predicts a poor prognosis in patients with diffuse large B cell lymphoma. Mol Med Rep. 2016;13:5125–5131. doi: https://doi.org/10.3892/mmr.2016.5190
- Wu S, Zheng C, Chen S, et al. Overexpression of long non-coding RNA HOTAIR predicts a poor prognosis in patients with acute myeloid leukemia. Oncol Lett. 2015;10:2410–2414. doi: https://doi.org/10.3892/ol.2015.3552
- Xing CY, Hu XQ, Xie FY, et al. Long non-coding RNA HOTAIR modulates c-KIT expression through sponging miR-193a in acute myeloid leukemia. FEBS Lett. 2015;589(15):1981–1987. doi: https://doi.org/10.1016/j.febslet.2015.04.061
- Shi X, Sun M, Liu H, et al. Long non-coding RNAs: a new frontier in the study of human diseases. Cancer Lett. 2013;339:159–166. doi: https://doi.org/10.1016/j.canlet.2013.06.013
- Li D, Feng J, Wu T, et al. Long intergenic noncoding RNA HOTAIR is overexpressed and regulates PTEN methylation in laryngeal squamous cell carcinoma. Am J Pathol. 2013;182:64–70. doi: https://doi.org/10.1016/j.ajpath.2012.08.042
- Yang Z, Zhou L, Wu LM, et al. Overexpression of long non-coding RNA HOTAIR predicts tumor recurrence in hepatocellular carcinoma patients following liver transplantation. Ann Surg Oncol. 2011;18:1243–1250. doi: https://doi.org/10.1245/s10434-011-1581-y
- Dong L, Hui L. HOTAIR promotes proliferation, migration, and invasion of ovarian cancer SKOV3 cells through regulating PIK3R3. Med Sci Monit Int Med J Exp Clin Res. 2016;22:325–331.
- Fang S, Gao H, Tong Y, et al. Long noncoding RNA-HOTAIR affects chemoresistance by regulating HOXA1 methylation in small cell lung cancer cells. Lab Invest. 2016;96:60–68. doi: https://doi.org/10.1038/labinvest.2015.123
- Luo ZF, Zhao D, Li XQ, et al. Clinical significance of HOTAIR expression in colon cancer. World J Gastroenterol. 2016;22:5254–5259. doi: https://doi.org/10.3748/wjg.v22.i22.5254
- Hao S, Shao Z. HOTAIR is upregulated in acute myeloid leukemia and that indicates a poor prognosis. Int J Clin Exp Pathol. 2015;8:7223–7228.
- Garzon R, Volinia S, Papaioannou D, et al. Expression and prognostic impact of lncRNAs in acute myeloid leukemia. Proc Natl Acad Sci USA. 2014;111:18679–18684. doi: https://doi.org/10.1073/pnas.1422050112