ABSTRACT
Context and Objective: Microcytic anaemia results from defective synthesis of haemoglobin in the erythroid precursors, causing a reduction in its mean corpuscular volume (MCV). The most common causes of microcytosis, without the increase in HbA2 levels, are iron deficiency anaemia (IDA) and α-thalassemia. The aim of this study was to identify the causes of microcytic anaemia and evaluate the haematological parameters from blood donors deemed ineligible (due to the low haematocrit level) that would differentiate the IDA and α-thal, whether isolated or in association.
Methods: Genomic DNA was submitted to the polymerase chain reaction multiplex for the diagnosis of the most common allele deletions of α-thal and erythrogram and in order to verify haematological parameters. Iron deficiency (ID) was determined through the measurement of serum ferritin.
Results: Of the 204 samples, 82 (40.2%) were identified with ID, 24 (17.8%) with α-thal and 10 (4.9%) with ID associated with α-thal. In the α-thal with ID group haemoglobin (Hb), MCV, mean corpuscular Hb concentration (MCHC) and mean corpuscular Hb (MCH) values were significantly lower compared to the isolated α-thal. In the group with ID Hb, MCV, MCHC and MCH values were significantly lower compared to those with isolated α-thal. The α-thal with ID group, showed Hb, MCV, MCHC and MCH significantly reduced when compared to those with IDA.
Conclusions: This study showed that the values of haematological parameters, especially haematocrit, Hb, MCV, MCH, MCHC and red blood cell distribution width (RDW), are lower in patients with IDA, especially when associated with α-thal and therefore it may be useful to discriminate between the different types of microcytic anaemia.
Introduction
Microcytic anemia occurs due to deficient synthesis of hemoglobin (Hb) in erythroid precursors, causing a reduction in the mean corpuscular volume (MCV) of red blood cells (RBCs) [Citation1,Citation2]. The most common causes of microcytosis without elevated HbA2 levels are iron deficiency anemia (IDA) and α-thalassemia (α-thal). Other less common diagnoses that should also be considered include anemia of chronic disease/inflammation and sideroblastic anemia [Citation3].
However, although iron deficiency (ID) is the most common cause of anemia when serum iron levels are normal, microcytosis may be related to α-thal, which affects about 20% of the population and, according to the World Health Organization (WHO), is one of the most common monogenic diseases in the world [Citation4–6].
The most common deletions associated with α-thal are −α3.7 and −α4.2. The −α3.7 deletion has been observed worldwide with the highest frequencies being reported in some African and Mediterranean populations. The −α4.2 deletion is more common in Asian countries and in some populations in the Mediterranean region [Citation4,Citation7].
Frequencies in Brazil vary greatly depending on the study region and the degree of ethnic mixture of the population [Citation8].
As in both ID and α-thal there are reductions in several hematimetric parameters that cause diagnostic difficulties, complementary laboratory exams are necessary for effective differentiation. Currently, the diagnosis of IDA is achieved by analyzing the iron metabolism, including iron dosage, total iron binding capacity and serum ferritin [Citation1,Citation3].
The diagnosis of α-thal is usually reached by evaluating and quantifying fractions of hemoglobin by electrophoresis and by screening for deletions that affect the α-globin gene [Citation4,Citation7,Citation9].
Knowledge and detection of blood donor candidates with these genetic alterations are extremely important for public health since, because of their high incidence and their chronic character with wide clinical variability, they affect the health system as a whole [Citation7–9]. Failure to identify α-thal in blood donors, who are usually physically healthy individuals without great comorbidities, may cause misdiagnoses and subsequently lead to incorrect or unnecessary treatment [Citation9,Citation10].
By using strategies to identify these individuals, blood banks can increase the efficiency of blood donations and provide donors with accurate information about the causes and possible treatments for their low hematocrit (Hct) levels. Furthermore, they can improve donor health and reduce the number of donor deferrals due to low Hct, which can have a major impact on the country’s blood supply [Citation11].
Objective
The aim of this study was to identify the causes of microcytic anemia and to determine whether hematimetric indices are effective in differentiating α-thal and IDA, alone or in combination, in samples from blood donor candidates who have been deferred due to low Hct levels.
Methods
This cross-sectional observational study was conducted in blood donor candidates deferred due to anemia who were attended at the Regional Blood Bank of Uberaba, Brazil (HRU). The present study was approved by the Ethics Research Committee of the Federal University of the Triângulo Mineiro (UFTM) and Hemominas Foundation (ID #2678/2013 and 304).
In the period from September 2011 to January 2014, the HRU received 44,582 candidates for blood donation, 8,989 (20.2%) of whom were considered clinically ineligible. Of these, 656 (1.5%) candidates had hematocrit levels below the minimum reference levels and they were therefore referred due to anemia: 572 (1.3%) were female and 84 (0.2%) were male. According to Brazilian legislation published by the Ministry of Health, the Hct cutoff points used in blood banks to accept candidates for donation is 39% for men and 38% for women. All blood donor candidates deferred due to anemia (n = 656) were approached for this study however only 204 (190 women and 14 men) agreed to participate.
After reading and signing informed consent forms, peripheral blood samples were collected from participants in tubes containing EDTA to perform laboratory tests and to investigate causes of anemia.
Blood counts were performed using the Coulter T890® hematology equipment (Coulter Electronics, Inc., Hialeach, FL, USA). Anemia was defined as hemoglobin <12 g/dL for women and <13 g/dL for men and microcytosis as MCV <80 fL.
ID was determined by measuring serum ferritin by means of a solid phase chemiluminescent immunometric assay (IMMULITE 2000, Diagnostic Products Corporation colours, Los Angeles, CA, USA). Values below 12 ng/dL for women and 20 ng/dL for men were indicative of ID.
Beta-thalassemia (β-thal) screening was carried out by quantification of HbA2 fractions by cellulose acetate electrophoresis at basic pH (8.5) and spectrophotometric determination of the fractions eluted. Diagnosis of β-thal was made based on HbA2 levels above 3.7%.
Genomic DNA was submitted to the multiplex-gap polymerase chain reaction technique for the diagnosis of the most common deletions of α-thal [−α3.7, −α4.2, – –SEA, – –FIL, – –THAI, - (α)20.5 and – –MED] [Citation12].
Subsequently, four groups of individuals were formed to compare the hematological parameters: α-thal without ID, α-thal with ID, IDA and others without changes (without anemia, ID and α-thal).
Data on hematological parameters were submitted to statistical analysis. Normality of the samples was verified using the Kolmogorov-Smirnov test and the homogeneity of the variances by the Bartlett test. The assumptions of normality and homogeneity were not satisfied, so the variables of interest were compared between the groups using the Kruskal-Wallis test followed by Dunn’s multiple comparison. Analysis using the receiver operating characteristic (ROC) curve was also implemented to verify the utility of hematological parameters to predict α-thal with ID, α-thal and ID. Differences were considered statistically significant when the p-value was <0.05.
Results
In this study, 204 blood donors were deferred from donation due to low Hct levels, of which 190 (93.1%) were female and 14 (6.9%) were male.
The number of first-time blood donors was 40 (19.6%) with 164 (80.4%) having had donated blood previously. Of these, 104 (63.4%) were considered repeat donors having donated one or more times a year and 60 (36.6%) were sporadic donors (donated more than 13 months previously).
A total of 82 (40.2%) blood donor candidates with IDA, 24 (11.8%) candidates with α-thal, ten (4.9%) with ID associated with α-thal and six (2.9%) with β-thal were identified (). The frequency of ID was higher in individuals who had previously donated blood compared to first-time donors (for men: 28.5% vs. 0%; p-value > 0.05 and women: 18% vs. 15%; p-value > 0.05); the differences were not being statistically significant. This difference was only significant on comparing the frequency of donations per year among deferred female donors who had donated two or more times (p-value < 0.05).
Table 1. Distribution of 204 samples from blood donors deferred due to low Hct levels as the cause of anemia.
When the erythrograms of 27 (13.2%) blood donors (all female) were analyzed, even though hematological screening had caused them to be deferred due to low Hct levels, the hematological parameters presented levels within reference values.
In 55 (27%) samples the cause of anemia was not identified by the tests performed (). The majority of donors with ID (93.4%) were women. The incidence of individuals with α-thal observed in this sample was 16.7% (34), all of whom had the −α3.7 deletion with 33 (16.2%) being heterozygous (−α3.7/αα) and one (0.5%) homozygous (−α3.7/ −α3.7).
Among the hematological parameters analyzed, significant differences were found for the Hct, Hb, MCV, mean corpuscular Hb (MCH), mean corpuscular Hb concentration (MCHC) and red blood cell distribution width (RDW; ). Thus, these indices were submitted to analyses by multiple comparisons.
Table 2. Hematologic parameters according to the different causes of anemia.
All parameters analyzed, except for the RBC count, showed significant differences between the α-thal groups without ID, α-thal with ID and IDA compared to the group without changes (without anemia, ID and α-thal) ().
Candidates with ID associated with α-thal presented significantly lower Hb (9.1 vs. 11.4 g/dL; p-value < 0.05), MCV (70 vs. 78.8 fl; p-value < 0.01), MCH (21 vs. 26.1 pg; p-value < 0.01), MCHC (29.7 vs. 32.9 g/dL; p-value < 0.05) and Hct (30.1 vs. 34.6; p-value < 0.05) values compared to those with α-thal alone.
Individuals in the IDA Group had lower values for some parameters (RBC, Hb, MCHC, Hct) compared to the α-thal Group, although the differences were not statistically significant.
Regarding the anisocytosis indices, the RDW value was significantly higher in candidates with isolated IDA (15.7 µm) or with IDA associated with α-thal (17.9 µm) compared to the group with only α-thal (15.2 µm: p-value < 0.01 for both).
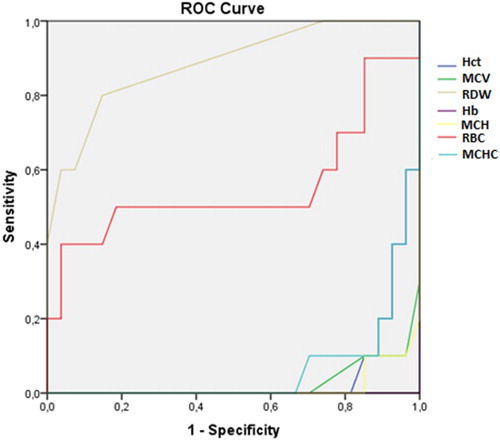
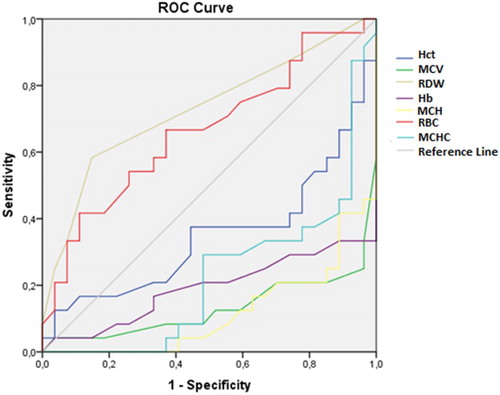
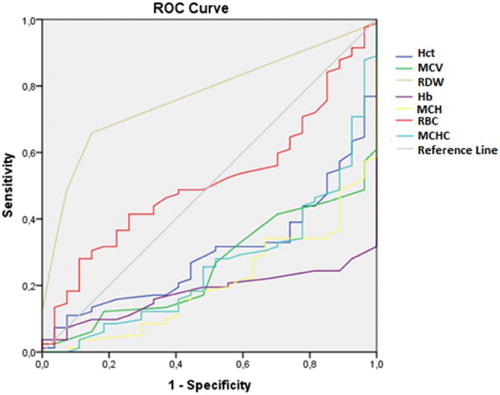
In addition to the comparisons of these hematological parameters between the groups, the ROC curve was also analyzed. From this analysis, the RDW and RBC had areas under the curve greater than 0.5 with only the RDW having an ideal area above 0.7 ().
Table 3. ROC curve of hematological parameters as predictors of α-thal with ID, α-thal and ID.
The other parameters, although presenting significantly different values between different groups according to the ANOVA (Kruskal-Wallis test), the ROC curve showed that these parameters did not demonstrate the sensitivity and specificity required differentiate between the different groups. – show the areas under the ROC curve for the α-thal with ID, α-thal and IDA Groups.
Discussion
Anemia, and particularly IDA, is one of the factors that leads to deferral of blood donor candidates with multiple blood donations being recognized as a cause of ID and anemia [Citation6,Citation9,Citation13,Citation14].
In the present study, the percentage of anemic blood donor candidates who had low serum ferritin levels was greater in those who had already donated blood. This was true for both genders with this percentage increasing for female donors depending on the number of donations per year.
Some studies have shown that IDA is the main cause of ineligibility in blood donors, especially among women, as observed in this study (Women: 38.2% vs. Men: 1.9%). This result is explained by blood loss during menstrual cycles or gestations [Citation1,Citation5,Citation9,Citation15,Citation16]. In addition to the main related causes, studies have also shown that the frequency of donations may be a potential risk factor for ID [Citation10,Citation14].
The different hypochromic microcytic anemias must be differentiated from ID, due to the specific treatments provided in each case [Citation3,Citation17,Citation18]. Some studies have shown that serum ferritin dosage is a good indicator to monitor iron stores in donors, thereby allowing the early detection of ID. However, in situations where ferritin levels are normal or increased, other tests such as serum iron dosage, total iron binding capacity, transferrin saturation, and hemoglobin electrophoresis may help to better characterize the type of anemia [Citation3,Citation17].
In this study, although IDA was the most common cause of anemia, a high frequency of α-thal was also identified (16.7%), reflecting the intense migration and ethnic mix that occurred in the study region. In addition, more than one third of α-thal cases also had IDA.
According to the technical regulations for hemotherapy procedures in Brazil, identification of the different causes of anemia is still a great challenge. This is because in many cases, in parallel with the hematological diagnosis, it is necessary to use different methodologies in the differential diagnosis [Citation16]. Even so, studies have shown good results using hematological parameters of the conventional complete blood count [Citation3,Citation18–22].
The lower Hct, Hb, MCV MCH and MCHC indices observed in candidates with IDA together with α-thal may be explained by the sum of two phenotypically similar diseases, showing alterations in the mean values of erythrocyte indices depending on iron level and/or the number of globin chains affected. Thus, when these two conditions coexist, the changes are more evident than each disease in isolation [Citation1,Citation3,Citation6,Citation9].
The significantly higher values of RDW in the groups with IDA and ID associated with α-thal should be attributed to the first condition, as demonstrated by other researchers [Citation23–25]. In IDA, the erythrocyte population is more heterogeneous than in thalassemia, with anisocytosis being presented due to the presence of erythrocytes produced during progressive stages of ID or increased iron intake [Citation2]. In thalassemia, erythrocytes are more homogeneous (mostly microcytic) due to mutations that alter the synthesis of globin chains. Thus, the RDW tends to be greater in IDA than in thalassemia [Citation23,Citation25].
The association between ID and α-thal alters the homogeneous pattern of erythrocytes and may obscure the presence of the hemoglobinopathy if this parameter is considered in isolation [Citation18,Citation20,Citation21]. Although there are discussions about the discriminatory efficiency of this index, some studies have tried to demonstrate the value and relevance of parameters that evaluate the degree of anisocytosis of blood cells to differentiate the microcytic anemias [Citation3,Citation9,Citation18,Citation20–22].
Considering the high degree of association between IDA and α-thal (10.9%) in this study, which confirms reports in the literature [Citation5,Citation13,Citation26], it is essential that all IDA donors who do not respond to iron supplementation should be investigated for α-thal. This conduct may, in some cases, prevent unnecessary supplementation and even avert the risk of iron overload and allow the proper counselling of the donor [Citation3,Citation4,Citation9,Citation15,Citation17,Citation22].
It should also be borne in mind that, even using the basic molecular tests, the etiology of anemia was not clarified in a significant number of referred donation candidates (27%). In these cases, it is necessary to use more advanced tests as recommended by the WHO [Citation6,Citation9,Citation10,Citation27,Citation28].
An interesting finding from this study is that 13.2% of referred blood donor candidates who had Hct levels below the cutoff limit at screening would have been considered suitable for donation after an analysis of the erythrogram. This finding reinforces the need for rigorous controls and periodic evaluations of the screening technique. In Brazilian blood centres, about 100,000 units are not collected each year due to candidates who present low Hct thus, the blood supply is directly affected [Citation10,Citation14].
There were some limitations in the present study. First, many blood donor candidates referred due to anemia did not agree to participate in this study. Efforts to control this problem are needed to address the issue of anemia and thereby lessen the deferral of potential blood donors. Determination Board of Directors Collegiate Resolution (RDC) #34 of the Ministry of Health shows that donor health can be improved through education and awareness [Citation14,Citation16,Citation29]. Second, IDA was classified based on low levels of ferritin only following the recommendations of the WHO, however some authors suggest that this biomarker may be imprecise, mainly due to factors such as possible inflammation and in the initial stages of ID [Citation6,Citation14,Citation30].
In conclusion, our data show that, although the hematological parameters, Hct, Hb, mean corpuscular volume (MCV), mean corpuscular hemoglobin(MCH) and mean corpuscular hemoglobin concentration (MCHC) were significantly reduced in all the anemic groups studied, only the RDW had an area under the ROC curve higher than 0.7 and could be useful to discriminate the main types of microcytic anemia in blood donor candidates. However, more accurate research is needed in cases of persistent microcytosis due to the high occurrence of an association between IDA and α-thal and/or other rare types of hereditary hemoglobinopathies or other nutritional deficiencies.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes
* Hospital of Clinics, Universidade Federal do Triângulo Mineiro (HC-UFTM), Hemocentro Regional de Uberaba (HEMOMINAS Foundation), Uberaba, Minas Gerais, Brazil.
References
- Sankar VH, Arya V, Tewari D, et al. Genotyping of alpha-thalassemia in microcytic hypochromic anemia patients from North India. J Appl Genet. 2006;47(4):391–5. doi: https://doi.org/10.1007/BF03194650
- Camaschella C. How I manage patients with atypical microcytic anaemia. Br J Haematol. 2013;160:12–24. DOI:10.1111/bjh.12081.
- Matos JF, Dusse LMS, Borges KBG, et al. A new index to discriminate between iron deficiency anemia and thalassemia trait. Rev Bras Hematol Hemoter. 2016;38(3):214–19. doi: https://doi.org/10.1016/j.bjhh.2016.05.011
- Higgs DR, Weatherall DJ. The alpha thalassaemias. Cell Mol Life Sci. 2009;66(7):1154–62. DOI:10.1007/s00018-008-8529-9.
- Sharma M, Pandey S, Ranjan R, et al. Prevalence of alpha thalassemia in microcytic anemia: a tertiary care experience from North India. Mediterr J Hematol Infect Dis. 2014;7(1):e2015004), DOI:10.4084/MJHID.2015.004. eCollection 2015.
- WHO. The global prevalence of anaemia in 2011. Geneva: World Health Organization; 2015; Available from: http://www.who.int/nutrition/publications/micronutrients/global_prevalence_anaemia_2011/en/. Accessed in 2017 (Mar 3)
- Piel FB, Weatherall DJ. The α-thalassemias. New Engl J Med. 2014;371(20):1908–16. DOI:10.1056/NEJMra1404415.
- Carlos AM, Souza RAV, Souza BMB, et al. Hemoglobinopathies in newborns in the southern region of the triângulo mineiro, Brazil. Cross-sectional study. São Paulo Med J. 2015;133(5):439–44. DOI:10.1590/1516-3180.2015.00042302
- Souza RA, Carlos AM, de Souza BM, et al. Α-thalassemia: genotypic profile associated with ethnicity and hematological differentiation of iron deficiency anemia in the region of Uberaba, Minas Gerais, Brazil. Hemoglobin. 2015;39(4):264–9. DOI:10.3109/03630269.2015.1037890. Epub 2015 Jul 16.
- da Silva MA, de Souza RA, Carlos AM, et al. Etiology of anemia of blood donor candidates deferred by hematologic screening. Rev Bras Hematol Hemoter. 2012;34(5):356–60. DOI:10.5581/1516-8484.20120092
- Delaney M, Schellhase KG, Young S, et al. Blood center practice and education for blood donors with anemia. Transfusion. 2011;51(5):929–36. DOI:10.1111/j.1537-2995.2010.02919.x. Epub 2010 Oct 26.
- Tan AS, Quah TC, Low PS, et al. A rapid and reliable 7-deletion multiplex polymerase chain reaction assay for α-thalassemia. Blood. 2001;98(1):250–1. doi: https://doi.org/10.1182/blood.V98.1.250
- Rahim F. Microcytic hypochromic anemia patients with thalassemia: genotyping approach. Indian J Med Sci. 2009;63:101–8. doi: https://doi.org/10.4103/0019-5359.49286
- Dauar ET, Patavino GM, Mendrone Júnior A, et al. Risk factors for deferral due to low hematocrit and iron depletion among prospective blood donors in a Brazilian center. Rev Bras Hematol Hemoter. 2015;37(5):306–15. doi: https://doi.org/10.1016/j.bjhh.2015.05.008
- Alberti SC, Teixeira ML. Prevalência de Anemia Ferropriva em Candidatos a Doação de Sangue do Serviço de Hemoterapia da Cidade de Concórdia-SC no Mês de Junho de 2009. Revista Saúde e Pesquisa. 2010;3(2):155–9.
- Brasil. Ministério da Saúde. Agência Nacional de Vigilância Sanitária. Resolução RDC n° 34, de 29 de maio de 2014. Determina o Regulamento Técnico para os procedimentos hemoterápicos. [Internet] Brasília: Ministério da Saúde; 2014 [cited 3 Mar 2017]. Available from: http://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2014/rdc0034_11_06_2014.pdf.
- Jalali MT, Mohseni A, Keikhaei B, et al. Evaluation of diagnostic efficacy of serum sTfR assay in iron-deficiency anemia and beta-thalassemia trait in Shafa hospital, Ahvaz, Iran 2010. Eur Rev Med Pharmacol Sci. 2012;16(10):1441–45.
- Giordano PC. Strategies for basic laboratory diagnostics of the hemoglobinopathies in multi-ethnic societies: interpretation of results and pitfalls. Int J Lab Hematol. 2013;35:465–79. DOI:10.1111/ijlh.12037. Epub 2012 Dec 7.
- Weatherall D. Thalassemia: the long road from the bedside through the laboratory to the community. Nat Med. 2010;16(10):1112–5. DOI:10.1038/nm1010-1112
- Buch AC, Karve PP, Panicker NK, et al. Role of red cell distribution width in classifying microcytic hypochromic anaemia. J Indian Med Assoc. 2011;109:297–9.
- Brancaleoni V, Di Pierro E, Motta I, et al. Laboratory diagnosis of thalassemia. Int J Lab Hematol. 2016;38(Supl 1):32–40. DOI:10.1111/ijlh.12527. Epub 2016 May 16.
- Chandra H, Shrivastava V, Chandra S, et al. Evaluation of platelet and red blood cell parameters with proposal of modified score as discriminating guide for iron deficiency anemia and β-thalassemia minor. J Clin Diagn Res. 2016;10(5):EC31–4. DOI:10.7860/JCDR/2016/17672.7843
- Bessman JD, Feinstein DI. Quantitative anisocytosis as a discriminant between iron deficiency and thalassemia minor. Blood. 1979;53(2):288–93.
- Schoorl M, Schoorl M, van Pelt J, et al. Application of innovative hemocytometric parameters and algorithms for improvement of microcytic anemia discrimination. Hematol Rep. 2015;7(2):5843), DOI:10.4081/hr.2015.5843
- Matos JF. Índice de anisocitose eritrocitária (RDW): diferenciação das anemias microcíticas e hipocrômicas. Rev Bras Hematol Hemoter. 2008;30(2):120–3. doi: https://doi.org/10.1590/S1516-84842008000200009
- Bryant BJ, Hopkins JA, Arceo SM, et al. Evaluation of low red blood cell mean corpuscular volume in an apheresis donor population. Transfusion. 2009;49(9):1971–6. DOI:10.1111/j.1537-2995.2009.02207.x
- Borges E, Wenning MR, Kimura EM, et al. Alta prevalência de alfa-talassemia entre os indivíduos com microcitose e hipocromia, sem anemia. Braz J Med Biol Res. 2001;34:759–62. doi: https://doi.org/10.1590/S0100-879X2001000600009
- So C-C, Liu AK, Tsang MH, et al. Genetic basis of persistent red blood cell microcytosis in the Chinese unexplained by phenotypical testing. J Clin Pathol. 2015;68(1):69–72. DOI:10.1136/jclinpath-2014-202568. Epub 2014 Oct 28.
- Agnihotri N. Whole blood donor deferral analysis at a center in Western India. Asian J Transfus Sci. 2010;4(2):116–22. DOI:10.4103/0973-6247.67035
- Thurnham DI, McCabe LD, Haldar S, et al. Adjusting plasma ferritin concentrations to remove the effects of subclinical inflammation in the assessment of iron deficiency: a meta-analysis. Am J Clin Nutr.. 2010;92(3):546–55. DOI:10.3945/ajcn.2010.29284. Epub 2010 Jul 7.