ABSTRACT
Background: There is a great risk of infection with viral-vaccine-preventable diseases like measles, mumps, and rubella (MMR) infections after the end of chemotherapy treatment of children with acute lymphoblastic leukemia (ALL), which could have been prevented with MMR vaccination. Previous studies reported widely variable rates of seropositivity (seroprotection) for MMR after ALL treatment ends. Also, few studies evaluated the response to MMR booster vaccinations after the end of ALL treatment and reported unclear and difficult to interpret results.
Material and methods: This retrospective cross-sectional study evaluated the prevalence of seropositive (protection) antibody titer levels for MMR among ALL childhood survivors who were followed-up at Jeddah Oncology Center, Saudi Arabia. The aim of the study was also to investigate and analyze the response of seronegative patients to a booster MMR vaccination.
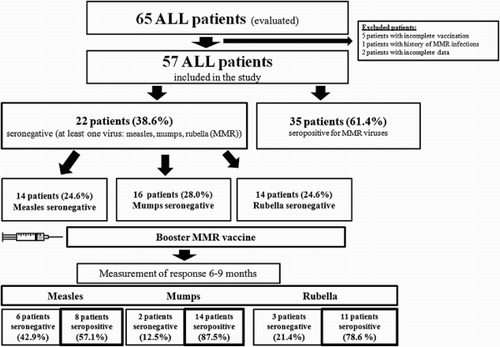
Results: Fifty-seven ALL children were evaluated. Thirty-five patients (61.4%) were seropositive/seroprotected and the remaining 22 patients (38.6%) were seronegative for MMR. ALL Children under the age of 5 years had a higher prevalence of seronegative titers. Interestingly, the prevalence of seroprotection decreased as the time interval increased post-treatment, while seroconversion rates after administering a booster MMR vaccine were 57.1%, 87.5%, and 78.6%, respectively for MMR.
Conclusion: We suggest the need for booster MMR vaccination, especially for ALL children under the age of 5 years and those who experienced a protracted time interval post-treatment.
Introduction
Cancer-afflicted children suffer from suppressed immune systems induced by the disease, chemotherapy, or both [Citation1]. Long-term chemotherapy treatments with prolonged immunosuppression carry a greater risk propensity for infection, which may have been preventable through vaccination [Citation2]. The immune system recovery may require six months to a year, before returning to normal levels [Citation3]. During this sensitive period, patients may exhibit an improper immune response thus exposing them to life-threatening infections like measles. Previous studies indicate that rates of seropositivity/seroprotection for measles, mumps, and rubella (MMR), after acute lymphoblastic leukemia (ALL) treatment ends, vary widely, with variances of ∼30–60% for measles, ∼30–92% for mumps, and ∼70–92% for rubella [Citation3,Citation4–15]. Few studies exist that evaluate the response to MMR booster vaccination, with unclear and difficult to interpret results, compounded by conflicting conclusions [Citation5,Citation7–10,Citation12–14]. This research endeavor aims to contribute and shed light in an area that requires further research and investigation.
Patients and methods
Patient population
Throughout January 2014 to December 2017, ALL childhood survivors’ follow-up examination data were reviewed from outpatient clinics of King Abdullah Medical City’s (KAMC) Oncology Center located in Jeddah, Saudi Arabia. After KAMC’s Institutional Review Board’s (IRB) approval for this retrospective cross-sectional study, patient’s medical charts, including their immunization card’s vaccination data, were examined for important clinical characteristics and historical information regarding previous MMR infection details and complaints. By evaluating the data, the prevalence of seropositive (seroprotection) antibody titer levels for MMR among the survivors and the seronegative patients’ response to a booster MMR vaccination were garnered.
Only ALL children between 1 and 18 years of age, who had their last chemotherapy treatment at least 12 months or more prior to data collection, and had received all the compulsory vaccines adhering to the National Saudi Vaccination Program (single measles vaccine dose at age of 9 months and two MMR vaccines at age of 12 and 18 months), were included in the sample size. All subjects were in remission. Any patient exhibiting low titers levels of antibody protection were categorized as seronegative/serounprotected. A letter was issued authorizing them to receive a booster MMR vaccination, from their nearest local primary health care vaccination facility. Measurement of antibody response was evaluated 6–9 months after MMR dose administration, and blood samples were routinely collected during their regular clinical visits. The patients received treatment based on modified Berlin-Frankfurt-Münster (BFM) 90 protocol standard/intermediate risk and high risk ALL protocols.
Children on active chemotherapy treatment, those in relapse, and those with a history of MMR infection or with incomplete compulsory vaccinations were excluded from the study. None of our patients were diagnosed with primary or acquired immune deficiency syndromes. All patients had a normal level of IgG in relation to age: total IgG level (mean ± SD) was 1274.82 ± 249.16 mg/dl, and the median was 1330.00 mg/dl (IQR:1090.00–1490.00 mg/dl).
Antibody assay
Anti-measles, anti-mumps, and anti-rubella virus immunoglobulin G (IgG) were tested using the ELISA method for detection of vaccine-specific antibodies using multiplex Flow Immunoassay (Rochester Lab, Rochester, NY, USA). Patients with the following levels (according to the manufacturer’s laboratory test references): Measles IgG = 13.5 AU/ml, Mumps = 9.0 AU/ml, Rubella = 10.0 IU/ml were considered to exhibit a protective titer thus seropositive/seroprotected against MMR specific infection, while those patients that had antibody titers below previously mentioned cutoff levels were considered to be seronegative/serounprotected.
Statistical analysis
Statistical analysis was conducted using SPSS software version 18.0 (SPSS, Chicago, IL, USA). The data were expressed as mean ± SD for numerical data or frequency and percentage for categorical data. Comparing means with normal distribution was completed by using Student’s t-test. Mann–Whitney U test was used for data not normally distributed. Chi-square test or Fisher’s exact test were used to compare the categorical data. Statistical significance level was present if p < 0.05.
Results
summarizes the sample groups’ characteristics. We found that 61.4% were seropositive and the remainder 38.6% were seronegative/unprotected. Collectively MMR seronegative patients were 14.0%. The seronegative rates for each vaccine are also indicated in .
Table 1. Differing ALL children characteristics.
Interestingly, for criteria such as sex of the patients, age of sampling, risk classification, treatment received, and time of sample collection from end of treatment were not significantly different between seropositive and seronegative patients (see ). ALL patients under the age of 5 years had significantly higher percentages of being seronegative with rates of 19.3%, 24.6%, and 21.1% for measles, mumps, and rubella, respectively. This is in stark contrast to patients diagnosed over the age of 5 years whose rates were 5.3%, 3.5%, and 3.5% for measles, mumps, and rubella, respectively. Although with no statistical significance, we found the seroprotective titers had decreased as the time duration increased post-therapy.
Table 2. Comparative differentiation criteria with respect to patients MMR response.
Seronegative patients administered with a booster dose of MMR vaccine (), were found afterwards to have seroconversion rates of 57.1% for measles, 87.5% for mumps, and 78.6% for rubella. No significant differences were found between the characteristics of seroconverted patients who responded to the vaccines compared to those who failed to respond to the vaccines and identified as failing revaccination shown in . Notably, no adverse effects or reactions were reported after MMR vaccination administration.
Table 3. Comparative differentiation criteria with respect to patients’ response after a booster MMR vaccine administration.
Discussion
Overall, this study found that childhood ALL survivors collective MMR seropositive rates were 61.4%, while on a discrete and individual level, the seropositive rates for each virus were 75.4%, 71.9%, and 75.4% for measles, mumps, and rubella, respectively. In comparison, previous research documented different seropositivity rates, as indicated in . This may be explained by our study sampling age range being older and the longer period experienced post-treatment. Other variables that may also contribute to and explain the comparative differences are population, treatment protocols, time period, and measurement cutoff points.
Table 4. Different studies evaluating seroprotection for measles, mumps, and rubella in ALL patients after the end of treatment.
The patients’ age on initial diagnosis of leukemia was of significance as the majority of the subjects were under the age of 5 years, exhibiting a significantly higher baseline protective antibodies titer rates to MMR. Although, of no statistical significance the response to MMR vaccination was better in the under 5 years of age. Patients’ vulnerable and immature immune system could be a contributing factor, as they greatly suffer from the suppressing effect of chemotherapy, possibly leading to slower and weaker vaccination response rates. Previous studies recorded similar findings and reported a more prevalent decrease in protective antibody levels in younger age patients [Citation7,Citation9,Citation12,Citation13].
No significant difference was found between our standard/intermediate and high-risk patients as regard patients’ initial antibody status nor their MMR booster response which was in keeping with previous research [Citation8,Citation9,Citation13].
Though of no significance, the extent of seroprotective titers decreased as the time increased, after the patients last treatment and was in line with similar research findings [Citation4,Citation15]. Conversely, Aytac et al. reported that seropositive patients, in comparison to seronegative, had a significantly longer time interval after their last chemotherapy session [Citation7].
More notably, seroconversion rates after a booster MMR vaccine were found to vary for different viruses. Other researchers reported similar results to ours (see ). The seroconversion rates of our seronegative measles patients were (57.1%), which were similar to some studies [Citation7,Citation13] yet lower than previous researchers reports [Citation8–10,Citation12]. We have no explanation why the response to measles booster vaccination was much lower than the other vaccines, namely mumps and/or rubella.
The vaccination response levels were found to be 87.5% for seronegative mumps patients, which were similar or slightly higher than other previous researchers’ findings [Citation7,Citation10,Citation13] Our rubella vaccine seroconversion rates were logged at 78.6%, again in line with other similar studies [Citation7,Citation13].
Many childhood cancer survivors persistently fail to produce/maintain protective antibody responses after receiving chemotherapy over prolonged periods of time with fluctuating expression of protective antibody to viral and/or bacterial vaccines [Citation11]. Children, who failed to produce protective antibodies, after booster vaccination, should be identified, investigated, and evaluated further as they are likely to be more at risk of contracting serious and severe infections.
Variations in the modulation of the immune response to measles vaccine, and this applies to other vaccines too, may be due to genetic host factors, including major histocompatibility human leukocyte antigen genes (HLA) and non-HLA genes, besides many other variables like: vaccine handling/storage, vaccine strain, immunization route, presence of maternal antibodies, age at immunization, time from immunization, inter-current illnesses and co-morbidities, nutritional status (vitamin deficiency, microbiome), race/ethnicity, and sex [Citation16].
After successful treatment of ALL, health care professionals (pediatricians and hematologist/oncologists) should evaluate humoral immune function [Citation11]. It is still difficult to decide between universal revaccination after ALL treatment, compared to the cost of individual testing for antibodies against each vaccine and providing only the needed vaccines. Comparatively, health care professionals should consider universal vaccine injection administration as opposed to preliminary blood sampling and then administering vaccine injection, because universal revaccination is seemingly and logistically an easier approach than the far more expensive endeavor of prior sampling/testing which adds other difficult obstacles of interpreting vaccine administration [Citation9].
It is suggested that, after the completion of chemotherapy, administration of booster vaccinations after 12 months may be a more simple and cost-effective method in restoring humoral immunity against vaccine-preventable diseases [Citation12]. Attenuated live viral vaccines (like MMR or varicella vaccines) should not be administered after the end of chemotherapy by at least 6 months and even better still after 12 months [Citation15].
Conclusion
Our study demonstrates that after ALL treatment ends, some ALL children survivors still exhibited unprotective titers against MMR viruses. Those patients who had failed to respond to a booster MMR vaccine, exhibited seroconversion rates ranging from ∼50% for measles, while rates for both mumps and rubella were logged at 78–87%. Unfortunately, we could not identify any unique patient characteristics that could predict failure to respond (seroconvert) to vaccination. Results may be due to the small sample size and one must not ignore other possible factors such as the high testing costs involved, difficultly encountered in recruiting and following-up patients in the long-term.
This study suggests the need for revaccination with a booster MMR vaccine for ALL children a year after their treatment ends, especially those diagnosed with ALL under the age of 5 years and those experiencing a protracted time interval post-treatment.
Limitation of the study
Performed in a single center on a relatively small sample size who experienced a protracted time interval after their last chemotherapy session, older aged patients were evaluated. There was no healthy control group to compare results to, even though such comparative measurements of protective titers in healthy children could be argued as having little validity, especially in the case of ALL survivors.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Ashraf E. Fouda http://orcid.org/0000-0003-1264-4156
Shaimaa M. Kandil http://orcid.org/0000-0002-9360-7295
References
- Mustafa MM, Buchanan GR, Winick NJ, et al. Immune recovery in children with malignancy after cessation of chemotherapy. J Pediatr Hematol Oncol. 1998;20(5):451–457. Available from: https://www.ncbi.nlm.nih.gov/pubmed/9787318 doi: https://doi.org/10.1097/00043426-199809000-00008
- van Tilburg CM, Sanders EA, Rovers MM, et al. Loss of antibodies and response to (re-)vaccination in children after treatment for acute lymphocytic leukemia: a systematic review. Leukemia. 2006;20(10):1717–1722. Available from: https://www.ncbi.nlm.nih.gov/pubmed/16888619 doi: https://doi.org/10.1038/sj.leu.2404326
- Kovacs GT, Barany O, Schlick B, et al. Late immune recovery in children treated for malignant diseases. Pathol Oncol Res. 2008;14(4):391–397. Available from: https://www.ncbi.nlm.nih.gov/pubmed/18575827 doi: https://doi.org/10.1007/s12253-008-9073-5
- Perkins JL, Harris A, Pozos TC. Immune dysfunction after completion of childhood leukemia therapy. J Pediatr Hematol Oncol. 2017;39(1):1–5. Available from: https://www.ncbi.nlm.nih.gov/pubmed/27820131 doi: https://doi.org/10.1097/MPH.0000000000000697
- Koochakzadeh L, Khosravi MH, Pourakbari B, et al. Assessment of immune response following immunization with DTP/Td and MMR vaccines in children treated for acute lymphoblastic leukemia. Pediatr Hematol Oncol. 2014;31(7):656–663. Available from: https://www.ncbi.nlm.nih.gov/pubmed/25007393 doi: https://doi.org/10.3109/08880018.2013.877111
- Bochennek K, Allwinn R, Langer R, et al. Differential loss of humoral immunity against measles, mumps, rubella and varicella-zoster virus in children treated for cancer. Vaccine. 2014;32(27):3357–3361. Available from: https://www.ncbi.nlm.nih.gov/pubmed/24793952 doi: https://doi.org/10.1016/j.vaccine.2014.04.042
- Aytac S, Yalcin SS, Cetin M, et al. Measles, mumps, and rubella antibody status and response to immunization in children after therapy for acute lymphoblastic leukemia. Pediatr Hematol Oncol. 2010;27(5):333–343. Available from: https://www.ncbi.nlm.nih.gov/pubmed/20469978 doi: https://doi.org/10.3109/08880011003767720
- Zengin E, Sarper N. Humoral immunity to diphtheria, tetanus, measles, and hemophilus influenzae type b in children with acute lymphoblastic leukemia and response to re-vaccination. Pediatr Blood Cancer. 2009;53(6):967–972. Available from: https://www.ncbi.nlm.nih.gov/pubmed/19544393 doi: https://doi.org/10.1002/pbc.22135
- Patel SR, Ortín M, Cohen BJ, et al. Revaccination of children after completion of standard chemotherapy for acute leukemia. Clin Infect Dis. 2007;44(5):635–642. Available from: https://www.ncbi.nlm.nih.gov/pubmed/17278052 doi: https://doi.org/10.1086/511636
- Ercan TE, Soycan LY, Apak H, et al. Antibody titers and immune response to diphtheria–tetanus–pertussis and measles–mumps–rubella vaccination in children treated for acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2005;27(5):273–277. Available from: https://www.ncbi.nlm.nih.gov/pubmed/15891564 doi: https://doi.org/10.1097/01.mph.0000163214.37147.5a
- Brodtman DH, Rosenthal DW, Redner A, et al. Immunodeficiency in children with acute lymphoblastic leukemia after completion of modern aggressive chemotherapeutic regimens. J Pediatr. 2005;146(5):654–661. Available from: https://www.ncbi.nlm.nih.gov/pubmed/15870670 doi: https://doi.org/10.1016/j.jpeds.2004.12.043
- Zignol M, Peracchi M, Tridello G, et al. Assessment of humoral immunity to poliomyelitis, tetanus, hepatitis B, measles, rubella, and mumps in children after chemotherapy. Cancer. 2004;101(3):635–641. Available from: https://www.ncbi.nlm.nih.gov/pubmed/15274078 doi: https://doi.org/10.1002/cncr.20384
- Nilsson A, De Milito A, Engström P, et al. Current chemotherapy protocols for childhood acute lymphoblastic leukemia induce loss of humoral immunity to viral vaccination antigens. Pediatrics. 2002;109(6):e91. Available from: https://www.ncbi.nlm.nih.gov/pubmed/12042585 doi: https://doi.org/10.1542/peds.109.6.e91
- Smith S, Schiffman G, Karayalcin G, et al. Immunodeficiency in long-term survivors of acute lymphoblastic leukemia treated with Berlin–Frankfurt–Münster therapy. J Pediatr. 1995;127(1):68–75. Available from: https://www.ncbi.nlm.nih.gov/pubmed/7608814 doi: https://doi.org/10.1016/S0022-3476(95)70259-8
- Esposito S, Prada E, Lelii M, et al. Immunization of children with secondary immunodeficiency. Hum Vaccin Immunother. 2015;11(11):2564–2570. Available from: https://www.ncbi.nlm.nih.gov/pubmed/26176360 doi: https://doi.org/10.1080/21645515.2015.1039208
- Haralambieva IH, Kennedy RB, Ovsyannikova IG, et al. Variability in humoral immunity to measles vaccine: new developments. Trends Mol Med. 2015;21(12):789–801. Available from: https://www.ncbi.nlm.nih.gov/pubmed/26602762 doi: https://doi.org/10.1016/j.molmed.2015.10.005