ABSTRACT
Objective: In China, the ability of the current immune thrombocytopenia (ITP) guideline to stratify recurrent risk at diagnosis is limited. This study aimed to investigate whether mean platelet volume at diagnosis (MPV) is a risk factor for ITP relapse in Chinese.
Methods: The present study was a retrospective cohort study. Two hundred thirty-three adult patients with newly diagnosed ITP were consecutively and nonselectively collected from March 2013 to June 2017. The exposure and outcome variable were MPV at baseline and relapse-free survival at 6 months. Other covariants included demographic data, general information, variables that can affect MPV reported by previous literature and risk factors of ITP relapse.
Results: After adjusting potential confounders, the non-linear relationship was detected between MPV and ITP relapse, and inflection point was 21. The effect sizes and the confidence intervals on the left and right sides of inflection point were 1.30 (1.22–1.39) and 0.89 (0.76–1.04), respectively. Subgroup analysis showed, in subjects with hyperuricemia (1.54 (1.24, 1.90)), MPV showed significant differences from non-hyperuricemia (1.19 (1.13, 1.25)), and the p for interaction was less than 0.05.
Conclusion: The relationship between MPV and ITP relapse is non-linear. MPV is an independent risk factor of ITP relapse when MPV is less than 21 fl.
Introduction
For adult patients with newly diagnosed immune thrombocytopenia (ITP), relapse is frequent after treatment with first-line therapy [Citation1]. Roughly 50% of patients relapse within 6 months [Citation2]. In China, the ability of the current ITP guideline to stratify recurrent risk at diagnosis is limited [Citation3]. Only a few studies have investigated the relationship between risk factors and recurrence in adult newly diagnosed ITP, and several candidate risk factors have been shown to be prognostic. For example, anti-Helicobacter pylori (HP) treatment can reduce the relapse rate in ITP patients with HP infection [Citation4–6]. Additionally, the incidence of ITP relapse and treatment resistance in elderly is higher than that of in young patients [Citation7,Citation8]. Thus, one pivotal step to improve outcomes in adult patients with newly diagnosed ITP is identifying sensitive markers that can improve our risk-stratification algorithms.
Mean platelet volume (MPV) is a hallmark of platelet activation [Citation9]. Internists widely use it in routine clinical practice. Previous findings have shown MPV have the potential for ITP diagnosis and differential diagnosis [Citation10–13]. However, only a few studies devoted to clarifying the correlation between MPV and ITP relapse, and these studies are underpowered to effectively address this issue [Citation14,Citation15]. The main reasons can be attributed to the study design and data analysis deficiencies.
In this study, we performed a retrospective cohort study to observe whether MPV is a risk factor for ITP relapse. We use MPV measured at baseline as exposure. The outcome variable is ITP relapse within 6 months. Other covariates include sex, age, height, weight, tobacco use, alcoholic consumption, HP infection status, diabetes history, cardiovascular diseases (CVD) history, hyperuricemia history, platelet count at baseline, bleeding symptoms (skin, mucosa, organ), first-line treatment strategy (oral prednisone, high-dose dexamethasone, intravenous immunoglobulin (IVIG)), mean platelet distribution width (MPD) at baseline.
Patients and methods
Study population
We conducted a retrospective cohort study at Department of Hematology of the People’s Hospital of Guizhou province, Guiyang city, China from March 2013 to June 2017. The patient’s clinical information is recorded in the hospital’s electronic medical record system. We retrospectively and nonselectively collected 437 patients with newly diagnosed ITP who were recorded in the system. The diagnosis of ITP was based on guidelines [Citation3]. We screened subjects referring to inclusion and exclusion standard. Explicit inclusion: (1) newly diagnosed ITP (defined as ITP diagnosed less than 3 months); (2) patients were excluded from myelofibrosis or other thrombocytopenic diseases by myeloid morphological examination and (3) normal spleen size. Exclusion: (1) secondary ITP; (2) drug-induced thrombocytopenia; (3) hepatitis B virus antigen, hepatitis C virus antigen or HIV virus antigen was positive; (4) patients did not respond to first-line treatment; (5)the participants were in gestational age; (6) severe dysfunction of heart, kidney, liver or lung and (7) patients took immunosuppressants within the past 3 months. Researchers obtained information (values) of MPV and other covariants at baseline. Because the study was a retrospective cohort study, the data were anonymous, and the requirement for informed consent was therefore waived.
Measurement of MPV at baseline
MPV at baseline was used as the exposure variable. MPV was specified as an untransformed continuous variable. We collected 2 ml of patient’'s venous blood and placed it in an EDTA-containing anticoagulant tube. The specimens were sent to the hospital center laboratory and analyzed by automatic hematology analyzer (BM830, Bao Ling Man Sunshine Technology Co. Ltd, China). The normal range of MPV was 6.5–12.5 fl in our institution.
Other covariates involved in this study
The following variables were used: sex, age, height, weight, tobacco use, alcoholic consumption, HP infection status, diabetes history, CVD history, hyperuricemia history, platelet count at baseline, bleeding symptoms (skin, mucosa, organ), first-line treatment strategy (oral prednisone, high-dose dexamethasone, IVIG) and MPD at baseline. All of these above covariants included demographic data, general information, variables that can affect MPV reported by previous literature, and risk factors of ITP relapse [Citation7,Citation16–23].
First-line treatment strategies
In this study, first-line treatment included oral prednisone, high-dose dexamethasone and IVIG. According to the guidelines, first-line treatment alternatives for newly diagnosed ITP patients were determined by the patient’s platelet count at diagnosis and bleeding symptoms. High-dose dexamethasone, oral prednisone and IVIG regimens were described in detail in the relevant guideline [Citation3].
Outcome variable definition
In the present study, we used relapse-free survival at 6 months as the outcome variable. We evaluated the curative efficacy according to guideline [Citation3]. Complete remission (CR) was defined as platelet count below100*109/l and absence of bleeding. Response (R) was defined as platelet count above 30*109/l and at least twofold increase of the baseline platelet count and absence of bleeding. No response (NR) was defined as a platelet count below 30*109/l or twofold increase of the baseline platelet count or bleeding. Relapse was defined as loss of CR (or R) during follow-up time (6 months). It was noted that determining relapse must meet the following conditions: platelet counts should be tested at least twice, and interval could not be less than 1 day. Where dates of follow-up were missing, the event records were excluded.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation (normal distribution) or median (quartile) (skewed distribution). Categorical variables were expressed in frequency or as a percentage. The one-way ANOVA (normal distribution), Kruscal–Wallis H (skewed distribution) test and chi-square tests (categorical variables) were used to determine any statistical difference between the means and proportions of the groups. Univariate Cox regression model was used to evaluate the associations between exposure and outcome. Both non-adjusted and multivariate-adjusted models were used. According to the recommendation of STROBE statement [Citation24], we simultaneously showed the results of unadjusted or minimally adjusted analyses and those from fully adjusted analyses. Whether the covariance was adjusted determined by the following principle: when added to this model, changed the matched odds ratio by at least 10% [Citation25]. Besides, we also used generalized additive model (GAM) to identify the non-linear relationship. If the non-linear correlation was observed, a two-piecewise linear regression model was performed to calculate the threshold effect of the MPV on ITP relapse according to the smoothing plot. The threshold level of MPV at which the relationship between relapse and MPV level began to change and became notable was determined using a recurrence method. The inflection point was moved along a pre-defined interval and detected the inflection point that gave the maximum model likelihood [Citation26]. The subgroup analyses were performed using stratified Cox regression models. Tests for effect modification by subgroup used interaction terms between subgroup indicators, followed by the likelihood ratio test. All the analyses were performed with the statistical software packages R (http://www.R-project.org, The R Foundation) and EmpowerStats (http://www.empowerstats.com, X&Y Solutions, Inc, Boston, MA, USA). p values less than 0.05 (two-sided) were considered statistically significant.
Results
The selection of patients
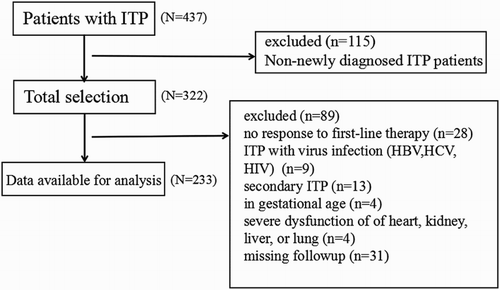
Of the 437 ITP patients (), 115 patients with non-newly diagnosed ITP were excluded from this study. In the remaining 322 patients, 89 patients were excluded. In 89 excluded subjects, 28 did not respond to first-line treatment, 9 have virus infection (including HBV, HCV, HIV), 13 were diagnosed as secondary ITP, 4 were in gestational age, 4 complicated with severe dysfunction of important organ and 31 loss follow-up, leaving 233 patients for data analysis.
Baseline characteristics of participants
The baseline characteristics of patients with newly diagnosed ITP were listed in . The mean age of patients was 41.1 ± 19.0 years, and approximately 66.1%(154/233) of the population was female. Among the 233 patients with newly diagnosed ITP, 85 relapsed within 6 months (36.48%), and the mean relapse time was 71.0 ± 15.8 day. We converted the exposure variable (MPV) from a continuous variable to a categorical variable (quartile) and compared baseline variability among different levels of MPV. There was no statistically significant difference in weight, baseline platelets, MPD, smoking status, alcoholic consumption, history of hyperuricemia and CVD, bleeding symptoms (skin, mucosa, organ) and the use of IVIG among different MPV groups. Compared with high-level (Q4) of MPV group, patients had a significantly lower age, the relapse rate in other three groups (Q1–Q3). In contrast, lower relapse time in Q4 was observed compared with other three groups (Q1–Q3) .
Table 1. Baseline characteristics of participants.
Univariate analysis
We listed the results of univariate analyses in . We found that age (1.03, 1.02–1.04), Hp infection (3.17, 2.03–4.94), bleeding in mucosa (2.07, 1.33–3.23) and organ (1.88, 1.04–3.39), MPV (1.20, 1.15–1.24) and MPD (1.06, 1.01–1.11) were the risk factors of ITP relapse. In contrast, univariate analysis showed that IVIG (0.60, 0.39–0.94) was correlated with low recurrence of ITP.
Table 2. The results of univariate analysis using cox regression model.
The results of unadjusted and adjusted models using
We used Cox regression models to enlighten whether baseline MPV was associated with ITP relapse. In the present study, we showed the non-adjusted and adjusted models in . In crude model (we did not adjust any covariate), for each 1 fl increase in MPV, the risk of recurrence within the first 6 months of ITP was increased by 20%(HR = 1.20, 95% confidence interval (CI): 1.15–1.24, p < 0.001). In minimally adjusted model (adjusted age, sex, height, weight), the trend of HR did not to be altered (HR = 1.21, 95%CI: 1.16–1.25, p < 0.001). In fully adjusted model (HR = 1.23, 95%CI: 1.17–1.29, p < 0.001), the change of HR was not obvious after adjusting age, sex, weight, height, smoking status, alcoholic consumption, HP infection, diabetes history, hyperuricemia, CVD, baseline platelets, bleeding in skin, bleeding in mucosa, bleeding in organ, treatment protocol (high-dose dexamethasone, oral prednisone, IVIG) and MPD. The trend of HR in different adjustment strategies showed that MPV was an independent risk factor for 6-month recurrence of ITP, and the results were stable. In addition, for the purpose of sensitivity analysis, we also handled MPV as a categorical variable (Quartile). The same trend was observed as well (p for trend was <0.001).
Table 3. The results of multivariate analysis using non-adjusted and adjusted cox regression models.
The analyses for non-linear relationship and the saturation or threshold effects
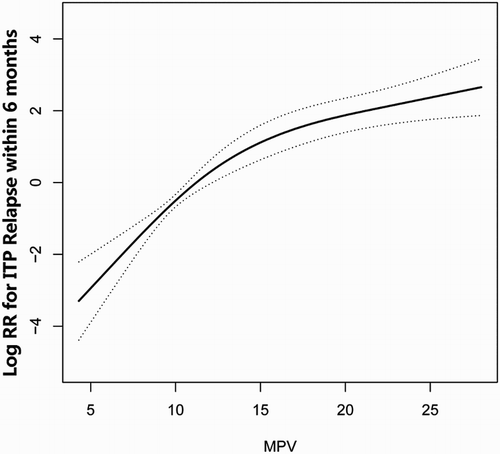
Given that MPV was a continuous variable, we considered the possibility of a non-linear conjunction between the MPV and ITP recurrence. In the present study (), we found that the relevancy between MPV and ITP relapse was non-linear (after adjusting for age, sex, weight, height, smoking status, alcoholic consumption, HP infection, diabetes history, hyperuricemia, CVD, baseline platelets, bleeding in skin, bleeding in mucosa, bleeding in organ, treatment protocol (high-dose dexamethasone, oral prednisone, IVIG) and MPD). By two-piecewise linear regression model, we calculated the inflection point was 21. On the left of inflection point, the effect size, 95%CI and p-value were 1.3, 1.2–1.4 and <0.001, respectively. However, we could not detect the relationship between MPV and ITP relapse on the right of inflection point (0.9, 0.8–1.0, 0.148) ().
Table 4. The results of two-piecewise linear regression model.
The results of subgroup analyses
In the present study, we showed the results of subgroup analyses in . We found the test for interaction was significant for hyperuricemia (p for interaction = 0.011). The test for interactions were not statistically significant for sex, age, smoking status, drinking, HP infection, diabetes ,CVD, bleeding in skin, mucosa, organ, high-dose dexamethasone, IVIG and oral prednisone (p for interaction = 0.695, 0.055, 0.403, 0.110, 0.07, 0.220, 0.680, 0.052, 0.730, 0.290, 0.327, 0.053 and 0.327, respectively). We observed there were evidences for the interactions MPV and hyperuricemia. In ITP patients without hyperuricemia, HR was 1.19 (1.13–1.25). In patients with hyperuricemia ITP, HR was 1.54(1.24–1.90). HR values between hyperuricemia and non-hyperuricemia had significant differences (detected by log-likelihood ratio test).
Table 5. The results of subgroup analysis and interaction analysis.
Discussion
This retrospective cohort study of 233 patients with newly diagnosed ITP revealed a stable association between partial detectable levels of MPV (≤21 fl) and an increased risk of ITP 6-months recurrence after adjustment of potential confounders. In addition, we also found there was a non-linear relationship between MPV and risk of ITP relapse. The linear increase between relapse risk and MPV reached a peak at an MVP of 21 fl (saturation effect), and an elevation in MPV above 21 fl did not increase the risk of ITP recurrence.
We conducted a PubMed search simultaneously using the key words‘MPV’and ‘ITP’. Thirty scientific papers were retrieved on database as of the end of January 2018. Of the 30 papers searched, only 2 was related to our study purpose. However, there were some potential drawbacks of study design and data analysis in these earlier studies, and therefore cannot provide credible evidence to substantiate the true relationship between MPV and ITP prognosis. In a study of 81 patients with newly diagnosed ITP, researchers observed MPV is gradually increasing before first and second relapses, and again normal values are being obtained after appropriate therapies [Citation15]. However, the researchers only compared MPV changes at different time points and did not take into account the individual differences of baseline platelets. In addition, they did not adjust potential confounding factors. The same mistakes were found in the other study. Ahmed et al. [Citation14]. reported MPV were found to be independent prognostic variables that predicted for the achievement of a durable CR in childhood ITP. However, their findings relied on linear extrapolation or on data unadjusted for important potential confounders. More importantly, the study design of these two articles was not based on the use of MPV at baseline to predict patients’ likelihood of recurrence in the future. In contrast to them, our purpose was to stratify recurrent risk at diagnosis. Besides, we not only adjusted the confounders reported in the previous literature that may affect the prognosis of ITP, we also further explored the non-linear relationship between MPV and ITP recurrence. To our knowledge, it is the first time to report the saturation effect of MPV and ITP recurrences and give the exact inflection point.
According to STROBE statement [Citation24], subgroup analysis can make better use of data to reveal the underlying truth. In this study, we performed an interaction test on the raw data (). We found hyperuricemia was the effect modifier to modify the relationship between MPV and ITP recurrence. In this study, we found the effect size of the relationship between MPV and ITP prognosis is magnified in patients with hyperuricemia compared with patients with non-hyperuricemia. Although we cannot find evidence from the previous literature to explain these findings, we at least observe patients with hyperuricemia have higher risk of relapse than non-hyperuricemia when patients have increased baseline MPV.
Our study has a number of strengths. First, compared with the previous studies, our study had a larger sample size. Second, cohort inclusion and exclusion criteria are more stringent. Many potential confounders were eliminated at the patient screening stage. Third, we observed the trends of the effects size of MPV as continuous variables and categorical variables (quartiles) and tested the p for trend when MPV was used as a categorical variable. The results were robust. Fourth, we not only use the generalized linear model to evaluate the linear relationship between MPV and ITP relapse but also use the GAM to clarify the non-linear relationship. GAM has obvious advantages in dealing with non-linear relations. This model can handle the non-parametric smoothing and will fit a regression spline to the data. The use of GAM will help us to better discover the real relationship between exposure and outcome [Citation27]. Fifth, this study is an observational study and therefore susceptible to potential confounding. We used strict statistical adjustment to minimize residual confounding. Sixth, the effect modifier factor analysis makes the use of data better.
There are some limitations in our study. First, the study population is only Chinese, its generalisability is limited. Second, in order to purify the cohort, we did not include non-newly diagnosed patients and did not analyze patients undergoing second-line treatment.
Acknowledgement
Authors appreciate Shou-Xin Zhang (a professional scientific editor) for the careful revision of this paper.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Peng-Xiang Guo http://orcid.org/0000-0002-4408-4053
References
- Neunert CE. Management of newly diagnosed immune thrombocytopenia: can we change outcomes? Blood Adv. 2017;1:2295–2301.
- Cuker A, Prak ET, Cines DB. Can immune thrombocytopenia be cured with medical therapy? Semin Thromb Hemost. 2015;41:395–404.
- Consensus of Chinese experts on diagnosis and treatment of thrombotic thrombocytopenic purpura (version 2012). Zhonghua Xue Ye Xue Za Zhi. 2012;33:983–984.
- Kuwana M. Helicobacter pylori-associated immune thrombocytopenia: clinical features and pathogenic mechanisms. World J Gastroenterol. 2014;20:714–723.
- Frydman GH, Davis N, Beck PL, et al. Helicobacter pylori eradication in patients with immune thrombocytopenic purpura: A review and the role of biogeography. Helicobacter. 2015;20:239–251.
- Ahn YS. Triple play of H pylori in ITP. Blood. 2010;115:4155–4156.
- Bizzoni L, Mazzucconi MG, Gentile M, et al. Idiopathic thrombocytopenic purpura (ITP) in the elderly: clinical course in 178 patients. Eur J Haematol. 2006;76:210–216.
- Michel M, Wasser J, Godeau B, et al. Efficacy and safety of the thrombopoietin receptor agonist romiplostim in patients aged ≥ 65 years with immune thrombocytopenia. Ann Hematol. 2015;94:1973–1980.
- Bath PM, Butterworth RJ. Platelet size: measurement, physiology and vascular disease. Blood Coagul Fibrinolysis. 1996;7:157–161.
- Tang YT, He P, Li YZ, et al. Diagnostic value of platelet indices and bone marrow megakaryocytic parameters in immune thrombocytopenic purpura. Blood Coagul Fibrinolysis. 2017;28:83–90.
- Negash M, Tsegaye A GMA. Diagnostic predictive value of platelet indices for discriminating hypo productive versus immune thrombocytopenia purpura in patients attending a tertiary care teaching hospital in Addis Ababa, Ethiopia. BMC Hematol. 2016;16:911.
- Khaspekova SG, Shustova ON, Golubeva NV, et al. Relationships of mean platelet volume and plasma thrombopoietin with glycocalicin levels in thrombocytopenic patients. Acta Haematol. 2015;133:295–299.
- Noris P, Klersy C, Gresele P, et al. Platelet size for distinguishing between inherited thrombocytopenias and immune thrombocytopenia: a multicentric, real life study. Br J Haematol. 2013;162:112–119.
- Ahmed S, Siddiqui AK, Shahid RK, et al. Prognostic variables in newly diagnosed childhood immune thrombocytopenia. Am J Hematol. 2004;77:358–362.
- Korkmaz S, Uslu AU, Aydin B, et al. Pre-treatment and post-treatment changes in platelet indices in patients with immune thrombocytopenia. Saudi Med J. 2013;34:591–596.
- Zaccardi F, Rocca B, Rizzi A, et al. Platelet indices and glucose control in type 1 and type 2 diabetes mellitus: A case-control study. Nutr Metab Cardiovasc Dis. 2017;27:902–909.
- Rusak T, Misztal T, Rusak M, et al. Involvement of hyperglycemia in the development of platelet procoagulant response: the role of aldose reductase and platelet swelling. Blood Coagul Fibrinolysis. 2017;28:443–451.
- Damaske A, Muxel S, Fasola F, et al. Peripheral hemorheological and vascular correlates of coronary blood flow. Clin Hemorheol Microcirc. 2011;49:261–269.
- Sertbas Y, Sertbas M, Okuroglu N, et al. Mean platelet volume changes before and after glycated hemoglobin (HbA1c) improvement in a large study population. Arch Med Sci. 2017;4:711–715.
- Wasilewska A, Tenderenda E, Taranta-Janusz K, et al. High-sensitivity C-reactive protein and mean platelet volume in paediatric hypertension. Pediatr Nephrol. 2010;25:1519–1527.
- Huczek Z, Kochman J, Kowara MK, et al. Baseline platelet indices and bleeding after transcatheter aortic valve implantation. Blood Coagul Fibrinolysis. 2015;26:527–532.
- Muscari A, De Pascalis S, Cenni A, et al. Determinants of mean platelet volume (MPV) in an elderly population: relevance of body fat, blood glucose and ischaemic electrocardiographic changes. Thromb Haemost. 2008;99:1079–1084.
- Chandan JS, Thomas T, Lee S, et al. The association between idiopathic thrombocytopenic purpura and cardiovascular disease: a retrospective cohort study. J Thromb Haemostasis. 2018;16(3):474–480.
- Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. PLoS Med. 2007;4:e297.
- Kernan WN, Viscoli CM, Brass LM, et al. Phenylpropanolamine and the risk of hemorrhagic stroke. N Engl J Med. 2000;343:1826–1832.
- Liu S, Wang X, Lu Y, et al. The effects of intraoperative cryoprecipitate transfusion on acute renal failure following orthotropic liver transplantation. Hepatol Int. 2013;7:901–909.
- Forest S. Nonlinear regularization operators as derived from the micromorphic approach to gradient elasticity, viscoplasticity and damage. Proc Math Phys Eng Sci. 2016;472:20150755.